Ag2S或CuS掺杂的Zn0.5Cd0.5S纳米晶体:溶剂热法合成及光致发光性能
2010-09-15朱金保畅晓莹朱国军袁高清
朱金保 畅晓莹 朱国军 袁高清
(华南理工大学化学与化工学院,广州 510640)
Ag2S或CuS掺杂的Zn0.5Cd0.5S纳米晶体:溶剂热法合成及光致发光性能
朱金保 畅晓莹 朱国军 袁高清*
(华南理工大学化学与化工学院,广州 510640)
以异丙醇为溶剂,醇热法制备Zn0.5Cd0.5S和Ag2S或CuS掺杂的Zn0.5Cd0.5S纳米晶体,考察了这些纳米晶体在可见光区域的光致发光性能。结果表明,反应温度和反应时间、掺杂剂的浓度和种类对Zn0.5Cd0.5S的发光性能有很大的影响,相比未掺杂Zn0.5Cd0.5S纳米晶体而言,Ag2S或CuS掺杂后其光致发光强度明显增强、半高宽更宽。
半导体;光致发光;纳米晶体;光学材料
In the last decade,Ⅱ-Ⅵ semiconductor nanomaterials,especially Cd1-xZnxS nanomaterials,have aroused considerable interest due to their potential applications in many areas,such as the window material for solar cells[1],photocatalysts for the production of hydrogen with visible light[2-4],and photoconductive devices[5].Besides,Cd1-xZnxS can have a tunable band gap from 2.4 to 3.7 eV via changing Cd content,which makes it a valuable optical material[6-8].
Many efforts have been devoted to improve photoluminescence (PL)properties of CdS or ZnS nanocrystals through doping.For example,ZnS nanocrystals doped with MnS and FeS could exhibit stronger PL emission spectra[9].The Mn2+-doped CdS nanocrystals capped with ZnS displayed much stronger and sharper yellow PL emission compared with the undoped sample[10].ZnS nanocrystals doped with CuS show a better PL properties especially when the concentrationof the dopant Cu2+ionsreaches 1mol%[11].In addition,it has been reported that the PL performance of ZnS could be also enhanced when doped with Al3+,Ga3+,In3+[12]and Ag+[13].To the best of our knowledge,there has been no report on PL properties related to the Zn0.5Cd0.5S nanocrystals doped with Ag2S and CuS although many efforts have been devoted to the synthesis and optical properties of Cd1-xZnxS nanoparticles[14-17].Herein,we report the enhancement of the PL intensity of Zn0.5Cd0.5S nanocrystals via changing concentration of dopants Ag2S and CuS as well as preparation conditions.
1 Experimental
1.1 Synthesis
All reagents(zinc acetate,copper acetate,cadmium nitrate,AgNO3,sodium sulfide and isopropanol)were purchased from Sinopharm Chemical Reagent Co.Ltd(Shanghai,China)in analytical grade and used without further purification.Cadmium nitrate,zinc acetate and sodium sulfide were used as the precursors for the synthesis of Zn0.5Cd0.5S.Silver nitrate was used as the dopant.In a typical experimental process,cadmium nitrate(2 mmol),zinc acetate(2 mmol),AgNO3(0.02 mmol)and isopropanol(20 mL)were added into a 50 mL Teflon-lined stainless-steel autoclave,followed by adding sodium sulfide (1.3 g)under continuously stirring.The autoclave was sealed and kept at 100℃for 3 h.The resulting yellow solid products were filtered and washed with absolute ethanol and water for several times,then dried in a vacuum oven at 60℃for 6 h.The Zn0.5Cd0.5S nanocrystals doped with Ag2S were finally obtained.The amount of Ag2S in Zn0.5Cd0.5S nanocrystals was adjusted by changing the concentration of silver nitrate from 0%to 2% (in molar ratio of silver ions to zinc ions or cadmium ions).Similarly,with copper acetate as the dopant,Zn0.5Cd0.5S nanocrystals doped with CuS could be obtained.
