Novel High Performance Ziegler-Natta Catalyst for Ethylene Slurry Polymerization
2009-05-14GuoZifang郭子芳ChenWei陈伟ZHOUJunling周俊领andYangHongxu杨红旭
Guo Zifang (郭子芳), Chen Wei (陈伟), ZHOU Junling (周俊领) and Yang Hongxu (杨红旭)
Novel High Performance Ziegler-Natta Catalyst for Ethylene Slurry Polymerization
Guo Zifang (郭子芳)1,2,*, Chen Wei (陈伟)2, ZHOU Junling (周俊领)2and Yang Hongxu (杨红旭)2
1Department of Material Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China2Sinopec Beijing Research Institute of Chemical Industry, Beijing 100013, China
A novel high performance MgCl2/TiCl4catalyst with tetrabutyloxsilicane as electron donor was prepared for ethylene slurry polymerization process. The properties of the catalyst such as particle size distribution, catalytic activity, hydrogen responsibility and copolymerization performance were investigated and compared with commercial catalyst (imported catalyst). Copolymerization of ethylene and 1-butylene using the catalyst was studied in a pilot plant. The composition, structure and property of the copolymer were characterized by13C nuclear magnetic resonance (13C NMR) and gel permeation chromatography-Infrared (GPC-IR), and compared with those of the copolymer obtained from a commercial catalyst. In comparison with the commercial catalyst, the novel catalyst had a higher activity (up to 34.6 kg·g-1) and a better particle size distribution (PSD), and produced polymers having higher bulk density (up to 0.37 g·cm-3) with less fine resin. Meanwhile, the novel catalyst showed a higher hydrogen responsibility and better copolymerization performance. The results indicated that the copolymer obtained from the novel catalyst has a higher branch in the high molecular weight fraction and lower branch in the low molecular weight fraction.
Ziegler-Natta catalyst, polyethylene, slurry polymerization process, structure and properties
1 INTRODUCTION
It is well known that Ti/Mg catalyst systems are commonly used in production of polyethylene in industry. The relevant researches are focused on catalytic activity, particle morphology, particle size distribution, hydrogen response and copolymerization performance [1-6]. For slurry phase polymerization processes of ethylene, besides the requirement of higher activity catalyst, the control of the particle size and its distribution of the resultant polyethylene are quite important [7-11]. It is known that slurry high density polyethylene (HDPE) processes to produce high value added bimodal resins is an important tendency, which requires the better performance catalysts. But there are some problems with commercial catalysts. First, the poor catalyst morphology results in polymer with unwanted fines and wide PSD. Especially in the production of bi-modal resins, excessive fines will lead to fouling in the system. Secondly, the poor co-polymerization performance leads to produce too much wax and thus pipe fouling.
During the ethylene polymerization, fine polymer particles will likely cause the generation of static electricity, the occurrence of “dust” phenomenon, and sometimes the formation of agglomerates which might block the transfer conduit systems after treatment. The most efficient approach to control particle size and its distribution of the polymer is to control the same parameters of catalyst used. Usually, two methods are typically used to prepare the main catalyst components in order to obtain catalysts having uniform particle diameter and good particle morphology. In the first method, a solid carrier consisting of an alcohol- adduct of magnesium dihalide is suspended into a medium such as hexane and reacts with a titanium or vanadium compound to obtain the catalyst components. The particle size and its distribution of the catalysts and the resultant polyethylene were difficult to be controlled [11]. The process of the second method is to dissolve a magnesium compound, such as magnesium dichloride, into a solvent to form a homogeneous solution, following the addition of a titanium compound to precipitate a solid comprising magnesium and titanium. And then the main catalyst component was obtained by treating such solid with excess liquid titanium compound and form catalyst by the combination with cocatalyst component [12, 13]. This method suffers several drawbacks: the particle size and its distribution of the catalysts are controlled completely by the precipitation process so that preparation stability is poor; recovery system and environment will face big problem and the cost of the catalysts is rather high due to the use of a large amount of liquid titanium compound. The particle size distribution of the resultant polymer powder is relatively broad and difficult to be controlled. Therefore, it is quite desired to provide a catalyst which will be suitable for slurry phase polymerization process of ethylene, exhibit high catalytic activity, show uniform particle diameter with narrow particle size distribution, and have good hydrogen response. In this article, a novel catalyst with tetrabutyloxsilicane as electron donor for ethylene slurry polymerization was prepared. The method of the catalyst prepared overcomes the forgoing drawbacks. The catalytic performance was compared with a commercial catalyst.
