DTNBP1 Gene Is Associated with Some Symptom Factors of Schizophrenia in Chinese Han Nationality△
2010-04-20YuhuiSunYanShenandQiXu
Yu-hui Sun, Yan Shen, and Qi Xu
National Laboratory of Medical Molecular Biology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100005, China
SCHIZOPHRENIA is a severe form of mental illness affecting about 1% of the adult population. It is often clinically described in terms of positive and negative symptoms, and cognitive deficits, which are assessed with Positive and Negative Syndrome Scale (PANSS) in clinical practice.1
DTNBP1 gene (6p22.3) was first reported to be associated with schizophrenia in 270 Irish high-density pedigrees.2In the next few years, DTNBP1 gene was recognized as one of the hottest schizophrenia susceptibility genes based on case-control and family-based association studies in different populations.3-10Yet consistent polymorphisms or haplotypes were not identified until now. There are also evidences showing that dysbindin is involved in the pathology of schizophrenia at both mRNA and protein levels.11-14However, some genetic results previously reported showed no association between DTNBP1 gene and schizophrenia.15-18In some other studies, that gene of interest was shown to be associated with negative symptoms of schizophrenia, cognitive deficits, working memory, and some other phenotypes.19-26
In the present study, we selected four single nucleotide polymorphisms (SNPs) (rs742106, rs909706, rs1011313, and rs2619539) in DTNBP1 gene previously reported associated with schizophrenia6,10,24,27,28to test whether these polymorphisms were associated with some symptom factors of that mental illness.
PATIENTS AND METHODS
Patients
A total of 284 unrelated schizophrenia patients (131 males and 154 females, aged 31.9 ± 10.9 years) of Han nationality were recruited by Department of Psychiatry, the First Hospital of Shanxi Medical University, from December 2004 to January 2009. These patients were diagnosed with schizophrenia by at least two consultant psychia- trists in a clinical interview based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (American Psychiatric Association, 1994). Patients were excluded from this study if they suffered from any organic brain disorders, mental retardation, severe head trauma, or demonstrate psychotic symptoms due to medical conditions or treatments. The included patients all pro- vided written informed consents to blood sample collection for genetic analysis. The protocol of this study was examined and approved by the Ethics Committee of Chinese Academy of Medical Sciences & Peking Union Medical College.
Clinical assessment of symptoms
For all the included patients, positive and negative symptoms were assessed according to the PANSS. The severity of each symptom was scored with a 7-grade scale (1: absent; 2: minimal; 3: mild; 4: moderate; 5: moderately severe; 6: severe; 7: extreme).
Genotyping of SNPs
Four SNPs present in the DTNBP1 gene were selected in the present study to be genotyped. Among those SNPs, rs909706 is located in intron 1, rs1011313 in intron 4, rs2619539 in intron 5, and rs742106 in intron 9.6,10,24,27,28
Genomic DNA was extracted from peripheral blood leukocytes following the standard phenol-chloroform procedure. The primers used for polymerase chain reaction (PCR) amplification were designed by the Primer Premier 5.0 software (Table 1). The reaction volume of PCR amplification was 25 μL, containing 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.001% (w/v) gelatin, 200 μmol/L of deoxyribonucleoside triphosphates (dNTPs), 0.4 μmol/L of the primers, 1.0 unit of Taq DNA polymerase (Tiangen, Beijing, China), and 50 ng of the extracted genomic DNA. The conditions for thermal cycling included an initial denaturation at 94°C for 5 minutes, 35 cycles at 94°C for 30 seconds, at 50-56°C for 30 seconds, and at 72°C for 30 seconds, followed by a final elongation at 72°C for 10 minutes on GeneAmp PCR System 2700 (Applied Biosystems, Foster City, CA, USA). The PCR products were purified and then sequenced bidirectionally with an ABI 3700 DNA sequencer (Applied Biosystems).
Statistical analysis
Hardy-Weinberg equilibrium for the genotypic distributions of each SNP was tested with the chi-square (χ2) goodness-of-fit test. Pair-wise linkage disequilibrium among these four SNPs was defined by the four-gamete rule using Haploview 4.2. The UNPHASED program (version 3.0.12) was applied to analyze genotype data.29The additive genetic value, which represents the difference in expected trait due to the allele compared with the reference, was estimated for determining the association between scored positive/negative symptoms and tested SNPs. The statistical significance level was set at 0.05 and adjusted by 10 000 permutations.