1.2 Characterization
The obtained samples were characterized by X-ray diffraction(XRD),scanning electron microscopy(SEM).An X-ray diffractometer(Rigaku D/max-ⅢA,Japan)with theCu Kα (λ=0.154 18 nm)radiation source was used for XRD analysis in the 2θ range from 20°to 60°,and the applied current and voltage were 30 mA and 40 kV,respectively.SEM images were taken in a LEO 1530VP field emission scanning electron microscope operated atan accelerating voltage of10 kV.Ultraviolet-Visible (UV-Vis)absorption spectra were recorded on an UV-Vis spectrometer(U3010 Hitachi,Japan).Photoluminescence spectra were obtained by using a Hitachi F-4500 fluorescence spectrometer.
2 Results and discussion
The XRD patterns of the undoped and doped Zn0.5Cd0.5S samples (with 1mol%Ag2S and CuS as the dopants,respectively)are shown in Fig.1.In the CuS doping case,no characteristic peaks of CuS are observed (Fig.1b)within the resolution limit of the diffraction.A similar phenomenon was also observed in Cu-doped Cd0.95Zn0.05S system[18].The reason may be that the radius of Cu2+(r=0.072 nm)approximates to that of Zn2+(r=0.074 nm)and Cd2+(0.097 nm)so that dopant CuS can easily enter the crystal lattice of Zn0.5Cd0.5S[19].With Ag2S as the dopant,one apparent characteristic peak of Ag2S is observed (Fig.1c),which is different from that in CuS-doped system.Since the radius of Ag+(r=0.126 nm)is much larger than that of Zn2+(r=0.074 nm)and Cd2+(0.097 nm),it is not easy for Ag2S to enter Zn0.5Cd0.5S crystal lattices.In this case,Ag2S with Zn0.5Cd0.5S probably constitutes a solid mixture or solid solution.In all cases,there appear broaden diffractions due to the formation of nanoparticles.It is worth noting that the doping with Ag2S or CuS does not affect theshapes of diffraction peaks of Zn0.5Cd0.5S.From the XRD patterns,the average diameter of the undoped and doped Zn0.5Cd0.5S crystals is estimated to be 2.7 nm according to the Debye-Scherrer formula.
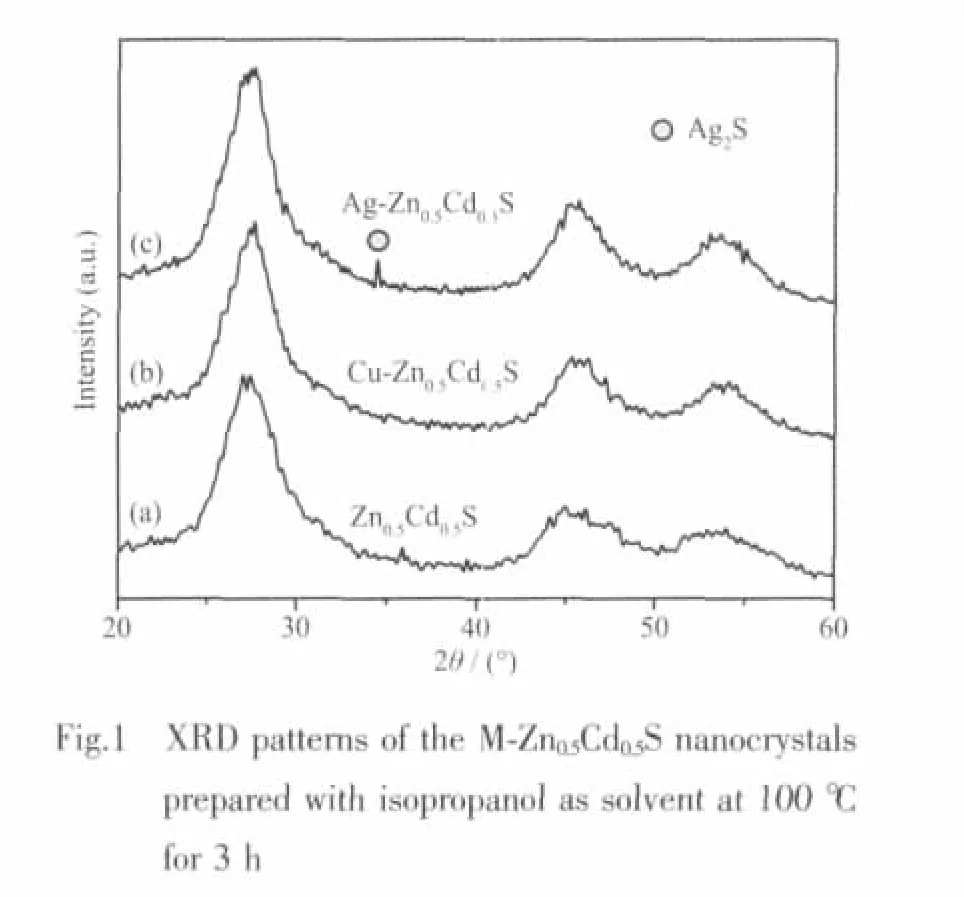
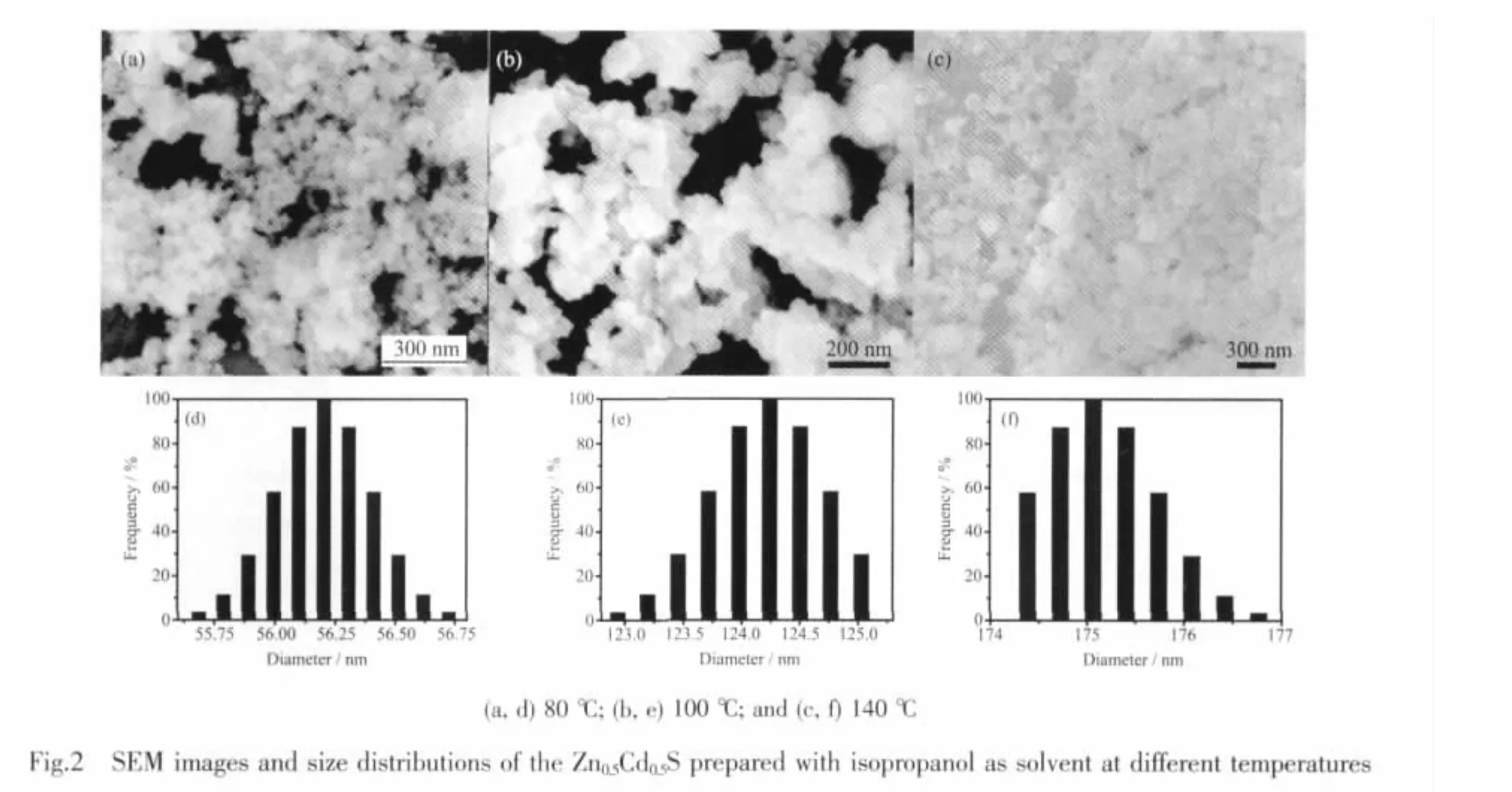
Fig.2 shows the SEM images and the size distributions of Zn0.5Cd0.5S prepared via solvothermal method at different temperatures.The as-synthesized Zn0.5Cd0.5S exhibits irregular nanogranular