一种新型蓝光发射聚合物的非线性光学性质和超快动力学
2010-03-06王耀川闫永丽楠冯钱士雄
王耀川 闫永丽 周 慧 何 楠冯 苗,4 钱士雄 陈 彧,*
(1复旦大学物理系,上海 200433;2复旦大学表面物理国家重点实验室,上海 200433;3华东理工大学化学系,教育部结构可控先进功能材料及其制备技术重点实验室,上海 200237;4福州大学材料科学与工程学院,福州 350002)
The materials with excellent two-photon absorption(TPA) properties are of great interest in many high-technology applications,e.g.,two photon fluorescence imaging[1],optical limiting[2], three-dimensional microfabrication[3-4],optical storage[5-6],two photon laser scanning microscopy[7],photodynamic therapy[8],and up-converted lasing[9].Design and syntheses of the organic and/or polymeric functional materials with excellent TPA performance have thus stimulated extensive research activities over the world in recent years[10].Although there are already a large number of papers and patents concerning fabrication of TPA materials,including one-dimensional dipolar[11-12],quadrupolar[13-20],octupolar[21-23],multibranched chromophores[24-25],and macrocyclic molecules[26-27],development ofthe molecular materials with large TPA cross section still draws much attention and presents an ongoing great challenge.
To design and synthesize more molecules with outstanding TPA properties,a proper understanding of the dynamics of twophoton excitation process in the materials is of great importance. Femtosecond(fs)pump-probe experiment is a very useful technique to obtain the information of the relaxation processes of the excited states.For example,Goodson III and co-workers employed pump-probe,time-resolved photoluminescence,and threepulse photon echo measurements to measure the dynamics of some molecules,including multibranched chromophores[24],organic conjugated dendrimers[28],and macrocyclic molecules[26-27]. Dynamics of molecules with two-photon absorption properties are,however,still inadequate,especially for the conjugated polymers.Of great important for the practical applications is that,these TPA materials must be easily fabricated into thin films[29].Polymerizing the suitable organic monomers,or embedding organic TPA materials as inclusions in a polymer host to form a composite material,would allow traditional methods, such as spin casting,to be employed to prepare thin films for solid-state applications.For the blend of organic TPA molecules and polymer host,a possible partial incompatibility of two components usually leads to difficulties in achieving homogeneous dispersions and to the ultimate phase separation at high loadings. The best way to overcome phase separation is to polymerize suitable organic monomers.In our previous work[30-34],we reported the TPA character and excited state dynamics of several molecules and polymers with both linear and tri-branched structure.Recently,we have synthesized two new functional materials:poly[5-(diphenylamino)-1,3-phenylenevinylene](Yu1)and its starting material 5-(N,N-diphenylamino)benzene-1,3-dicarbaldehyde(Yu0)[35],as shown in Fig.1.As expected,the resultant polymer Yu1 displays good thermal stability and highly solubility in common organic solvents.
In this contribution,we explore the optical properties and the excited state dynamics of these two materials using two-photon fluorescence(TPF),Z-scan,and pump-probe experiments.In contrast to the monomer Yu0,the TPA properties of Yu1 were found to be greatly enhanced upon polymerization.The investigation of the ultrafast dynamics demonstrates the different relaxation processes happened in Yu0 and Yu1 samples.
1 Experimental
1.1 Materials and reagents
In our previous paper[35],we reported the synthesis and structural characterization of the samples Yu0 and Yu1 in details. Due to large steric hindrance effect of N,N-diphenylamino,the ethenyl between the repeating unit is in transconfiguration.The number average molecular weight(Mn)and the weight average molecular weight(Mw)for Yu1 were 1.71×103and 2.10×103,respectively.All chemicals were purchased from Aldrich(USA) and used without further purification.Organic solvents were purified,dried,and distilled under dry argon.Unless noted otherwise,other analytically pure reagents were used as received.The operations for synthesis prior to the termination reaction were carried out under purified argon.
1.2 Instrumentation and measurements

Fig.1 Synthesis of polymer Yu1
The samples investigated were dilute solutions in tetrahydrofuran(THF)with the concentration of about 10-5mol·L-1,with the exception in the Z-scan and TPF experiments.
