Development on the Technique of Total Recovery of Benzoic Acid Residue
2009-05-15XUJiao徐姣HEJie何杰ZHANGWeijiang张卫江YANGTao杨焘JIAOShujun焦书军andHUXuedong胡雪东
XU Jiao (徐姣), HE Jie (何杰),*, ZHANG Weijiang (张卫江), YANG Tao (杨焘), JIAO Shujun(焦书军) and HU Xuedong (胡雪东)
Development on the Technique of Total Recovery of Benzoic Acid Residue
XU Jiao (徐姣)1, HE Jie (何杰)1,*, ZHANG Weijiang (张卫江)1, YANG Tao (杨焘)1, JIAO Shujun(焦书军)2and HU Xuedong (胡雪东)2
1Chemical Engineering Research Center, Tianjin University, Tianjin 300072, China2Hebei Tongyuan Material Circulation Company Limited, Shijiazhuang 050032, China
Benzoic acid residue is solid waste produced from the production of benzoic acid by oxidizing toluene. Because it contained important chemical raw materials such as benzoic acid, benzyl benzoate and fluorenone, it is necessary to recover them from the residue. In this work the technique featured with high efficiency evaporation and vacuum distillation was developed to obtain total recovery and utilization of the benzoic acid residue. By controlling the operation temperature at 260°C and the pressure of 16 kPa in the rising and falling film evaporators, heavy components separated efficiently from the residue can be polymerized and the light components consisting of 63% of the residue entered into a benzoic acid vacuum distillation column. Keeping the temperature of polymerization at (280±10)°C, coumarone resin was produced after adjusting the softening point according to the market requirements. Vacuum distillation was operated under the following conditions: top temperature at 186°C, top pressure of 16 kPa, bottom temperature at 240-250°C, reflux ratio being 3︰1. Benzoic acid of 98% purity was distilled out from the column as a side stream and the bottom product was crude benzyl benzoate. By the developed technique, the benzoic acid residue was splitted into three products, benzoic acid, crude benzyl benzoate and coumarone resin without any surplus waste.
benzoic acid residue, benzoic acid, crude benzyl benzoate, coumarone resin, high-efficiency evaporation, vacuum distillation
1 INTRODUCTION
A large amount of benzoic acid residue is formed in the process of production of benzoic acid by oxidizing toluene, which is a mixture of benzoic acid, benzyl benzoate, fluorenone, diphenyl benzoic acid and many other materials [1, 2]. Under the normal temperature, benzoic acid residue appears black solid and inflammability. When heated up to 50°C, it starts to melt and brings strong stimulation on human skin, eyes and respiratory passage and heavy causticity on devices. So, it is very important to treat benzoic acid residue. Since there are many foreign substances with different characters in it and could not be separated easily, a good solution to this problem has not been found till today. Torviscosa Company of Italy buries it deep underground and the common disposal method in China is to burn. Nevertheless, all these methods cannot solve the problems of secondary pollution and the waste of resources.
Benzoic acid is an important organic raw material in chemical industry. It serves as food additive, antiseptic, medicine and cosmetic intermediates and is widely applied in the synthetic techniques of phenol and caprolactam [3-5]. Also, benzyl benzoate and fluorenone are raw materials for manufactures of medicine and fine chemicals [6-10]. And coumarone resin is a kind of rubber additive widely used in the rubber industry. To utilize the resources and reduce secondary pollution, the effective recovery technique of benzoic acid residue is studied, which combined high efficiency evaporation-vacuum distillation. Now an industrial setup has been established to recover three products, including benzoic acid, crude benzyl benzoate (a mixture of benzyl benzoate and fluorenone) and the heavy bottom residue (which can be used as a substitute of coumarone resin on the market after further polymerization), from the benzoic acid residue without any surplus waste.
2 EXPERIMENTAL
2.1 Benzoic acid residue
Every year 6000 tons of benzoic acid residue formed during the process of synthesizing caprolactam by benzoic acid was provided by Shijiazhuang Chemical Fiber Company Ltd. after most light components and catalyst had been recovered. Its physical properties and chemical composition as we determined are given in Tables 1 and 2. In Table 2 others represent the rest about thirty components in benzoic acid residue, and for each of which the mass fraction is less than 1%.