shapes(Fig.2).The average size of Zn0.5Cd0.5S nano-granules prepared at 80℃is 56 nm (Fig.2d),which is smaller than the average size of 175 nm(Fig.2f)obtained at 140℃.The reason may be that raising temperature could promote the growth and aggregation of the Zn0.5Cd0.5S crystals.Moreover,the obvious discrepancy with XRD related results is attributed to the fact that the prepared Zn0.5Cd0.5S nanocrystals are agglomerates and not individual nano-grains themselves.
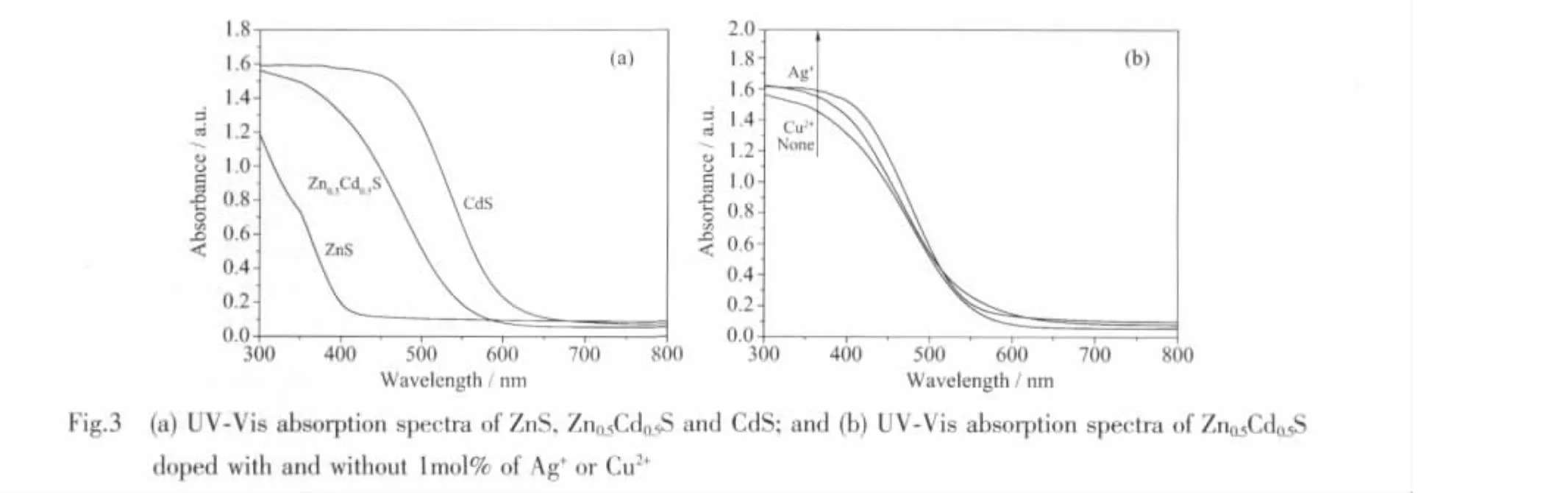
Fig.3a shows the UV-Vis absorption spectra of ZnS,Zn0.5Cd0.5S and CdS nanocrystals.The band gap values calculated from the derivative of absorption spectra of ZnS,Zn0.5Cd0.5S and CdS are 3.65 eV(340 nm),2.76 eV (450 nm)and 2.38 eV (520 nm),respectively.The blue shift in absorption edge for Zn0.5Cd0.5S is due to the doping effect of ZnS in CdS crystal lattices.Fig.3b exhibits the UV-Vis absorption spectra of the Zn0.5Cd0.5S nanocrystals doped with and without Ag+or Cu2+.Compared with the undoped one,the Zn0.5Cd0.5S nanocrystals doped with Ag2S or CuS seem to have higher UV-Vis absorption.