The absorption spectra in the ultraviolet and visible region were measured with a Shimadzu UV-2450 spectrophotometer (made in Japan).Steady-state fluorescence spectra were measured on a Shimadzu RF-5300 PC spectrofluorophotometer (made in Japan).
We employed a femtosecond laser system(Spitfire,Spectra Physics,made in USA)to study the nonlinear optical properties and the ultrafast dynamics of two materials.The system consists of a mode-locked Ti:sapphire oscillator and a regenerative amplifier.The output femtosecond laser beam from the spitfire has an average power of 300 mW at wavelength of 800 nm,the pulse duration of 140 fs and the repetition rate of 1 kHz.
The open aperture Z-scan technique was used to measure the TPA cross section of materials.The laser beam with the pulse energy about 1.0 μJ(the solvent tetrahydrofuran(THF)does not show detectable nonlinear properties at this input power)was focused by a lens of 10 cm focal length on the solution filled in a 1 mm cell,and the transmitted light after the solution was collected by a photodiode detector connected with a Lock-in amplifier. The two-photon excited fluorescence spectra were recorded by a spectrometer 500-Pi(made in USA).To study the excited state dynamics of Yu0 and Yu1,one-color and two-color pumpprobe experiments,where the femtosecond laser beam was splitted into two beams by a beam splitter with an intensity ratio of about 1:10,have been done.The detailed pump-probe experiment under the configurations of parallel and perpendicular polarizations was described in a previous paper[36].
2 Results and discussion
2.1 Linear absorption and fluorescence spectra

Fig.2 Linear absorption spectra(solid)and fluorescence spectra(dot-dashed)of Yu0 and Yu1 in THF solution
The linear absorption and one-photon fluorescence spectra of Yu0 and Yu1 solutions in THF are shown in Fig.2.The electronic absorption spectrum of monomer Yu0 exhibits typical absorption characteristics of the phenyl units in the molecular structure.Excited by the 300 nm light,two fluorescence emission bands of Yu0 appear at about 362 and 532 nm,respectively.Upon polymerization,the polymer Yu1 has highly delocalized π-electron conjugated structure which would facilitate electron/hole to transfer in the whole conjugated system.The density of chromophore of Yu1 is much higher than that of the starting material Yu0.All of these will result in different properties of the materials,including the linear absorption and fluorescence spectra.For Yu1,a strong absorption peak at 303 nm is observed in the absorption spectrum,while the fluorescence emission peak is blue-shifted to 466 nm compared with that of Yu0.The Stokes shift observed in Yu1 reveals that the bottoms of the potential curves of the ground state and the fluorescent excited state may not locate at the same coordinate,quite different from those of the Yu0 sample.
2.2 Z-scan and two-photon excited fluorescence spectra
The TPA cross sections of Yu0 and Yu1 were measured by using the open aperture Z-scan technique with the laser pulses at wavelength of 800 nm with 140 fs pulse duration.The concentrations were 0.035 and 0.015 mol·L-1(repeat unit)for Yu0 and Yu1 in THF solutions,respectively.The results are shown in Fig.3.
The values of TPA absorption coefficient β were determined by fitting the experimental result with self-compiled programs given in Ref.[37].The TPA cross section σ2can be calculated by using the equation of σ2=hνβ/N0,where N0=NAC is the number density of the absorption centers,NAis the Avogadro constant, and C represents the solute molar concentration.The TPA cross sections of Yu0 and Yu1 are determined to be 2 GM(1 GM=1× 10-50cm4·s·photon-1)and 143 GM (20 GM per repeating unit), respectively.It can be clearly seen that the TPA ability is significantly enhanced upon the polymerization,and the TPA cross section of Yu1(per repeating unit)is about 10 times of that for monomer Yu0.These findings are logically associated with the structural difference between Yu0 and Yu1.Unlike the molecualr structure of Yu0 with the A-D-A mode(here A is electron acceptor,and D is donor),the polymer Yu1 possesses the highly delocalized aromatic π-electron conjugated structure.In a previ-ous research work,we reported TPA properties of(E)-4,4′-bis (diphenylamino)stilbene(BDPAS)and its derivatives[37].For comparison purpose,we also show the corresponding experimental data of these organic compounds in Table 1.The TPA cross section of BDPAS,[4-((4-bromo-phenyl)-{4-[2-(4-diphenylaminophenyl)-vinyl]-phenyl}-amino)-phenyl]-methanol(sample-2)[38],and 4-(bis-{4-[2-(4-diphenylamino-phenyl)-vinyl]-phenyl}-amino)-vinyl-benzene(sample-4)[38]are 33,25,and 50 GM,respectively,while the values of σTPA/N are 16.5,12.5,and 16.5 GM,respectively.N is the number of triphenylamine[39].Among these materials listed in Table 1,the conjugated polymer Yu1 displays the best TPA performance.The materials with aldehye as the acceptor groups,for example,Yu0,4-((4-bromo-phenyl)-{4[2-(4-diphenylaminophenyl)-vinyl]-phenyl}amino)benzaldehyde(sample-1)[38],and 4-(bis-{4-[2-(4-diphenylamino-phenyl)-vinyl-phenyl}-amino)-benzaldehyd(sample-3)[38],exhibit poor TPA behavior.