Table 1 Basic physical properties of benzoic acid residue
①When the heating temperature rises to 100°C, benzoic acid immediately sublimes from benzoic acid residue.
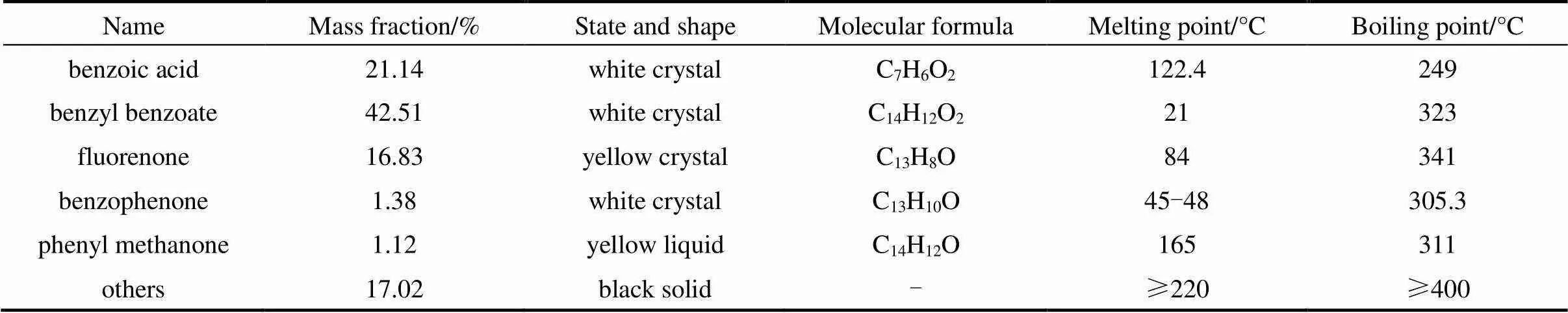
Table 2 Physical properties of main components in benzoic acid residue
2.2 Technological flow sheet
Benzoic acid residue is a byproduct produced in the process of toluene oxidation by air. It contains about several tens of kinds of organic materials of high melting and boiling points. And benzoic acid starts to sublime at 100°C, which can bring about blocking in the apparatus. So, it is very difficult to separate and purify by common distillation only. A technique combining high efficiency evaporation- vacuum distillation was developed to recover benzoic acid and other chemical products thoroughly based on laboratory investigation. The simple technological flow chart is shown in Fig. 1.
In this technique, high efficiency evaporation was a preparatory process in order to separate light components mainly including benzoic acid, benzyl benzoate and fluorenone from heavy ones, so as to reduce the burden of the vacuum distillation column. Through two steps of high efficiency evaporation the mass fraction of benzoic acid in light components increases up to 60% and was sent into the benzoic acid distillation column for further purification. The heavy ones were sent into polymerization kettle to produce coumarone resin.
In addition, normal pressure distillation does not work effectively because of high boiling points of the components. But vacuum distillation might work on it through increasing the mixture relative volatility and reducing the boiling points of the components [11]. So, vacuum distillation was adopted in this technique. Gaseous materials out of from high efficiency evaporators entered the benzoic acid distillation column for further separation. At the reflux ratio of 3︰1, benzoic acid product was collected from the column as a side stream after its purity reached at 98%, and the mixture of benzyl benzoate and fluorenone was collected from the bottom as the product of crude benzyl benzoate. Of course, crude benzyl benzoate can be further separated into benzyl benzoate, fluorenone and diphenyl benzoic acid.
During the process of high efficiency evaporation- vacuum distillation, benzoic acid residue was totally transformed into three products: benzoic acid, crude benzyl benzoate and coumarone resin without any surplus waste. The mass fractions of the three products in the benzoic acid residue were about 21%, 42%, 37% respectively. As a plasticizer, crude benzyl benzoate produced by this technique can meet the requirements of different industries. The coumarone resin can attain the national special grade standard because of its softening point 80-90°C, foreign materials less than 0.15% and water mass fraction less than 0.3%.