It has been reported that PL properties of semiconductor nanocrystals are strongly dependent on their surface states[20],surface passivation[21]capped effect[22]and size distributions[23].These are related to their preparation method and reaction conditions(suchas reaction time and reaction temperature).Fig.4 illustrates the room-temperature PL spectra of the samples prepared at different reaction temperatures and different reaction times.It could be seen from Fig.4a that the intensity and FWHM of PL spectra of Zn0.5Cd0.5S obviously increase with reaction time from 1 to 3 h.However,the intensity and FWHM of the PL spectra decrease greatly when reaction time is extended to 6 h.In addition,it can be seen from Fig.4b that the PL spectra become stronger and their FWHM gets wider as reaction temperature increases from 80℃to 100 ℃,whereas the Zn0.5Cd0.5S nanocrystals prepared at 140 ℃ do not display better PL properties.This is related to the size or the surface defect state of Zn0.5Cd0.5S nanoparticles.During the photoluminescence process,defects can bind photo-induced electrons to form excitons so that PL signals can easily occur.The more the amount of defects,the stronger the PL intensity[24].The defect states in semiconductors nanoparticels play an important role in the energy transfer[25].Raising reaction temperature (140℃)or extending reaction time (6 h)could lead to the aggregation of Zn0.5Cd0.5S nanoparticles so that the amount of the defect states decreases.Our SEM results show that raising reaction temperature results in the formation of larger Zn0.5Cd0.5S nanoparticles (Fig.2c and f).Therefore,furtherraising reaction temperature orextending reaction time does not enhance the PL intensity of Zn0.5Cd0.5S nanocrystals.Based on these results,we further synthesized Zn0.5Cd0.5S nanocrystals doped with Ag2S orCuS underthe appropriate preparation conditions(reaction time 3 h and reaction temperature 100℃),and examined their PL properties in the visible light region.