Fig.3 Experimental and fitting results of open aperture Z-scan experiments for Yu0 and Yu1 dissolved in THF using 800 nm fs pulses T:transmittance
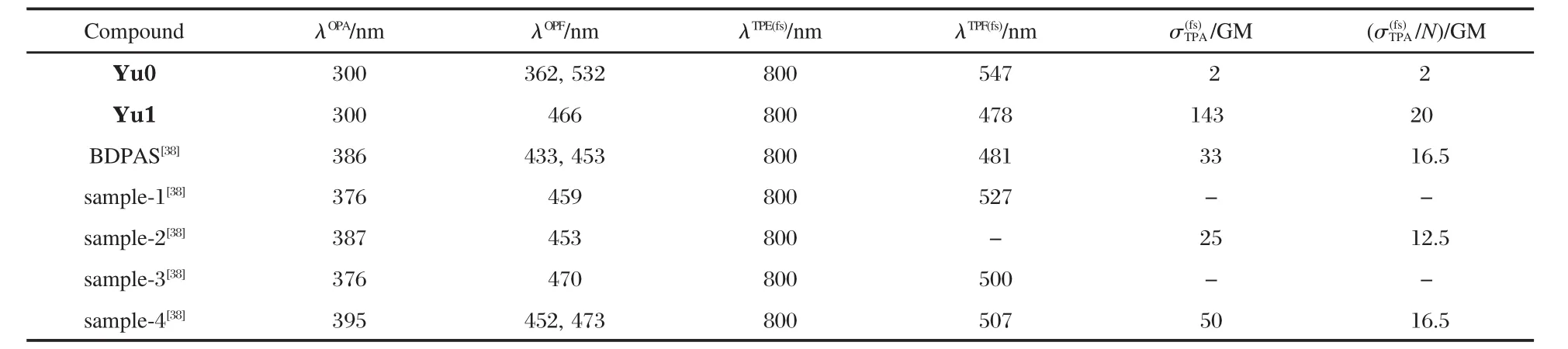
Table 1 One-photon and two-photon properties of some correlative compounds in THF solution
Both Yu0 and Yu1 emit intense fluorescence under the excitation of femtosecond pulses at wavelength of 800 nm.The fluorescence spectra were recorded on a Spectra 500-Pi spectrometer.The fluorescence spectra of Yu0 and Yu1 in THF solution excited by laser beam at different power densities are shown in Fig.4.The linear dependence of fluorescence intensity on the square of the excitation intensity confirms that the intense fluorescence emission excited by the 800 nm femtosecond pulses really comes from the TPA process.The TPF intensity is greatly enhanced upon polymerization of Yu0,and the fluorescence emission peaks of Yu0 and Yu1 appear at 547 and 478 nm,respectively.The observed red-shift effect in TPF spectra could be explained by the reabsorption effect,as we used solutions with a much higher concentration in the TPF experiment than those in linear case.However,for Yu0,another fluorescence emission peak at 362 nm disappears in TPF spectrum.The main reason for this is that the excitation wavelength used in TPF measurement is the 800 nm pulses,which can only excite the lower excited state,different from the 300 nm excitation beam used in one-photon fluorescence.