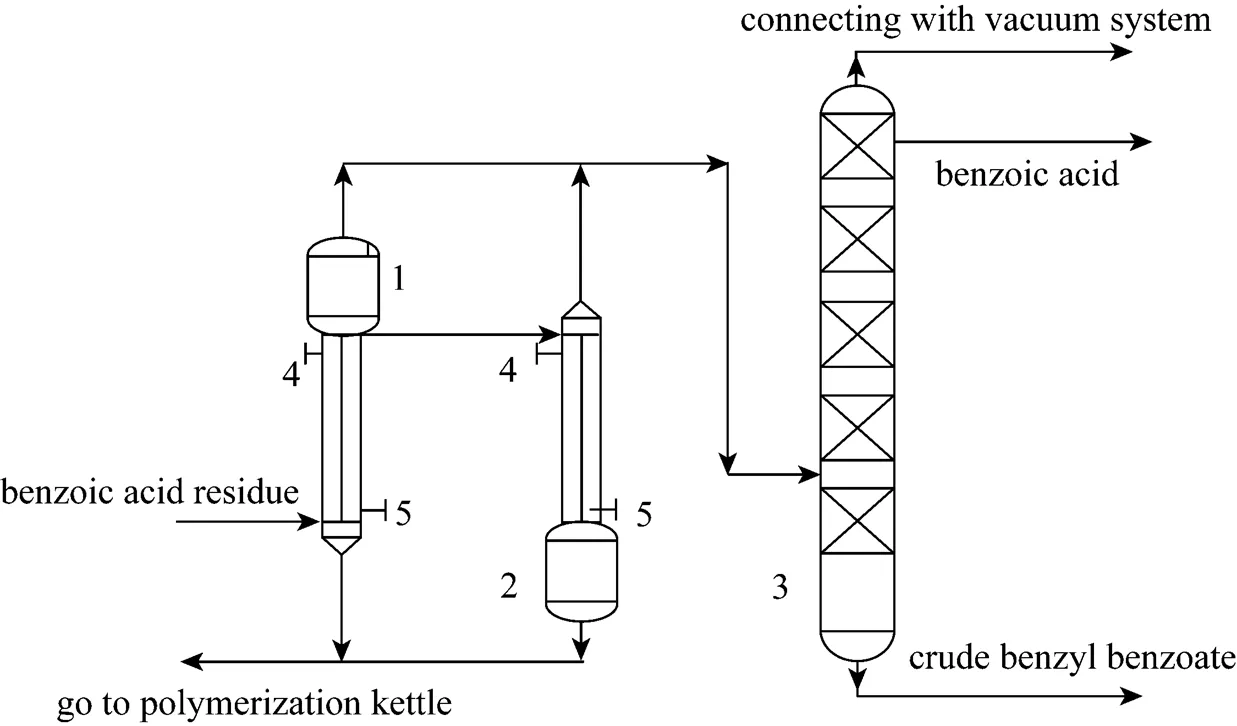
Figure 1 Technical flow chart for recovery of benzoic acid residue1—rising film evaporator; 2—falling film evaporator; 3—benzoic acid vacuum distillation column; 4—heat conducting oil outlet; 5—heat conducting oil inlet
2.3 Apparatus and procedure
2.3.1
High efficiency evaporation contained two steps of flashing, a rising film evaporator (12HJS-006) with 16 m2heat-exchanging areas and a falling film evaporator (12HJS-007) with 22 m2heat-exchanging areas (manufactured by Shijiazhuang Sub-company of the 12th Construction Co. of China Chemical Engineering Cooperation). Every evaporator consists of a heating chamber filled with heating pipes and a separation compartment. Firstly, preheated benzoic acid residue kept in 120°C in the storage tank was pumped into the heating room of the rising film evaporator. Driven by high-speed moving vapor inside the heating pipes, the liquid flows in the form of film along the wall of pipes and evaporate by exchanging heat with heat conducting oil of 320°C. Controlled temperature at 260°C and pressure at 16 kPa, most of light components are distilled out and evaporate into the vacuum distillation column. The rest liquid of light components in the separation room of the rising film evaporator entered into the falling one, where light components evaporate further and enter into the vacuum distillation column and the heavy residue is sent into a polymerization kettle with that from the rising film evaporator together to produce coumarone resin.
