Role of Acetylated p53 in Regulating the Expression of map2 in Retinoic Acid-induced P19 Cells△
2010-04-20LiZhangLiYanYeZhangNinghuaWuandYufeiShen
Li Zhang, Li Yan, Ye Zhang,Ning-hua Wu, and Yu-fei Shen
National Laboratory of Medical Molecular Biology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100005, China
MAMMALIAN microtubule-associated protein 2 (MAP2) is a group of proteins belonging to the MAP family, expressed mainly in the dendrites of neurons, in particular, of the developed neurons. Experimental evidence shows that MAP2 can bind both microtubules and F-actin with its microtubule-bind- ing-repeat domain in vivo1and in vitro2. As a cytoskeletal integrator in the dendrites, MAP2 links microtubule, actin microfilaments, and neurofilaments together to support dendrite elongation. Meanwhile, abnormal interaction of MAP2 with microtubes is involved in the occurrence of neurodegenerative diseases.
In spite of its importance in neuronal development and neurodegenerative diseases, the regulation of MAP2 is still elusive. To explore into this issue, we investigated the regulatory effect of acetylated p53 on map2 gene expression in retinoic acid (RA)-induced differentiation of P19 cells. p300/CBP associated factor (PCAF) was also detected in this study for its mediating role in the acetylation of p53. P19 cell line was used as a model of neuronal differentiation for its ability to differentiate into neurons and glial cells after treated by RA.3
MATERIALS AND METHODS
Cell culture and RA treatment
P19 cells were cultured in α-minimum essential medium (Invitrogen, Carlsbad, CA, USA) containing 10% (v/v) fe- tal bovine serum (FBS) and incubated in a humidified chamber with 5% CO2at 37°C. The cells were then cultured in medium containing 0.5 μmol/L all-trans RA (Sigma- Aldrich, St. Louis, MO, USA) for 4 days, and the aggregates, plated into a single layer, were cultured for another 3-4 days in fresh RA-free medium and turned into neuron-like shape with neurite networks.4,5P19 cells cultured under the same conditions except for RA treatment were used as control.
Plasmids
Eukaryotic expression plasmids of wild-type p53 (pC53- SN3) and those of p53 mutant at 143 residue (pC53-SCx3) were gifts from Dr. Bert Vogelstein (Johns Hopkins Oncology Center). Eukaryotic expression plasmid of FLAG (an octapeptide protein tag) tagged PCAF (pCX-Flag-PCAF) was given by Dr. Hua Lu (Department of Biochemistry and Molecular Biology, Oregon Health & Science University). pGL3-map2-1.3k contains 1.3 kb nucleotides of the map2 promoter (-1258/+47 bp). The Renilla luciferase reporter (pRL-TK) was purchased from Invitrogen.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Real-time RT-PCR was carried out as previously described.4The relative expression of map2 was normalized against gapdh, using the comparative CT method following the manufacturer's instructions (Rotor-Gene RG-3000A Real- time PCR System, Corbett Research, Mortlake, NSW, Australia). Primers used in PCR were as follows: ngn1, 5'-TCTCTAAAGAACATCCGTCAC-3' (forward primer), 5'- CTGTCAATCTTCACATTACCAC-3' (reverse primer); gapdh, 5'-TCCACCACCCTGTTGCTGTA-3' (forward primer), 5'- ACCACAGTCCATGCCATCAC-3' (reverse primer) (Invitrogen, Shanghai, China). The level of map2 mRNA expression was detected at seven different time points: before RA treatment, every day during the 4-day treatment, on the second day after the treatment, and on the fourth day after the treatment. The experiments were repeated at least three times and results presented as means±SD.
Western blot
Whole cell extracts were separated in 8% sodium dodecyl sulfate (SDS)-polyacrylamide gel before analyzed by Western blot.6Antibodies used in this assay included anti-MAP2 polyclonal (Cell Signal Technology, Danvers, MA, USA), anti-p53 monoclonal (DO-1) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-ac-p53 (Upstate, Syracuse, NY, USA), and anti-PCAF monoclonal (Santa Cruz Biotechnology), and anti-Lamin B (Santa Cruz Biotechnology). The level of MAP2 protein expression was detected at the same seven time points as in real-time RT-PCR.