2.3 Ultrafast excited state dynamics
Femtosecond one-color and two-color pump-probe experiments were carried out to clarify the excited state dynamics of Yu0 and Yu1.Fig.5(a,b)show the results of one-color(at 800 nm)and two-color(with 400 nm pumping and 800 nm probe) pump-probe experiments.The average pump power is about several milliwatt.After fitting the experimental result by biexponential process analysis with the deconvolution of the instrumental response function,one can obtain the values of the decay times of the excited states for Yu0 and Yu1 in THF.The transient dynamics of the monomer Yu0 in one-color pump-probe at wavelength of 800 nm shows an ultrafast absorption peak with a lifetime of 230 fs,followed by a fast process with lifetime of 8.4 picosecond(ps)and a long decay process with lifetime of more than several hundreds ps.After polymerization,the dynamic of polymer Yu1 shows only two components:an ultrafast process of 225 fs and a long decay process of 103 ps.It is thus sugges-tive of that,the ultrafast component for these samples is effectively generated by a special TPA process,simultaneous absorption of one photon from the pump beam and another photon from the probe beam,resulting in the transition from the ground state to two-photon excited state[30].

Fig.4 TPF spectra of Yu0(a)and Yu1(b)in THF solution at different excitation intensities(Ipump)TPF:two-photon fluorescence;Insets are the TPF intensity versus the square of the excitation intensity.

Fig.5 (a)One-color pump-probe dynamics using 800 nm femtosecond pulses for Yu0 and Yu1 in THF solution; (b)Two-color pump-probe dynamics(400/800 nm pump-probe)for Yu0 and Yu1 in THF solution;One-color(400 nm)pump-probe dynamics for(c)Yu0 and(d)Yu1 in THF solution under two polarization configurations
As shown in Fig.5(b),the pump beam at 400 nm can effectively excite the molecules via linear absorption process.In these two-color pump-probe measurements,the ultrafast process of polymer Yu1 disappears,the dynamics decay shows only a long decay process of 108 ps.But for Yu0,there still exist three decay components with lifetimes of 303 fs,10 ps,and more than several hundreds ps.From these results,combining with onecolor pump-probe experimental results at 800 nm,we can conclude that the ultrafast process of Yu0 in both one-color at wavelength of 800 nm and two-color case at 400/800 nm were generated by TPA process.Yu0 could simultaneously absorb one photon from the 400 nm pump beam and another photon from the 800 nm probe beam in the transition from the ground state to a higher excited state.
Another one-color pump-probe transient measurement at wavelength of 400 nm was carried out to further explore their excited state dynamics.The obtained temporal responses in parallel and perpendicular polarization configurations are shown in Fig.5(c,d).The calculated time constants and the percentages of the corresponding components for parallel configuration are summarized in Table 2.The dynamics of Yu0 show no apparent difference under parallel and perpendicular polarization configurations.While for Yu1,there is a considerable difference between two polarization configurations at the initial decay time, which indicates that this sharp component is not caused by the coherent artifact effect.When two beams are parallelly polarized,the decay traces can be decomposed into two processes. The lifetime of fast decay process,corresponding to exciton migrations,is determined to be 0.19 ps which is much shorter than compounds P1,P2,and P3[34]with the D-π-A structure reported before.Basically,the short lifetimes could be considered as a direct demonstration of effective exciton migration processes. The main difference between Yu1 and P1,P2,P3 lies in thedifference of their structures.Yu1 has a D-π-D structure,which is different from the D-π-A structure of P1-P3.Thus we guess that the D-π-D structure polymer may possess better TPA properties.As π-conjugated polymers can be regarded as a one-dimensional system and the exciton migration is mainly along the backbone of the polymer[34]showing large optical anisotropy. The process of exciton migration will be undetectable in the direction perpendicular to that of the backbone.Thus,the fast process of dynamics disappeared due to the high orientation of exciton migration when the probe beam and pump beam were set in perpendicular polarization configuration.For Yu0 and Yu1,the lifetimes of long decay processes are 311 and 211 ps,respectively,which are assigned to exciton recombination processes with fluorescence emission.