2.3.2
The benzoic acid vacuum distillation column was packed with JWB-500X stainless wire corrugated packing (the packing parameters in Table 3) produced by Tianjin University Tianjiu Chemical Co., column inner diameter was 800 mm, whole height of the column was 25 m and height of packing layer was 16 m, and the total theoretical plate number is estimated to be 64.
Because the freezing point of benzoic acid is 122.4°C, it is easy to freeze into the condenser and block the system. Total reflux was adopted at beginning and the top temperature was kept at the range of 180-186°C to prevent benzoic acid from condensing. Under the condition of stable temperature and pressure, samples were taken out at certain reflux ratio. Samples were taken and analyzed by gas chromatography.
Light components out of from evaporators entered into the benzoic acid vacuum distillation column and was separated under the following conditions: top temperature 186°C, top pressure 16 kPa, bottom temperature 240-250°C, reflux ratio 3︰1. Benzoic acid with purity of 98% was distilled out from the column as a side stream. The rest heavy distil fraction was collected from the bottom of column as the product of crude benzyl benzoate.
Considering trace benzoic acid in the gaseous phase may enter the vacuum pump through the vacuum lines, condense in it and block the system, a series of sublimation/crystallizing columns with 3 m2cooling areas of each operated alternately in front of the vacuum system to collect benzoic acid crystal, which can collect benzoic acid 3-5 kg∙h-1. The process is shown in Fig. 2.
2.3.3
High boiling point component out of from rising and falling film evaporators was pumped into the polymerization kettle, where some kinds of organic materials react with each other and polymerize into larger molecular materials such as anhydrides and esters at the temperature of (280±10)°C. After a period of 30 h, the softening point can rise up to (85±3)°C, and it can be adjusted by varying the reaction time to meet different requirements of the market. After having exchanged heat with crude material of benzoic acid residue, temperature of coumarone resin dropped to 150°C. Then it was sent to cool down and crystallize. At last the aim product of coumarone resin was shaped into thin pieces for package. As a kind of rubber additive, this product was used as coumarone resin widely in the tyre manufacture industry.

Table 3 Parameters of the structured packing of JWB-500X

Figure 2 Schematic drawing of sublimation crystallizing columns 1—sublimation crystallizing columns; 2—vacuum system; 3—benzoic acid gas; 4—exhaustion pipe; 5—ethanol inlet
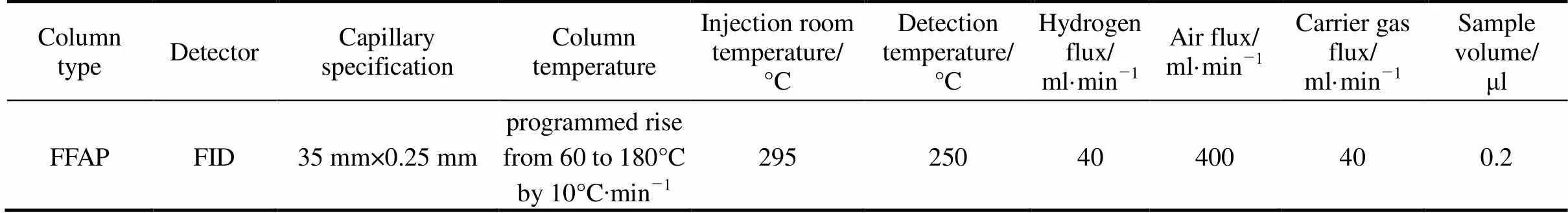
Table 4 Conditions of gas chromatography
In addition, a little waste gas consisting of low boiling point components was released in the polymerization kettle. It was absorbed by ethanol in an absorption kettle. After the temperature of saturated solution of ethanol was lowered to the room temperature, ethanol was reused as absorbent. The waste gas emitted into a fuel system.
2.4 Analyses
Samples were analyzed [12] by type SP-2100 gas chromatograph (Beijing Analytical Instruments Factory). The analytic conditions are described in Table 4. In this experiment, the area unitary method was adopted to the quantitative analyses of benzoic acid samples.
3 RESULTS AND DISCUSSION
3.1 Effect of evaporation temperature
Temperature is the key operation factor in the process of evaporation. When it is less than 260°C, part of light components that is not separated would enter the polymerization kettle with other heavy components so that the difficulty of polymerization would be increased and the polymerization time prolonged. When the evaporation temperature is higher than 260°C, part of heavy components were evaporated into the vacuum distillation column, which increased the burden of vacuum distillation and affected the purity of benzoic acid. So, the optimal evaporation temperature was 260°C.