Luciferase reporter assay
To examine the effects of p53 on map2 expression, P19 cells were co-transfected with map2-Luc reporter plasmid (pGL3-map2-promoter-Luc, pGL3-Luc basic vector inserted with 1.3 kb map2 promoter), pRL-TK, and pC53- SN3, pC53-SCx3, or pCMV-Neo-Bam (empty vector). The role of PCAF in the regulation of map2 expression was investigated by co-transfecting P19 cells with pGL3-map2- promoter-Luc, pRL-TK, and pCX-Flag or pCX-Flag-PCAF. The activities of luciferases were determined by means of a Dual-Luciferase Reporter System (Promega, Madison, WI, USA), following the manufacturer's instructions. The assay was repeated 3 times, each in triplicate.
Immunofluorescence (IF) staining
We assessed the intracellular MAP2 distribution in P19 cells with IF assay. MAP2 was detected by IF staining in P19 cells at three different time points: before RA treatment, on the fourth day of RA treatment, and on the second day after the removal of RA. Images were collected with a Nikon Eclipse TE2000-U microscope (Melville, NY, USA).
In this step, wild-type p53 or PCAF were transfected into the P19 cells grown on coverslips in 6-well plates. Forty-eight hours after the transfection, the cells were washed twice and rewashed for 3 times, both with 1× phosphate-buffered saline (PBS) and lasting for 5 minutes. The cells were then fixed with 4% paraformaldehyde for 10-15 minutes at 37°C. With 0.1% Triton X-100 (Fisher, Waltham, MA, USA), the cells were permeablized for 10 minutes at room temperature, followed by 3 times of washing. After incubated in 3% bovine serum albumin (BSA) (Sigma-Aldrich)/PBS at 37°C for 30 minutes, the cells were cultured with p53 or PCAF antibody in 3% BSA/PBS overnight at 4°C and then washed with 1×PBS for 15 minutes. The fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:100) was added before incubation for 30 minutes at room temperature. Finally, after washed with 1×PBS for 15 minutes, the cells were counterstained with 4'6-diamidino-2-phenylindole (DAPI) in the last wash to visualize the nuclei.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were carried out as previously described,4,7using anti-ac-p53. The primers for amplification of map2 promoter were: P1/P2 (-1050/-780 bp), 5'-AGTCCCAGT- CCTCAAA-3', 5'-AAAGGTATGTATTAAAGA-3'; P3/P4 (-574/-375 bp) 5'-ACAAAACCTCAAACTA-3', 5'-TCATCATCTGG- TCTCCT-3'.
The operating cycle number required to reach a threshold in the linear range was determined and compared with a standard curve for the primer set generated by five 10-fold dilutions of genomic DNA samples of known concentration. The percentage of immunoprecipitated DNA relative to input was calculated based on the results from three independent ChIP experiments and presented as means±SD.
RESULTS
Induction of map2 expression by RA in P19 cells
IF staining showed an orderly internuclear network of MAP2 formed only on the sixth day in the P19 cells (Fig. 1A). Real time RT-PCR assays revealed that the mRNA of map2 increased 34 folds after the 4-day RA treatment and 730 folds 2 days after the treatment, compared with the level of control cells (Fig. 1B). Western blot also showed an increase in the protein level of MAP2 (Fig. 1C). These results suggested that map2 gene was induced by RA treatment in P19 cells during neuronal differentiation.