RESULTS
The χ2goodness-of-fit test showed that the genotypic distributions of the four studied SNPs were all in Hardy- Weinberg equilibrium in the schizophrenia patients (Table 2).
According to the plotting by Haploview 4.2, rs1011313 and rs2619539 were in one lingkage disequilibrium block in our samples, while the other two were not.
The quantitative trait test showed allelic association of rs909706 with the excitement symptom of schizophrenia (χ2=7.352, P<0.05, adjusted by 10 000 permutations), and allele G with higher additive values than allele A (Table 3). In addition, rs2619539 was shown to be associated with lack of spontaneity and flow of conversation (χ2=9.151, P<0.05, adjusted by 10 000 permutations), and the genotype C/G with a higher additive values than the other genotypes (Table 4).
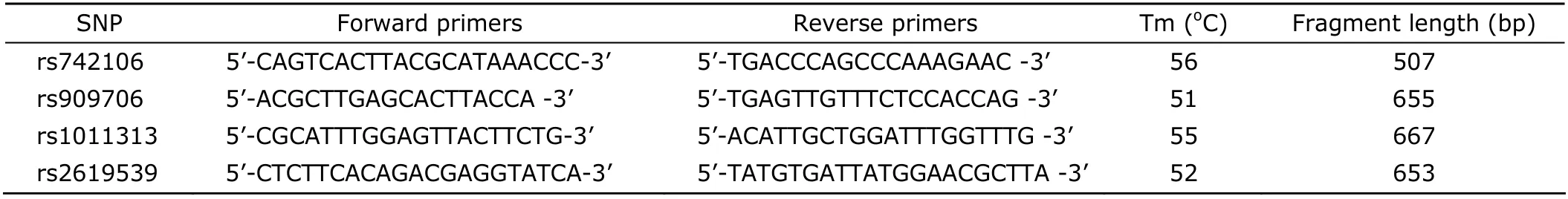
Table 1. Polymerase chain reaction primers for genotyping of DTNBP1 SNPs
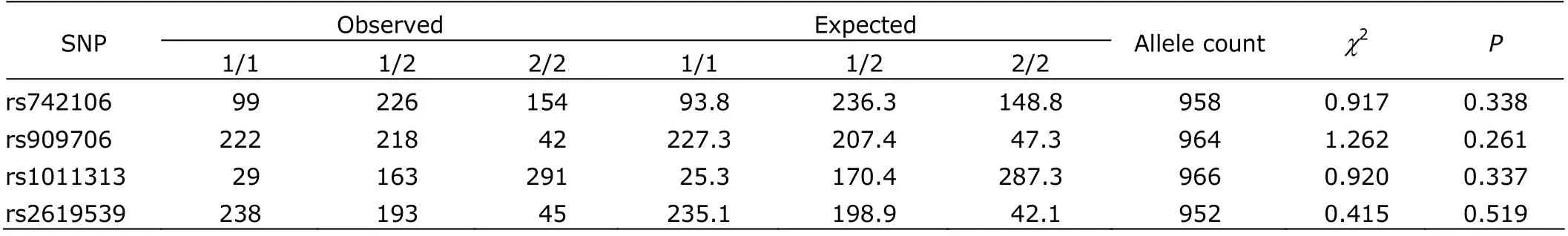
Table 2. Hardy-Weinberg equilibrium test of the four SNPs in schizophrenia patients
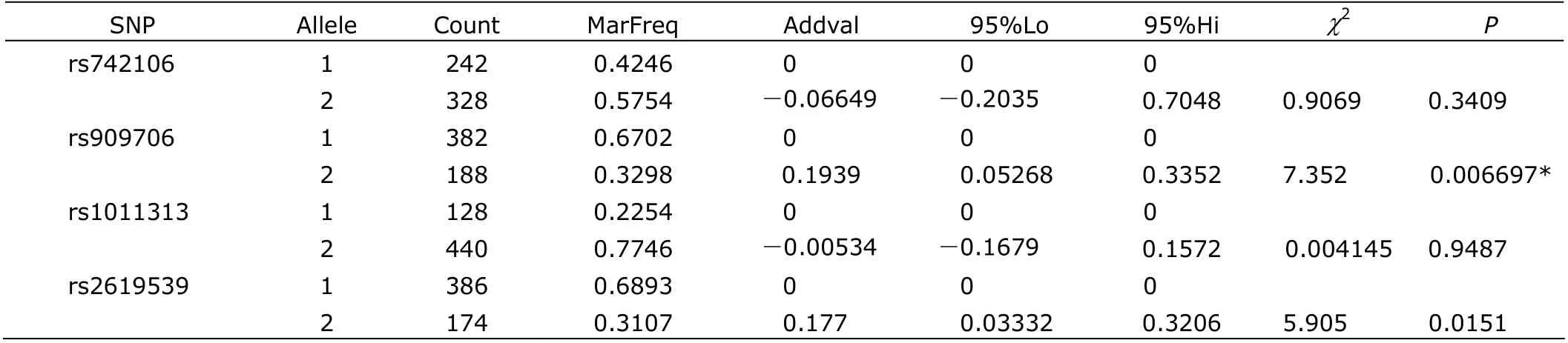
Table 3. Allelic association of four SNPs and excitement
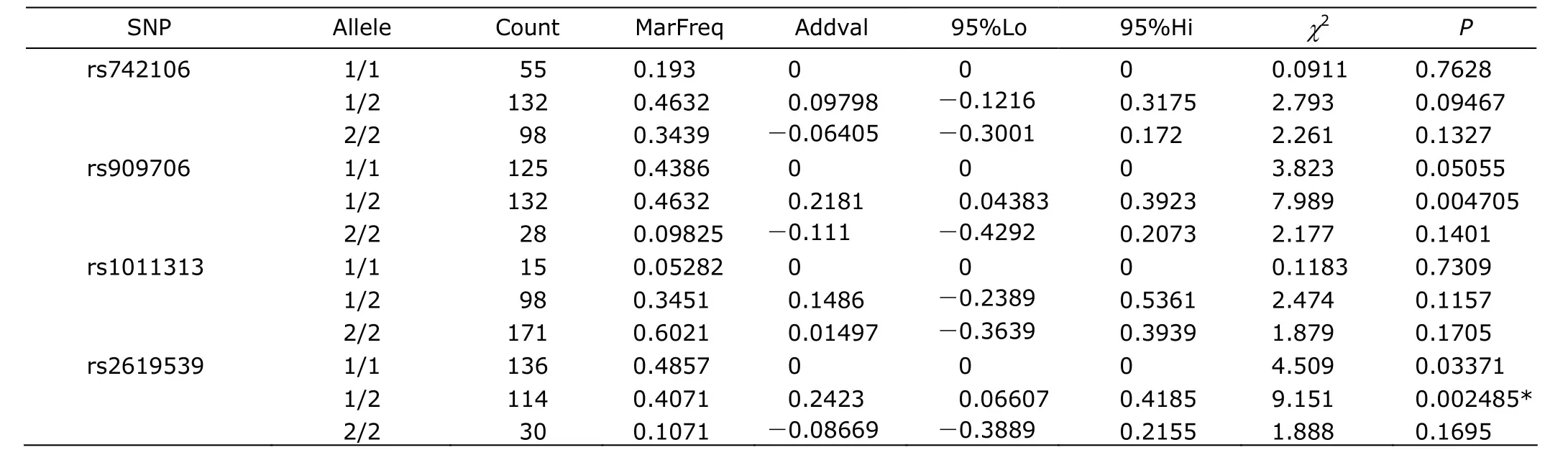
Table 4. Genotypic association of four SNPs and lack of spontaneity and flow of conversation
We also carried out quantitative trait tests for other symptoms covered in PANSS, yet no associations were found between those symptoms and the studied SNPs.