3.2 Effect of top pressure of vacuum distillation column
The boiling point of components in the vacuum distillation column was affected by top pressure, and the collection temperature was also affected. When operated under lower top pressure, light fraction can be distilled easily at lower collection temperature. When the top pressure of the benzoic acid vacuum distillation column was controlled at 16 kPa, benzoic acid purity could reach 98%.
3.3 Effect of reflux ratio of vacuum distillation column
Many light and heavy components existed in the benzoic acid residue, and their separation was affected by the reflux ratio. In this experiment, the effect of reflux ratio on the purity of benzoic acid was investigated.
As shown in Table 5, with the increase in reflux ratio, the purity of benzoic acid increased evidently. When reflux ratio was increased to 3, the purity of benzoic acid reached 98%. Hence, the reflux ratio of 3︰1 was considered an optimal operation parameter.
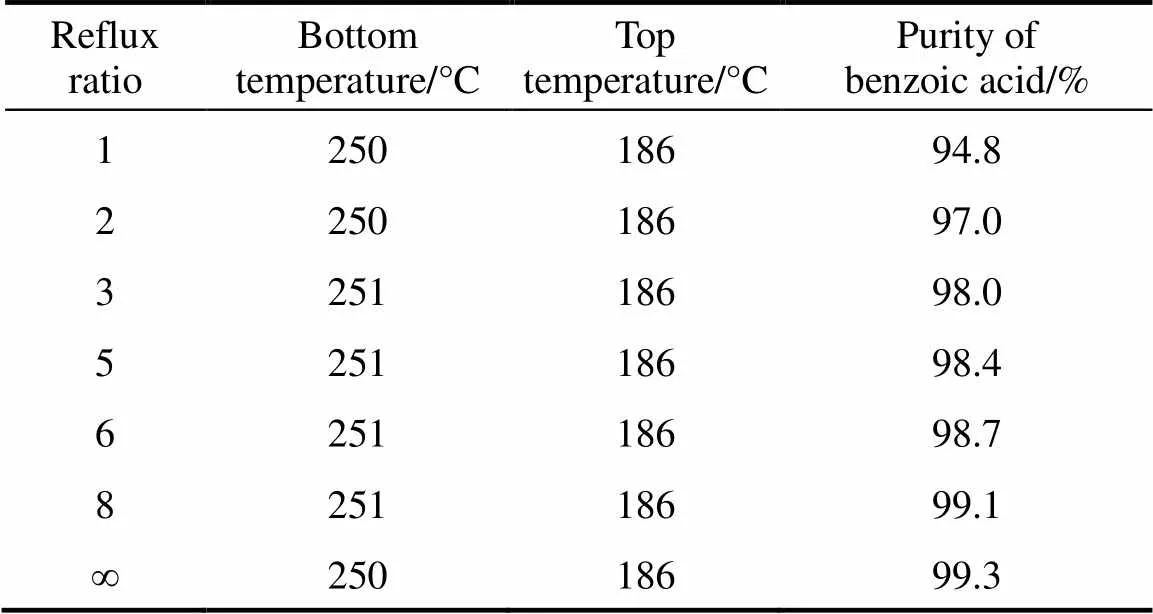
Table 5 Effect of reflux ratio on purity of benzoic
3.4 Effect of top and bottom temperature of vacuum distillation column
In this experiment, the effect of top and bottom temperature on the purity of benzoic acid were investigated in the vacuum column at the optimal reflux ratio 3︰1 and top pressure 16 kPa.
As shown in Table 6, when operated at the constant reflux ratio during the phase of 1-5 h, high purified benzoic acid was distilled out at the top temperature of 181−186°C and at the bottom temperature of 240-250°C, with the average purity of benzoic acid reaching 98%. After that, the top and bottom temperatures increased continually, and the purity of benzoic acid decreased below 80%, probably because heavy fractions such as benzyl benzoate and fluorenone appeared in the product.