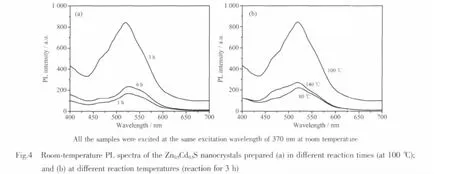
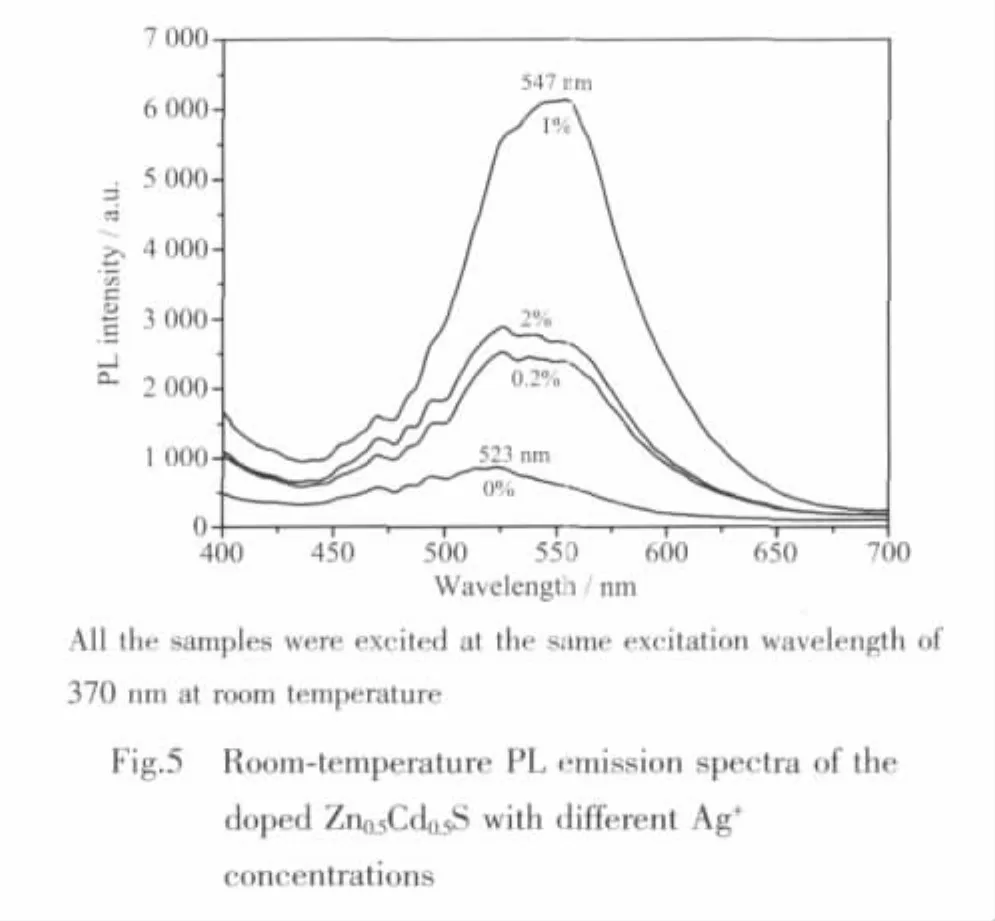
Fig.5 shows the PL spectra of Zn0.5Cd0.5S nanocrystals doped with different Ag+concentrations(0 ~2mol%).As the amount of Ag+doped in Zn0.5Cd0.5S varies from 0 to 1mol%,the PL peak shifts from 523 to 547 nm and FWHM increases from 65 to 100 nm.When the dopant concentration is 1mol%,the intensity of PL emission reaches the maximum,about 7 times higher than that of the undoped Zn0.5Cd0.5S sample.In the present work,even a small quantity of Ag2S (0.2%)incorporation could also result in obvious enhancement of the PL intensity(Fig.5).However,when the dopant concentration reaches 2mol%,the luminescence intensity is diminished.A similar concentration quenching phenomenon was also observed in the Cu-dopedZnS[11,19].These results indicate that dopant plays a dual role.On the one hand,the dopant Ag2S may act as active luminescence centers so that doping with a small quantity of Ag2S can greatly enhance the PL intensity of Zn0.5Cd0.5S nanocrystals.On the other hand,however,Ag2S may serve as nonradiative recombination centers to reduce the PL intensity.Such a negative effect will become obvious when the doping amount of Ag2S increases to a certain extent (as shown in Fig.5).Practically,the exact mechanism for the dopant Ag2S is still not clear.Further studies are needed.
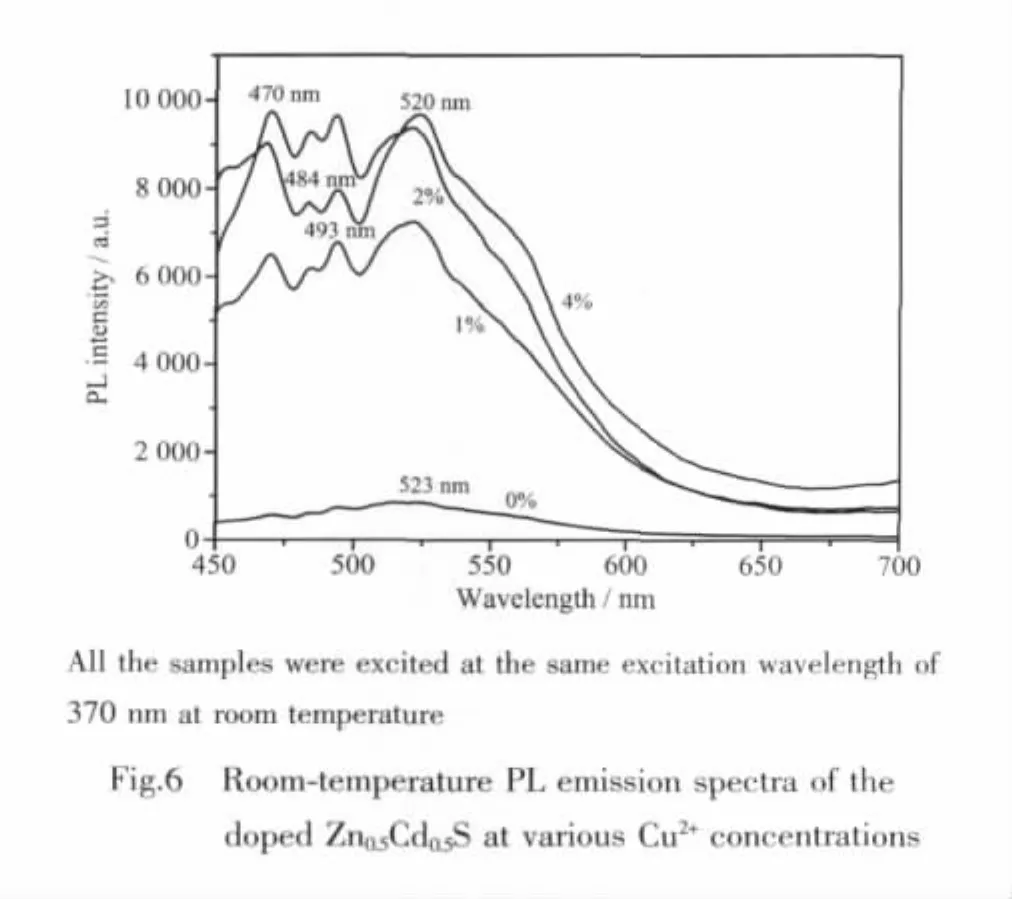