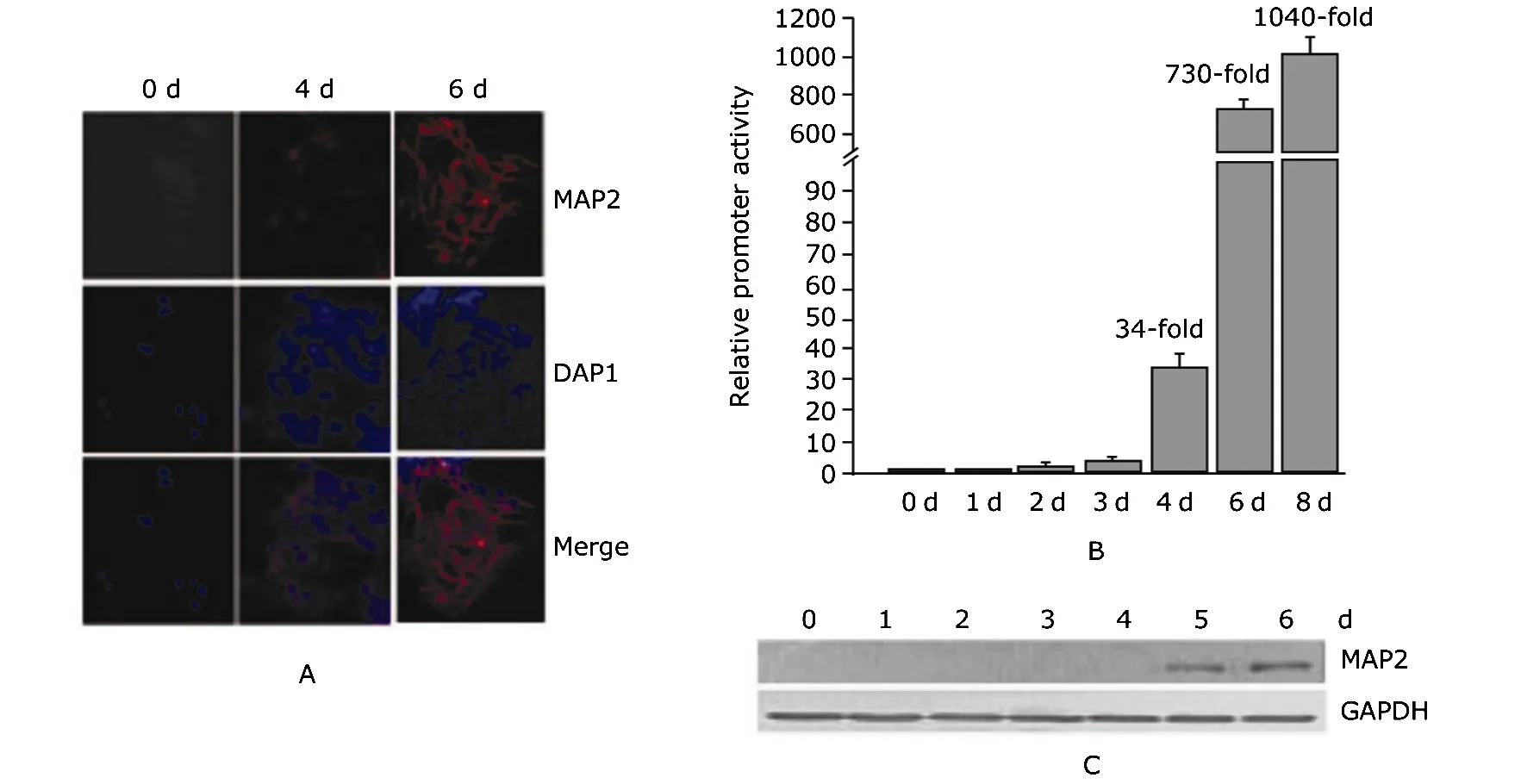
Figure 1. Microtubule-associated protein-2 (MAP2) expression in P19 cells induced with retinoic acid (RA). A. Immunofluorescence staining of P19 cells before and after a 4-day RA treatment. Red: anti-MAP2; blue: DAPI. 0 d: before RA treatment; 4 d: treated with RA for 4 days; 6 d: treated with RA for 4 days, then cultured for 2 days without RA.B. Real-time reverse transcription-polymerase chain reaction (RT-PCR) result of map2 mRNA expression in RA-treated P19 cells. Error bars represent normalized mean values based on the results from at least three independent experiments at each time point. 0 d: before RA treatment; 1-4 d: each day during the 4-day RA treatment; 6 d: 2 days after the RA treatment; 8 d: 4 days after the RA treatment. C. Western blot result of the expression of MAP2 protein in RA-treated P19 cells.
Effect of p53 on RA-induced map2 expression
In the nuclear extract of RA-treated P19 cells, p53 was acetylated and the total protein level of p53 slightly increased (Fig. 2A). IF assay showed that p53 was enriched in the nuclei of P19 cells after RA induction (Fig. 2B).
Luciferase reporter assay showed that wild-type p53, but not p53 mutant, could enhance the promoter activity of map2 compared with the control (Fig. 2C). Two p53 putative binding sites were identified at-494/-475 bp (5'-CG- TAGGCTTATAGACTTGACTCCTGGGGACAC-3', designated as p53bs1) and-810/-789 bp (5'-TTTGTGTAGCTTAAC- TTGCCTGTAATCCTATAT-3', designated as p53bs2) respec- tively. ChIP with antibody against acetylated p53 in P19 cells showed that the occupancy of acetylated p53 on both p53bs1 and p53bs2 were enhanced after RA treatment. The percentage of input increased from 0.61 to 1.15 for p53bs1, and from 0.32 to 0.71 for p53bs2. These results suggested that p53 was acetylated and enriched in the nuclei of p19 cells treated with RA, which is required for map2 expression in neuronal differentiation.