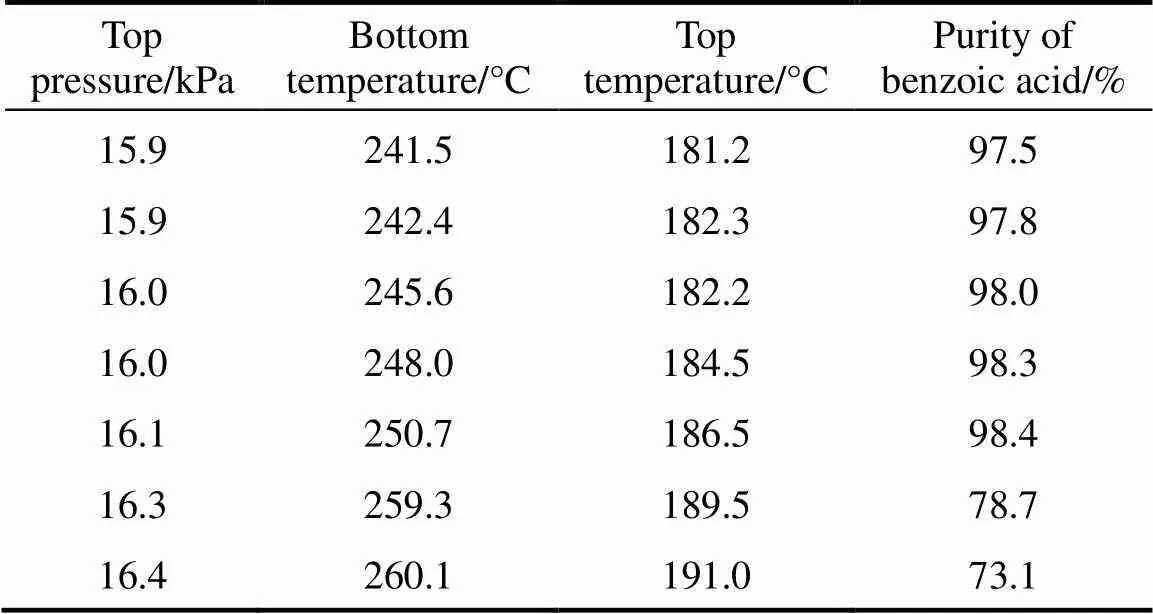
Table 6 Effect of the top and bottom temperatures on benzoic acid purity
3.5 Effect of feed rate to vacuum distillation column
In this experiment, the effect of feed rate on vacuum distillation was investigated under the conditions: reflux ratio 3︰1, top pressure 16 kPa, top temperature 181-186°C, bottom temperature 240-250°C. As shown in Table 7, with the increase of feed rate, the purity of benzoic acid increased gradually. But when feed velocity reached over 0.4 L∙h-1, the purity of benzoic acid increased very slowly and kept stable level of 98%. So, 0.4 L∙h-1was chosen as the optimal feed velocity.
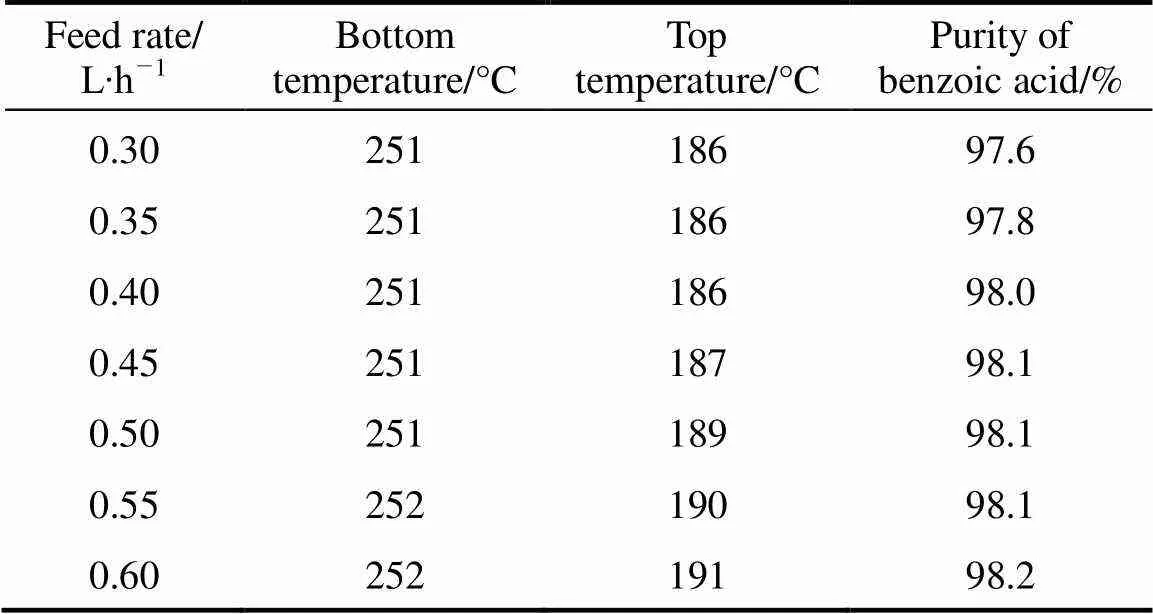
Table 7 Effect of feed velocity on purity of benzoic acid
4 CLOSING REMARKS
The technique featured with high efficiency evaporation and vacuum distillation was adopted by Hebei Tongyuan Material Circulation Co. Ltd to recover benzoic acid residue totally and succeeded in Oct. 2007. The troublesome pollution of benzoic acid residue transformed into three products: benzoic acid, crude benzyl benzoate and coumarone resin without any surplus waste by solving four difficult problems: separation of various components of high melting and boiling points by vacuum distillation; application of high efficient evaporation with the rising and falling film evaporators; prevention of benzoic acid blocking in the vacuum system by its sublimation and improvement of the quality of coumarone resin by polymerization. Averagely, the company benefits over 10 million CNY per year from this technique.
1 Liu, G.Q., Ma, L.X., Liu, J., Chemical Engineering Material Property Data Handbook, Chemical Industry Press, Beijing, 447 (2002). (in Chinese)
2 Wang, Z.Y., Zhang, Y.X., “Benzoic acid produced by liquid air oxidized from toluene”,, 7 (1), 1-3 (1996). (in Chinese)