The PL spectra of the Zn0.5Cd0.5S nanocrystals doped with various concentrations of Cu2+(0~4mol%)are shown in Fig.6.It could be seen from Fig.6 that there are four emission peaks at about 470,484,493 and 520 nm.The position of these emission peaks does not change with the increase of Cu2+concentration,which demonstrates that CuS has been doped in Zn0.5Cd0.5S crystals equably.The intensity of main peak at 520 nm (Fig.6)increases as Cu2+concentration increases from 0 to 4mol%.When the dopant concentration is 4mol%,the intensity of PL emission peak at 520 nm is about 11 times higher than that of the undoped one.ThePL spectraoftheCu-doped Zn0.5Cd0.5S are obviously different from those of the Agdoped samples(Fig.5).These results further show that the PL performance of Zn0.5Cd0.5S could be efficiently tuned by changing the nature and concentration of dopants.In general,moredefectstateswillbe generated when Ag2S or CuS are doped into Zn0.5Cd0.5S.Therefore,it is reasonable that the defect-related PL intensities are enhanced for the Ag2S or CuS-doped samples compared with the undoped one.Moreover,in the CuS-doped Zn0.5Cd0.5S case,the PL emission peaks at around 470,484 and 493 nm are related to CuS.As reported in the CuS-doped ZnS system[11],the emission peak at 493 nm may originate from the recombination between the shallow donor level(sulfur vacancy)and the t2energy level of Cu2+(splitting from Cu3d).The emission peak at around 484 nm may arise from the transition between the conduction band of Zn0.5Cd0.5S and the t2level of Cu2+,and the peak at 470 nm is due to the transition from the shallow defect state to the t2level of Cu2+.
3 Conclusions
A solvothermal process was utilized to synthesize the Zn0.5Cd0.5S nanocrystals doped with and without Ag2S or CuS.The Zn0.5Cd0.5S nanocrystals obtained under the appropriate preparation conditions have a better PL performance.The doping with Ag2S or CuS could greatly improve the PL intensity of the Zn0.5Cd0.5S nanocrystals in the visible light region.