Table 2 Calculated time constants and the percentages(in the parenthesis)of the corresponding decay components in one-color pump-probe experiment at wavelength of 400 nm
3 Conclusions
Two novel TPA materials,Yu0 and Yu1,were studied by femtosecond laser spectroscopic techniques,including Z-scan, TPF,one-color and two-color pump-probe.Both two materials exhibit intense TPF emission under the excitation of fs pulses at wavelength of 800 nm.In contrast to the monomer Yu0,the TPA properties of Yu1 was found being significantly enhanced upon polymerization.The investigation of the ultrafast dynamics demonstrates the different relaxation processes happened in the samples Yu0 and Yu1.From 400 nm one-color pump-probe experiment of the polymer Yu1,one can observe apparent optical anisotropy.The fast component observed in parallel configuration of Yu1 disappears in the perpendicular polarization configuration due to the high orientation of exciton migration.The nonlinear optical study and the ultrafast dynamics results really reflect an enhancement effect caused by the polymerization of the monomer.Our study also reveals that the polymer Yu1 may have promising applications in photonics fields,such as two photon imaging,optical limiting and/or polymeric light-emitting diodes(PLED).
1 Kasischke,K.A.;Wishwasrao,H.D.;Fisher,P.J.;Zipfel,W.R.; Webb,W.W.Science,2004,305:99
2 He,G.S.;Xu,G.C.;Prasad,P.N.;Reinhardt,B.A.;Bhatt,J.C.; Dillard,A.G.Opt.Lett.,1995,20:435
3 Kawata,S.;Sun,H.B.;Tanaka,T.;Takada,K.Nature,2001,412: 697
4 Zhou,W.H.;Kuebler,S.M.;Braun,K.L.;Yu,T.Y.;Cammack,J. K.;Strickler,J.H.;Webb,W.W.Science,2002,296:1106
5 Strickler,J.H.;Webb,W.W.Opt.Lett.,1991,16:1780
6 Cumpston,B.H.;Ananthavel,S.P.;Barlow,S.;Dyer,D.L.; Ehrlich,J.E.;Erskine,L.L.;Heikal,A.A.;Kuebler,S.M.;Lee,I. Y.S.;McCord-Maughon,D.;Qin,J.G.;Röckel,H.;Rumi,M.; Wu,X.L.;Marder,S.R.;Perry,J.W.Nature,1999,398:51
7 Denk,W.;Strickler,J.H.;Webb,W.W.Science,1990,248:73
8 Bhawalkar,J.D.;Kumar,N.D.;Zhao,C.F.;Prasad,P.N.J.Clin. Laser Med.Surg.,1997,15:201
9 Bhawalkar,J.D.;He,G.S.;Park,C.K.;Zhao,C.F.;Ruland,G.; Prasad,P.N.Opt.Commun.,1996,124:33
10 He,G.S.;Tan,L.S.;Zheng,Q.D.;Prasad,P.N.Chem.Rev., 2008,108:1245
11 Albota,M.;Beljonne,D.;Bredas,J.L.;Ehrlich,J.E.;Fu,J.Y.; Heikal,A.A.;Hess,S.E.;Kogej,T.;Levin,M.D.;Mardar,S.R.; McCrod-Maughon,D.;Perry,J.W.;Rumi,H.R.;Subramaniam, G.;Webb,W.W.;Wu,X.L.;Xu,C.Science,1998,281:1653
12 Mongin,O.;Porres,L.;Moreaux,L.;Mertz,J.;Blanchard-Desce, M.Org.Lett.,2002,4:719
13 Chung,S.J.;Kim,K.S.;Lin,T.C.;He,G.S.;Swiatkiewicz,J.; Prasad,P.N.J.Phys.Chem.B,1999,103:10741
14 Yoo,J.;Yang,S.K.;Jeong,M.Y.;Ahn,H.C.;Jeon,S.J.;Cho,B. R.Org.Lett.,2003,5:645
15 Brousmiche,D.W.;Serin,J.M.;Fréchet,M.J.;He,G.S.;Lin,T. C.;Chung,S.J.;Prasad,P.N.;Kannan,R.;Tan,L.S.J.Phys. Chem.B,2004,108:8592
16 Pond,S.K.;Rumi,M.;Levin,M.D.;Parker,T.C.;Beljonne,D.; Day,M.W.;Brédas,J.L.;Marder,S.R.;Perry,J.W.J.Phys. Chem.A,2002,106:11470
17 Ventelon,L.;Morel,Y.;Baldeck,P.;Moreaux,L.;Mertz,J.; Blanchard-Desce,M.Nonlinear Optics Principles Materials Phenomena and Devices,2001,27:249
18 Porres,L.;Mongin,O.;Blanchard-Desce,M.;Ventelon,L.; Barzoukas,M.;Moreaux,L.;Pons,T.;Mertz,J.Proceedings of the Society of Photo-Optical Instrumentation Engineers(SPIE),2003, 4797:284