2 EXPERIMENTAL
2.1 Materials
Polymerization grade ethylene was obtained from Beijing Yanshan Petrochemical Co., Ltd. (BYPC), used after passage through 4A molecular sieve. Triethylaluminium (TEA)(Ethyl Co., 95% purity)was used without further purification. Handling of the air and moisture sensitive materials was conducted in a nitrogen-filled dry-box or under nitrogen protection. Titanium tetrachloride, tributyl phosphate, epoxy chloropropane, tetrabutyloxsilicane,n-hexane and anhydrous magnesium chloride were obtained from Beijing Chemical Reagents Co., Ltd. (Beijing, China). Commerical catalyst was afforded by Beijing Research Institute of Chemical Industry (BRICI).
2.2 Preparation of the catalyst
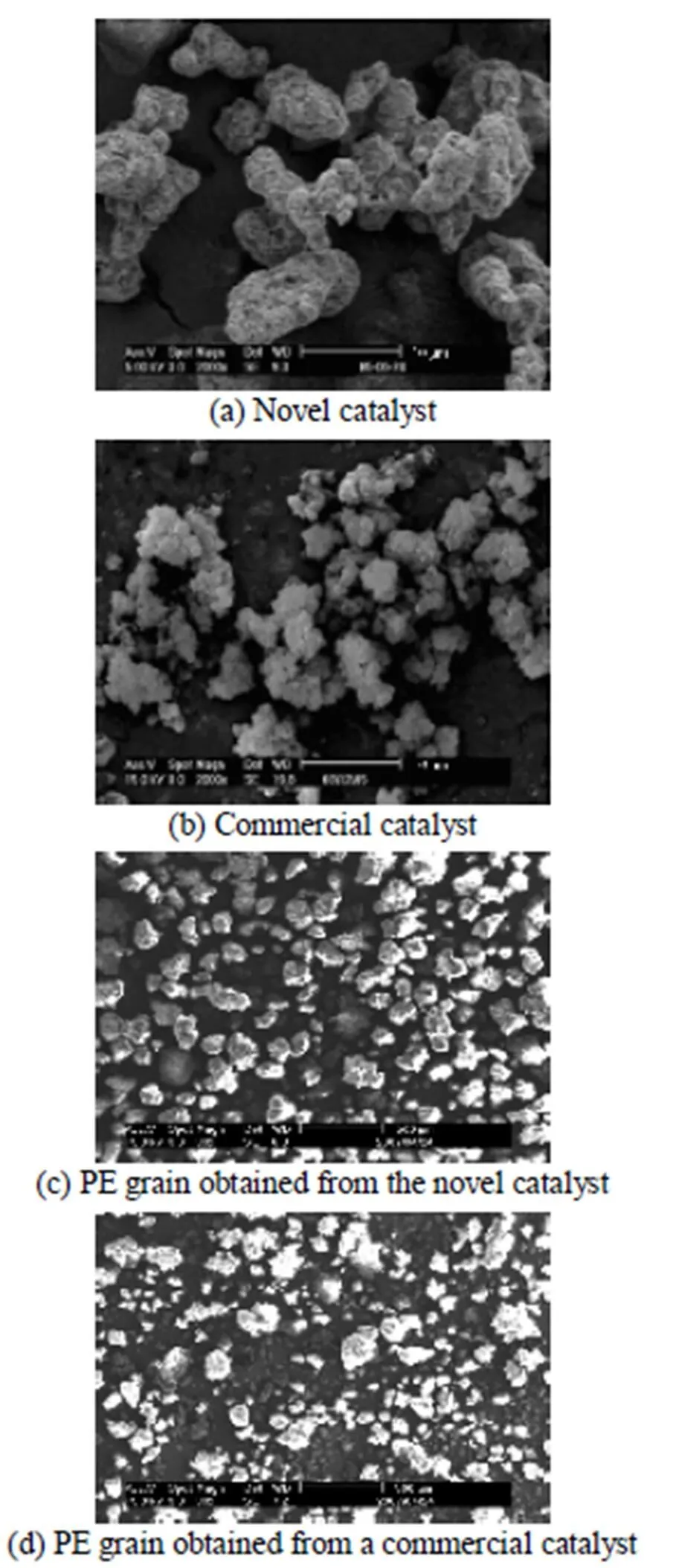
Figure 1 SEM pictures of the catalyst and PE grain
2.3 Polymerization of ethylene
One liter of hexane, 1.0 ml of 1 mol·L-1solution of AlEt3in hexane, and a certain amount of the above-prepared solid catalyst component (containing 0.25 milligrams of titanium) were added to a 2 liters stainless steel autoclave, in which atmosphere had been well replaced with highly pure N2. After the reactor was heated to 75°C, hydrogen was introduced until the pressure in the reactor reached 0.28 MPa (gauge pressure). Then, ethylene was introduced until total pressure in the autoclave reached 0.73 MPa (gauge pressure). The polymerization reaction was continued at 80°C for 2 hours and then extinguished by slowly releasing the gas in the autoclave.
2.4 Characterization of catalyst and polymers