Effect of PCAF on p53 in RA-induced map2 expression
PCAF increased in RA-treated P19 cells, along with the progress of differentiation (Fig. 3A). IF assay showed that PCAF was slightly enriched in the nuclei after RA treatment (Fig. 3B). After co-transfection of pGL3-map2-promoter- Luc with pCX-Flag-PCAF into P19 cells, it was found that PCAF enhanced the promoter activity of map2 compared with the pCX-Flag control (Fig. 3C).
Taken together, the effect of PCAF on the map2 promoter activity was possibly mediated by p53 which was in turn acetylated by the elevated PCAF in RA-induced P19 cells.
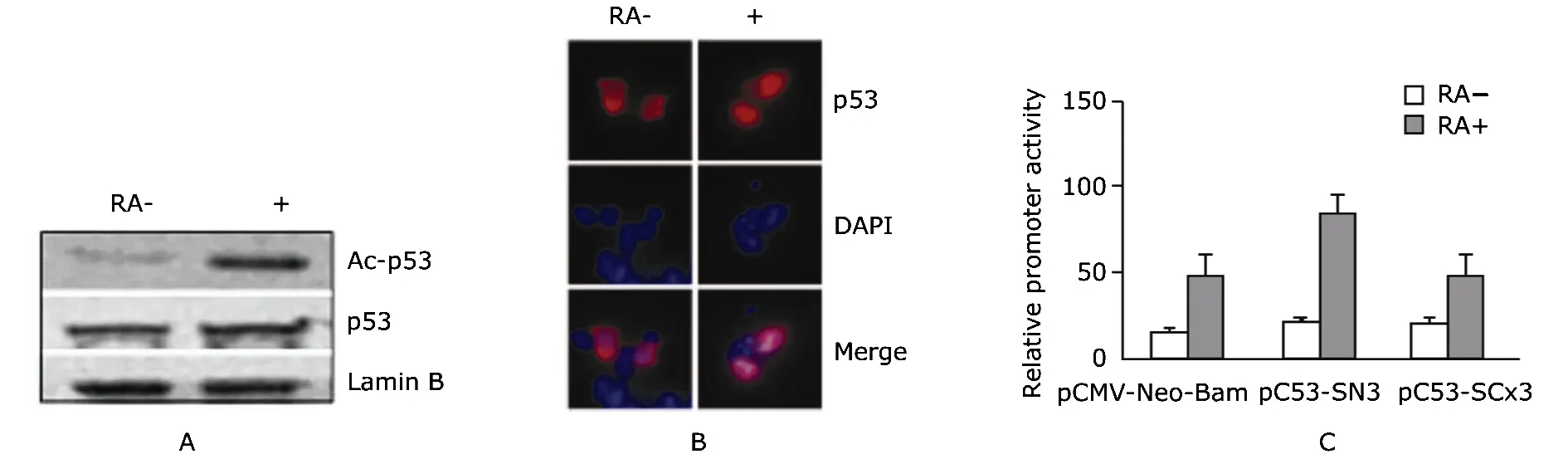
Figure 2. Effect of p53 on map2 gene expression in P19 cells after RA induction. A. P19 cells with or without RA treatment are blotted with antibodies against acetylated p53 (ac-p53), p53, and Lamin B; B. Immunofluorescence staining of p53 in P19 cells with or without RA treatment; Red: anti-p53; blue: DAPI. C. Promoter activity assays of map2 gene in P19 cells treated with or without RA treatment; Expression plasmids of pC53-SN3 (wild-type p53), pC53-SCx3 (p53 mutant), or pCMV-Neo-Bam (empty vector) were individually co-transfected with pGL3-map2-promoter-Luc and pRL-TK.
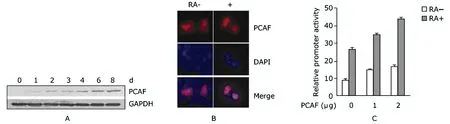
Figure 3. Effect of p300/CBP associated factor (PCAF) in RA-induced map2 gene expression in P19 cells. A. Western blot result of PCAF expression in the whole cell extracts of P19 cells after RA induction, with GAPDH as control; B. Immunofluorescence staining of PCAF in P19 cells with or without RA treatment; Red: anti-PCAF; blue: DAPI. C. Effect of PCAF on the promoter activity of map2 by luciferase reporter assay.