[1]Oladej I O,Chow L.Thin Solid Films,2005,474:77-83
[2]Deshpande A,Shah P,Gholap R S,et al.J.Colloid Interf.Sci.,2009,333:263-268
[3]Zhang K,Jing D W,Xing C J,et al.Int.J.Hydrogen Energ.,2007,32:4685-4691
[4]Xing C J,Zhang Y J,Yan W,et al.Int.J.Hydrogen Energ.,2006,31:2018-2024
[5]Ray S C,Karanjai M K,DasGupta D.Thin Solid Films,1998,322:117-122
[6]YANG Ping(杨 萍),LÜ Meng-Kai(吕孟凯),XU Dong(许东),et al.Chinese J.Mater.Res.(Cailiao Xuebao),2001,6:635-638
[7]ZHOU Jian-An(周建安),LI Dong-Mei(李冬梅),SANG Wen-Bin(桑 文 斌),et al.Chinese J.Chem.Phys.(Huaxue Wuli Xuebao),2004,17:637-640
[8]CAI Xiao-Jun(蔡晓军),YU Guo(于 郭),CUI Wen-Guo(崔文国),et al.J.Funct.Mater.(Gongneng Cailiao),2009,10:1643-1646
[9]Kang T,Sung J,Shim W,et al.J.Phys.Chem.C,2009,113:5352-5357
[10]Yang H S,Holloway P H.Appl.Phys.Lett.,2003,82:1965-1968
[11]Peng W Q,Cong G W,Qu S C,et al.Opt.Mater.,2006,29:313-317
[12]Yang P,Lu M K,Xu D,et al.Mater.Res.Bull.,2001,36:1301-1306
[13]QU Hua(曲 华),CAO Li-Xin(曹立新),SU Ge(苏 革),et al.Spectrosc.Spect.Anal.(Guangpuxue Yu Guangpu Fenxi),2009,2:305-308
[14]Wang W Z,Germanenko I,El-Shall M S.Chem.Mater.,2002,14:3028-3033
[15]Chen Y Q,Zhang X H,Jia C,et al.J.Phys.Chem.C,2009,113:2263-2266
[16]Singh K,Verma N K,Bhatti H S.Phys.B,2009,404:300-304
[17]Li Y C,Ye M F,Yang C H,et al.Adv.Funct.Mater.,2005,15:433-441
[18]Lui T Y,Zapien J A,Tang H,et al.Nanotechnology,2006,17:5935-5940
[19]MU Chun-Hong(慕春红),LIU Peng(刘 鹏),ZHU Gang-Qiang(朱刚强),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,23:844-848
[20]De Mello Donega C,Hickey S G,Wuister S F,et al.J.Phys.Chem.B,2003,107:489-496
[21]Talapin D V,Rogach A L,Kornowski A,et al.Nano Lett.,2007,1:207-211
[22]Ghosh G,Naskar M K,Patra A,et al.Opt.Mater.,2006,28:1047-1053
[23]Qu L H,Peng X G.J.Am.Chem.Soc.,2002,9:2049-2055
[24]Wu X,Li K W,Wang H.J.Alloy Compd.,2009,487:537-544[25]Ehrhart G,Capoen B,Robbe O,et al.Opt.Mater.,2008,30:1595-1602
Zn0.5Cd0.5SNanocrystals Doped with Ag2S or CuS:Solvothermal Synthesis and Photoluminescence Properties
ZHU Jin-Bao CHANG Xiao-Ying ZHU Guo-Jun YUAN Gao-Qing*
(School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510640)
Zn0.5Cd0.5S nanocrystals and their derivatives doped with Ag2S or CuS were synthesized via a solvothermal process using isopropanol as the solvent.The optical properties of the as-prepared samples were investigated in the visible light region.The results show that the nature and concentration of dopants as well as preparation conditions(reaction temperature and time)have a great influence on the photoluminescence (PL)spectra of Zn0.5Cd0.5S nanocrystals.The Zn0.5Cd0.5S nanocrystals doped with CuS and Ag2S exhibit a stronger PL intensity and wider FWHM(full width at half maximum)in the visible light region compared with the undoped Zn0.5Cd0.5S.
semiconductor;photoluminescence;nanocrystals;optical materials
O611.4
A
1001-4861(2010)12-2203-06
2010-05-30。收修改稿日期:2010-07-26。
广东省科技计划项目(No.2007B010600041)和华南理工大学SRP项目资助。
*通讯联系人。E-mail:qyuan@scut.edu.cn
朱金保,男,26岁,硕士研究生;研究方向:纳米材料及电化学合成。