The titanium contents of the catalyst were determined using inductively coupled plasma (ICP Swiss 3410 ARL). The13C NMR (nuclear magnetic resonance) spectrum of the polymer was recorded on a Bruker DMX-400. Gel permeation chromatography- Infrared (GPC-IR) analysis was determined at 150°C by a GPC150II+IR5, 1,2,4-trichlorobenzene stabilized with 300 mg·L-1of 2,6-di-butyl-hydroxyl toluene (BHT) as solvent with a flow rate of 1.0 ml·min-1. Short chain branches per 1000 total carbon (SCB/1000TC) by subtracting the number of methyl end groups per 1000 TC assuming the absence of vinyl chain ends.
3 RESULTS AND DISCUSSION
3.1 Morphology evaluation of the catalyst and polyethylene
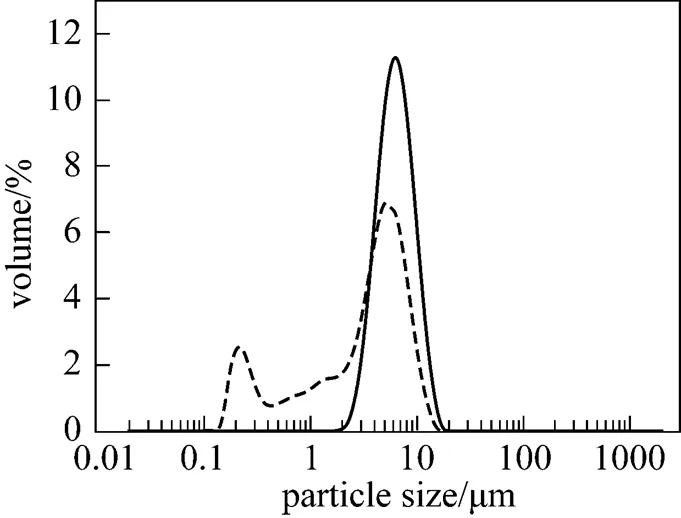
It is well known that particle size and dispersity of catalyst have important influence on the morphology and bulk density of the polymerization product. The scanning electron microscope (SEM) pictures of the catalyst and polyethylene are shown in Fig. 1. It is found that the catalyst particles have good morphology and well distribution as shown in Fig. 1 (a). The polyethylene grains have good particle morphology and well distribution because of the duplication of the catalyst morphology [Fig. 2 (c)]. Fig. 2 demonstrates that the novel catalyst has a narrower particle size distribution and less fine content than those of the commercial catalyst.

3.2 Hydrogen responsibility evaluation of the catalysts
Hydrogen is the most widely used chain-transfer agent for molecular weight control with Ziegler-Natta systems in industry. Hydrogen is the only commercially applicable chain-transfer agent in the low-pressure olefin polymerization process over the Ziegler-Natta catalysts [3]. The effects of H2concentration on ethylene polymerization using the novel catalyst were showed in Figs. 3 to 5 and were compared with the commercial catalyst.