DISCUSSION
Our findings show that p53 drives the expression of neuronal map2 by binding to two specific elements in the promoter of the gene, which are the classical consensus binding sites for p53.8Acetylation of p53 by p300/CBP and PCAF enhances the protein stability of p53 and its transactivation potential via facilitating its interactions with specific promoters and other proteins in the transcriptional machinery.9,10We showed in this study that the occupation of acetylated p53 on the two p53 binding sites in map2 promoter in P19 cells after RA induction might be responsible for the elevated map2 promoter activity.
The biological significance of acetylation on each of the lysine residues in p53 is distinct.10,11For instance, the acetylation of p53 specifically at K320 favors cell survival, while the acetylation at K372 is prone to promote cell death.9RA treatment could induce the expression of PCAF, which is concomitant with the increase of acetylated p53, in agreement with the findings reported in previous researches.12It is thus possible that acetylation of p53 at K320 could be specifically responsible for the regulation of the expression level of map2. p300/CBP can acetylate lysines located in the last COOH-terminal portion of p53, in particular, K373 and K382.13
In conclusion, our work demonstrates the effect of acetylated p53 on the regulation of map2 in neuronal differentiation of P19 cells and the role of PCAF in this process. Drug development for neurite re-growth and clinic treatments for neurodegenerative diseases could both benefit from this study.
1. Takemura R, Okabe S, Umeyama T, et al. Polarity orientation and assembly process of microtubule bundles in nocodazole-treated, MAP2c-transfected COS cells. Mol Biol Cell 1995; 6:981-96.
2. Roger B, Al-Bassam J, Dehmelt L, et al. MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr Biol 2004; 14:363-71.
3. Jones-Villeneuve EM, McBurney MW, Rogers KA, et al. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol 1982; 94: 253-62.
4. Wu M, Zhang Y, Wu NH, et al. Histone marks and chromatin remodelers on the regulation of neurogenin1 gene in RA induced neuronal differentiation of P19 cells. J Cell Biochem 2009; 107:264-71.
5. Fang HB, Mi Y, Zhang Y, et al. HDAC3 augments the autoregulation of neuroD gene in P19 cells. Neuroreport 2009; 21:19-23.
6. Shen JH, Wu M, Wu NH, et al. The role of JDP2 in the differentiation of neuroblastoma SH-SY5Y cells. Chin Sci Bull 2008; 53:227-32.
7. Li ZY, Yang J, Gao X, et al. Sequential recruitment of PCAF and BRG1 contributes to myogenin activation in 12-O- tetradecanoylphorbol-13-acetate-induced early differentiation of rhabdomyosarcoma-derived cells. J Biol Chem 2007; 282:18872-8.
8. Hoh J, Jin S, Parrado T, et al. The p53MH algorithm and its application in detecting p53-responsive genes. Proc Natl Acad Sci U S A 2002; 99:8467-72.
9. Knights CD, Catania J, Di Giovanni S, et al. Distinct p53 acetylation cassettes differentially influence gene-expression patterns and cell fate. J Cell Biol 2006; 173: 533-44.
10. Luo J, Li M, Tang Y, et al. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci U S A 2004; 101:2259-64.
11. Prives C, Manley JL. Why is p53 acetylated? Cell 2001; 107:815-18.
12. Di Giovanni S, Knights CD, Rao M, et al. The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. EMBO J 2006; 25:4084-96.
13. Tang Y,Zhao W, Chen Y, et al . Acetylation is indispensable for p53 activation. Cell 2008; 133:612-26.
杂志排行
Chinese Medical Sciences Journal的其它文章
- Lipids-induced Apoptosis Is Aggravated by Acyl-coenzyme A: Cholesterol Acyltransferase Inhibitor△
- Gaussia Luciferase Reporter Assay for Assessment of Gene Delivery Systems in Vivo△
- A Case of Thoracic Spinal Stenosis Secondary to Paget's Disease
- DTNBP1 Gene Is Associated with Some Symptom Factors of Schizophrenia in Chinese Han Nationality△
- Association between the Epidermal Growth Factor Gene and Intelligence in Major Depression Patients△