DISCUSSION
Schizophrenia is a genetically complex disorder with an unknown pathophysiology. It is suggested that to study its specific symptoms or clinical phenotypes could help to establish a better understanding of this disorder.30The PANSS assessing the positive and negative symptoms of schizophrenia includes positive scales (delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, suspiciousness/persecution, and hostility), negative scales (blunted affect, emotional withdrawal, poor rapport, passive/apathetic social withdrawal, difficulty in abstract thinking, lack of spontaneity and flow of conversation, and stereotyped thinking), and general psychopathology scale (somatic concern, anxiety, guilt feelings, tension, mannerisms and posturing, depression, motor retardation, uncooperativeness, unusual thought content, disorientation, poor attention, lack of judgment and insight, disturbance of volition, poor impulse control, preoccupation, and active social avoidance).1
rs909706 and rs2619539, the two SNPs found in this study to be related with some symptoms of schizophrenia, are both intronic polymorphisms. rs909706 was found to contribute to schizophrenia,10and associated with change of patients' response to anti-psychotic agent haloperidol.28And rs2619539 was associated with methamphetamine psychosis,31less severe manic-type symptoms in psychosis,32and cognitive functions.23
Complementary to those previous findings, the quantitative trait test in our study showed association of some SNPs in DTNBP1 gene with some symptom factors of schizophrenia. In specific, rs909706 was associated with excitement, and rs2619539 with lack of spontaneity and flow of conversation. Their locations in different linkage disequilibrium blocks may explain the fact that those two symptoms belong to positive and negative scales respectively.
Our study revealed a possible association between DTNBP1 and the symptoms of schizophrenia, which perhaps could partly explain the relationship between DTNBP1 variations and that mental illness. Further investigation in large samples and different populations is still needed for clarifying the mechanisms of how the DTNBP1 gene affects the symptoms of schizophrenia.
1. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261-76.
2. Straub RE, Jiang Y, MacLean CJ, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet 2002; 71:337-48.
3. Schwab SG, Knapp M, Mondabon S, et al. Support for association of schizophrenia with genetic variation in the 6p22.3 gene, dysbindin, in sib-pair families with linkage and in an additional sample of triad families. Am J Hum Genet 2003; 72:185-90.
4. Kirov G, Ivanov D, Williams NM, et al. Strong evidence for association between the dystrobrevin binding protein 1 gene (DTNBP1) and schizophrenia in 488 parent-offspring trios from Bulgaria. Biol Psychiatry 2004; 55:971-5.
5. Williams NM, Preece A, Morris DW, et al. Identification in 2 independent samples of a novel schizophrenia risk haplotype of the dystrobrevin binding protein gene (DTNBP1). Arch Gen Psychiatry 2004; 61:336-44.
6. De Luca V, Voineskos D, Shinkai T, et al. Untranslated region haplotype in dysbindin gene: analysis in schizophrenia. J Neural Transm 2005; 112:1263-7.
7. Li T, Zhang F, Liu X, et al. Identifying potential risk haplotypes for schizophrenia at the DTNBP1 locus in Han Chinese and Scottish populations. Mol Psychiatry 2005; 10:1037-44.
8. Duan J, Martinez M, Sanders AR, et al. DTNBP1 (Dystrobrevin binding protein 1) and schizophrenia: association evidence in the 3' end of the gene. Hum Hered 2007; 64:97-106.
9. Riley B, Kuo PH, Maher BS, et al. The dystrobrevin binding protein 1 (DTNBP1) gene is associated with schizophrenia in the Irish Case Control Study of Schizophrenia (ICCSS) sample. Schizophr Res 2009; 115:245-53.
10. Zuo L, Luo X, Kranzler HR, et al. Association study of DTNBP1 with schizophrenia in a US sample. Psychiatr Genet 2009; 19:292-304.
11. Weickert CS, Straub RE, McClintock BW, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry 2004; 61:544-55.
12. Bray NJ, Preece A, Williams NM, et al. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Hum Mol Genet 2005; 14:1947-54.
13. Weickert CS, Rothmond DA, Hyde TM, et al. Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res 2008; 98: 105-10.
14. Tang J, LeGros RP, Louneva N, et al. Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Hum Mol Genet 2009; 18: 3851-63.