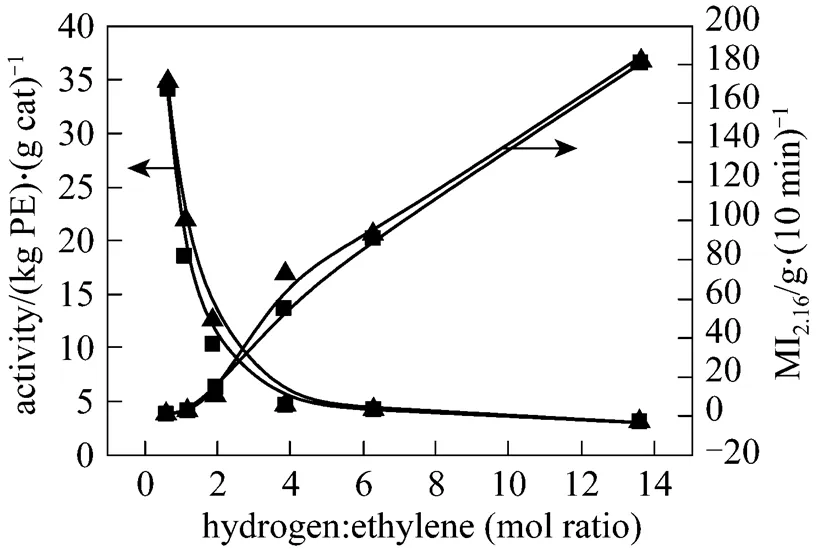
Figure 3 Comparison of hydrogen responsibility of two catalysts▲ novel cat.;■ commercial cat.
Figure 3 shows that hydrogen has a great effect on not only the product melt index (MI ) but also the catalyst activity. With increasing ratio of hydrogen/ ethylene, the polymer MI increases (molecule weight becomes smaller). The polymer MI of the novel catalyst is higher than that of the commercial catalyst. The rate of chain-transfer reaction increases with an increase in hydrogen, resulting in the increase of MI. Thus, the novel catalyst has a better hydrogen responsibility than the commercial catalyst. With the ratio of hydrogen/ethylene increases, the activities of both catalysts decrease and reach the same level.
The bulky density of two kinds of polymers decreased with the increase of H2loading as shown in Fig. 4. But the bulky density of the novel catalyst polymers was higher than that of the commercial catalyst due to the better catalyst particle morphology of the novel catalyst.
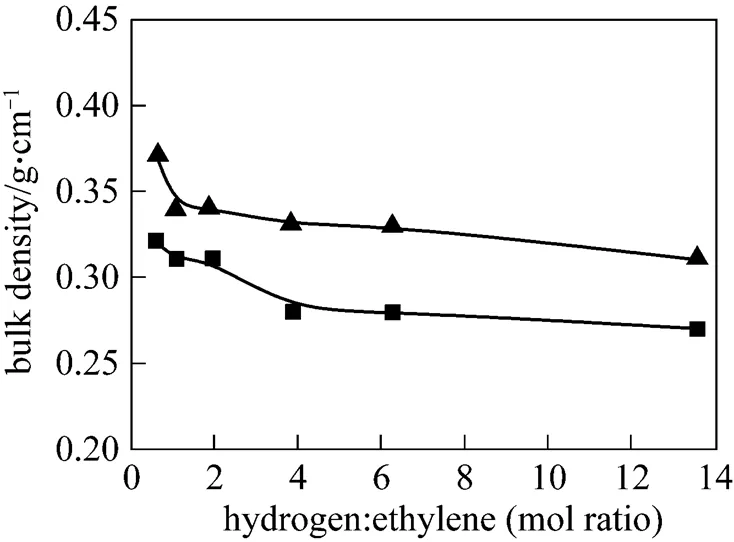
Figure 4 Effect of hydrogen on PE bulk density of two catalysts ▲ novel cat.;■ commercial cat.