19 Ventelon,L.;Moreaux,L.;Mertz,J.;Blanchard-Desce,M.Synth. Met.,2002,127:17
20 Cho,B.R.;Son,K.H.;Lee,S.H.;Song,Y.S.;Lee,Y.K.;Jeon,S. J.;Choi,J.H.;Lee,H.;Cho,M.J.J.Am.Chem.Soc.,2001,123: 10039
21 Cho,B.R.;Piao,M.J.;Son,K.H.;Lee,S.H.;Yoon,S.J.;Jeon,S. J.;Cho,M.Chemistry-A European Journal,2002,8:3907.
22 Hua,J.L.;Li,B.;Meng,F.S.;Ding,F.;Qian,S.X.;Tian,H. Polymer,2004,45:7143
23 Wang,X.M.;Yang,P.;Xu,G.B.;Jiang,W.L.;Yang,T.S.Synth. Met.,2005,155:464
24 Bhaskar,A.;Ramakrishna,G.;Lu,Z.;Twieg,R.;Hales,J.M.; Hagan,D.J.;Stryland,E.V.;Goodson III,T.J.Am.Chem.Soc., 2006,128:11840
25 Drobizhev,M.;Karotki,A.;Dzenis,Y.;Rebane,A.;Suo,Z.; Spangler,C.W.J.Phys.Chem.B,2003,107:7540
26 Bhaskar,A.;Ramakrishna,G.;Hagedorn,K.;Varnavski,O.;Mena-Osteritz,E.;Bäuerle,P.;Goodson III,T.J.Phys.Chem.B,2007, 111:946
27 Williams-Harry,M.;Bhaskar,A.;Ramakrishna,G.;Goodson III, T.;Imamura,M.;Mawatari,A.;Nakao,K.;Enozawa,H.; Nishinaga,T.;Iyoda,M.J.Am.Chem.Soc.,2008,130:3252
28 Varnavski,O.;Yan,X.Z.;Mongin,O.;Blanchard-Desce,M.; Goodson III,T.J.Phys.Chem.C,2007,111:149
29 Belfield,K.D.;Schafer,K.J.Chem.Mater.,2002,14:3656
30 Mi,J.;Li,B.;Zhu,R.;Liu,W.;Qian,S.;Meng,F.;Tian,H.Appl. Phys.B,2005,80:541
31 Li,B.;Tong,R.;Zhu,R.;Meng,F.;Tian,H.;Qian,S.J.Phys. Chem.B,2005,109:10705
32 Li,B.;Tong,R.;Zhu,R.;Hua,J.;Tian,H.;Qian,S.J.Luminesc., 2006,116:119
33 Yan,Y.;Li,B.;Liu,K.;Dong,Z.;Wang,X.;Qian,S.J.Phys. Chem.A,2007,111:4188
34 Yan,Y.;Li,B.;Liu,K.;Dong,Z.;Qian,S.;Li,W.;Wang,X.Appl. Phys.B,2007,88:249
35 Chen,Y.;Lin,Y.;Ei-khouly,M.E.;He,N.;Yan,A.;Liu,Y.;Cai, L.;Ito,O.J.Polym.Sci.Part A-Polym.Chem.,2008,4:4259
36 Ma,G.;Guo,L.;Mi,J.;Liu,Y.;Qian,S.;Pan,D.;Huang,Y.Solid State Commun.,2001,118:633
37 Sheik-Bahae,M.;Said,A.A.;Wei,T.H.;Hagan,D.J.;Van Stryland,E.W.IEEE Journal of Quantum Electron,1990,26:760 38 Huang,Z.Z.;Wang,X.M.;Li,B.;Lv,C.G.;Xu,J.;Jiang,W.L.; Tao,X.T.;Qian,S.X.;Chui,Y.P.;Yang,P.Opt.Mater.,2007,2: 1084
39 Drobizhev,M.;Karotki,A.;Dzenis,Y.;Rebane,A.;Suo,Z.; Spangler,C.W.J.Phys.Chem.B,2003,107:7540