15. Morris DW, McGhee KA, Schwaiger S, et al. No evidence for association of the dysbindin gene [DTNBP1] with schizophrenia in an Irish population-based study. Schi- zophr Res 2003; 60:167-72.
16. Datta SR, McQuillin A, Puri V, et al. Failure to confirm allelic and haplotypic association between markers at the chromosome 6p22.3 dystrobrevin-binding protein 1 (DTNBP1) locus and schizophrenia. Behav Brain Funct 2007; 3:50.
17. Liu CM, Liu YL, Fann CS, et al. No association evidence between schizophrenia and dystrobrevin-binding protein 1 (DTNBP1) in Taiwanese families. Schizophr Res 2007; 93:391-8.
18. Peters K, Wiltshire S, Henders AK, et al. Comprehensive analysis of tagging sequence variants in DTNBP1 shows no association with schizophrenia or with its composite neurocognitive endophenotypes. Am J Med Genet B Neuropsychiatr Genet 2008; 147B:1159-66.
19. Burdick KE, Goldberg TE, Funke B, et al. DTNBP1 genotype influences cognitive decline in schizophrenia. Schi- zophr Res 2007; 89:169-72.
20. Donohoe G, Morris DW, Clarke S, et al. Variance in neurocognitive performance is associated with dysbindin-1 in schizophrenia: a preliminary study. Neuropsychologia 2007; 45:454-8.
21. Zinkstok JR, de Wilde O, van Amelsvoort, et al. Association between the DTNBP1 gene and intelligence: a case-control study in young patients with schizophrenia and related disorders and unaffected siblings. Behav Brain Funct 2007; 3:19.
22. Hashimoto R, Noguchi H, Hori H, et al. A genetic variation in the dysbindin gene (DTNBP1) is associated with memory performance in healthy controls. World J Biol Psychiatry 2010; 11:431-8.
23. Hashimoto R, Noguchi H, Hori H, et al. Association between the dysbindin gene (DTNBP1) and cognitive functions in Japanese subjects. Psychiatry Clin Neurosci 2009; 63:550-6.
24. Luciano M, Miyajima F, Lind PA, et al. Variation in the dysbindin gene and normal cognitive function in three independent population samples. Genes Brain Behav 2009; 8:218-27.
25. Narr KL, Szeszko PR, Lencz T, et al. DTNBP1 is associated with imaging phenotypes in schizophrenia. Hum Brain Mapp 2009; 30:3783-94.
26. Wolf C, Jackson MC, Kissling C, et al. Dysbindin-1 genotype effects on emotional working memory. Mol Psychiatry 2009 [Epub ahead of print].
27. Pae CU, Drago A, Kim JJ, et al. DTNBP1 haplotype influences baseline assessment scores of schizophrenic in-patients. Neurosci Lett 2008; 440:150-4.
28. Zuo L, Luo X, Krystal JH, et al. The efficacies of clozapine and haloperidol in refractory schizophrenia are related to DTNBP1 variation. Pharmacogenet Genomics 2009; 19:437-46.
29. Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol 2003; 25:115-21.
30. Fanous AH, Kendler KS. Genetic heterogeneity, modifier genes, and quantitative phenotypes in psychiatric illness: searching for a framework. Mol Psychiatry 2005; 10:6-13.
31. Kishimoto M, Ujike H, Motohashi Y, et al. The dysbindin gene (DTNBP1) is associated with methamphetamine psychosis. Biol Psychiatry 2008; 63:191-6.
32. Corvin A, Donohoe G, Nangle JM, et al. A dysbindin risk haplotype associated with less severe manic-type symptoms in psychosis. Neurosci Lett 2008; 431:146-9.
杂志排行
Chinese Medical Sciences Journal的其它文章
- Lipids-induced Apoptosis Is Aggravated by Acyl-coenzyme A: Cholesterol Acyltransferase Inhibitor△
- Gaussia Luciferase Reporter Assay for Assessment of Gene Delivery Systems in Vivo△
- A Case of Thoracic Spinal Stenosis Secondary to Paget's Disease
- Association between the Epidermal Growth Factor Gene and Intelligence in Major Depression Patients△
- Role of Acetylated p53 in Regulating the Expression of map2 in Retinoic Acid-induced P19 Cells△