Figure 5 indicates that the novel catalyst polymers had higher bulk density, narrower particle size distribution and less fines than those of the commercial catalyst because of duplicating the morphology of the catalysts.
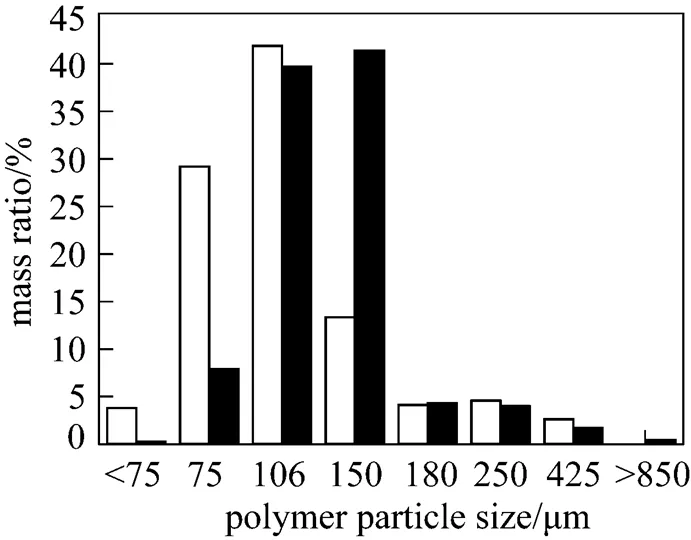

3.3 Copolymerization performance evaluation of the catalysts
In order to investigate copolymerization performanceof the novel catalyst, different amount of 1-butylene has been added to reactor and the results are listed in Table 1. The catalyst productivity and bulky density of polymers decreased with the increase of 1-butylene. Branch degree of the polymers increased with the increase of 1-butylene. At the same amount of 1-butylene, the polyethylene with higher branch degree was obtained by the novel catalyst, indicating that the novel catalyst has better copolymerization performance than the commercial catalyst.
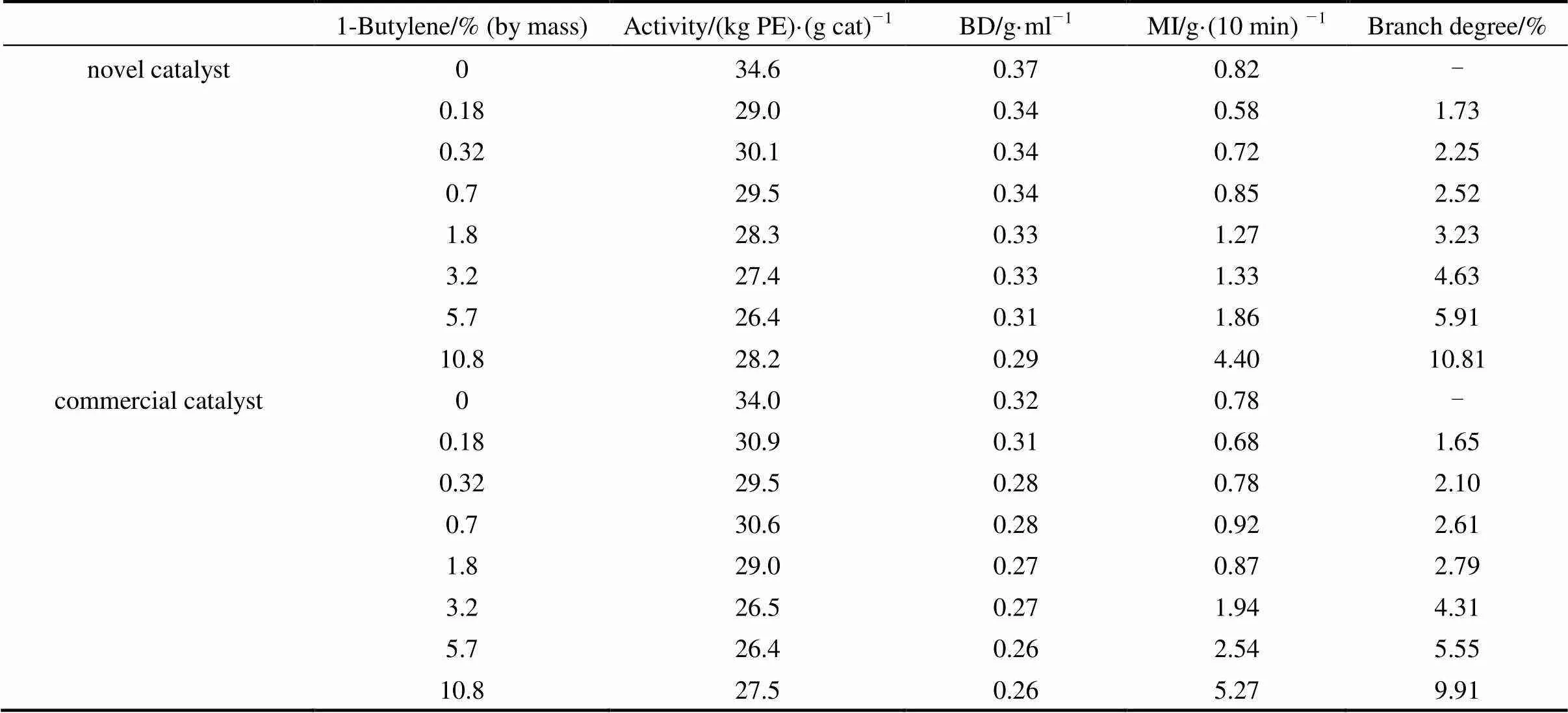
Table 1 Copolymerization performance evaluation of the catalysts
3.4 GPC-IR evaluation of the copolymer
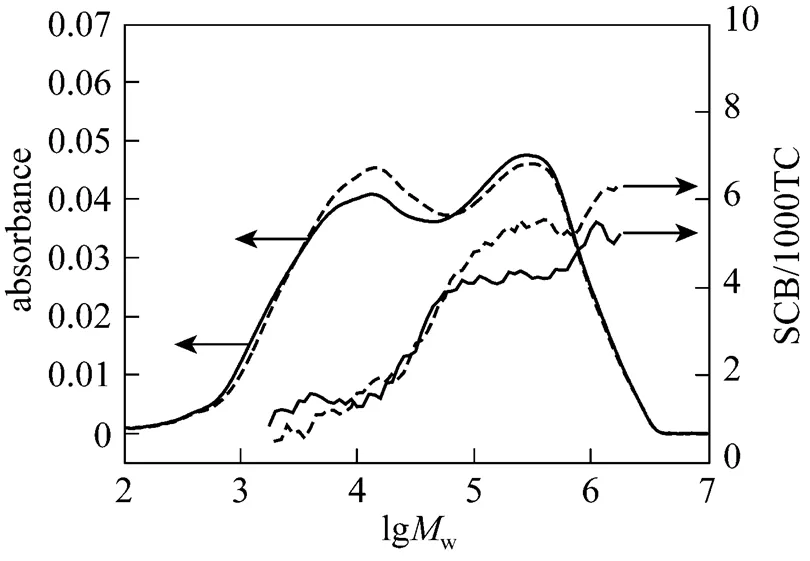
The polymer properties are largely determined by the characteristics of the polymer such as molecular weight and its distribution, and degree of branching. The breadth of the molecular weight distribution,Mw/n, also influences the processability of the polymer. The degree of short chain branching strongly influences some variables such as crystallinity and density, which in turn determines the ultimate properties of the material. The slurry polymerization processes provides resins that have excellent mechanical properties maintaining outstanding process-ability. It can be realized through bi-modal high molecular weight HDPE. The low molecular weight component produced in one reactor provides good processability, while the high molecular weight component created in the other reactor gives excellent mechanical strength. GPC-IR has been used to characterize the copolymer and the results are given in Fig. 6. It is found that the bi-modal molecular weight has been formatted. The copolymer obtained from the novel catalyst has a higher branch degree in the high molecular weight fraction and lower branch degree in the low molecular weight fraction, which is favorable to improve the resin mechanical properties.