3 Wu, X.G., Mo, D., “Statement of high purity benzoic acid refined process”,, 30, 1-3 (2000). (in Chinese)
4 Sieber, R., Bosset, J.O., “Benzoic acid as a natural compound in cultured dairy products and cheese”,., 5, 227-246 (1995). (in Chinese)
5 Lin, G.T., Handbook of Food Additives, 3rd edition, Chemical Industry Press, Beijing, 645 (2003). (in Chinese)
6 Sbarbati Nudelman, N., Mendiara, S., “Conversion of benzaldehyde into benzyl benzoate from the reaction with lithium metal in hexane”,., 38(13), 2245-2248 (1997).
7 Li, Y., “Study on the process of benzyl benzoate composes”,, 2, 23-24 (1999). (in Chinese)
8 Liu, C.Y., Liang, T.S., Shu, D.M., “The application of fluorenone in the field of macromolecule composed”,, 3, 134-136 (2003). (in Chinese)
9 Nizink, G.E., “Preparation of fluorenone”, US Pat., 3875237 (1975).
10 Zhao, X.F., Wang, G.Y., Xiao, R.H., “Study on fluorenone produced by air liquid oxidized”,, 32 (3), 145-147 (2001). (in Chinese)
11 Lei, Z.G., Chen, B.H., Ding, Z.W., Special Distillation Process, Elsevier, Amsterdam (2005).
12 Li, H.C., Analytical Chemistry Handbook—Analysis of Gas Chromatogram, 2nd edition, Chemical Industry Press, Beijing (1999). (in Chinese)
2008-09-22,
2009-05-18.
* To whom correspondence should be addressed. E-mail: wjzh@tju.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Challenges in Study of Single Particles and Particle Swarms*
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- An Unsteady Heterogeneous Mass Transfer Model for Gas Absorption Enhanced by Dispersed Third Phase Droplets*
- A Review of Techniques for the Process Intensification of Fluidized Bed Reactors
- Influences of the [Co2+]/[Co3+] Ratio on the Process of Liquid-phase Oxidation of Toluene by Air*
- Modeling and Kinetic Study on Absorption of CO2 by Aqueous Solutions of N-methyldiethanolamine in a Modified Wetted Wall Column*