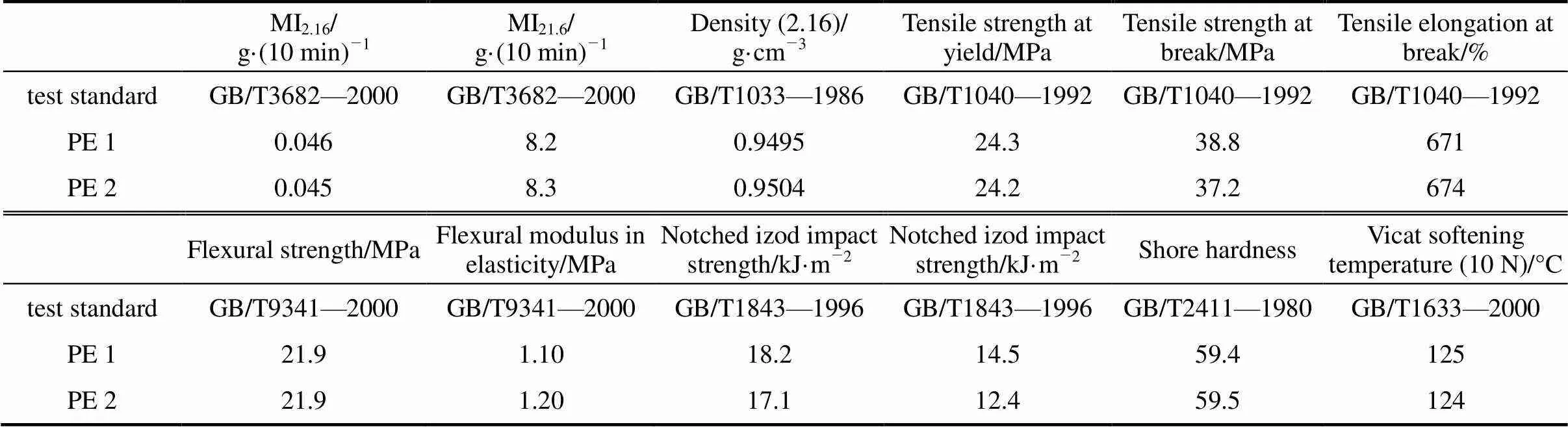
Table 2 Mechanical properties of two kinds of bimodal resin by the novel catalyst and commercial catalyst
Note: PE 1 denotes the polyethylene obtained from the novel catalyst; PE 2 denotes the polyethylene obtained from the commercial catalyst.
3.5 Mechanical property evaluation of the polymer
The mechanical properties of the foregoing bimodal polyethylene obtained from the commercial catalyst and the novel catalyst have been compared and the results are listed in Table 2. It is shown that the polyethylene obtained from the novel catalyst has better notched izod impact strength than that from the commercial catalyst.
A novel high performance MgCl2/TiCl4type catalyst with tetrabutyloxsilicane as electron donor was prepared and compared with the commercial catalyst in ethylene slurry polymerization process. Novel catalyst has higher catalytic activity, better hydrogen responsibility and better copolymerization performance for ethylene polymerization and copolymerization than the commercial catalyst. The polyethylene obtained from the novel catalyst has narrower particle size distribution, less resin fine content, higher polymer bulk density than those from the commercial catalyst. The copolymer obtained from the novel catalyst has a higher branch degree in the thigh molecular weight fraction and lower branch degree in the low molecular weight fraction.

1 Diedrich, B., “Second generation Ziegler polyethylene processes”,Appl..., 26, 1-11 (1975).
2 Ludwig, L.B., “The ethylenen polymerization with Ziegler catalysts: Fifty years after the discovery”,Chem..., 42, 5010-5030 (2003).
3 Galli, P., Luciani, L., Gecchin, G., “Advances in the polymerization of polyolefins with coordination catalysts”,Angew..., 94, 63-90 (1981).
4 Auriemma, F., Talarico, G., Corradini, P., Progress and Development of Catalytic Olefin Polymerization, Technology and Education Publishers, Tokyo, 7-15 (2000).
5 Böhm, L.L., “High mileage Ziegler catalysts: Excellent tools for polyethylene production”,Macromol.., 173, 55-63 (2001).
6 Montedison, S.P.A., “Catalyst components and catalysts for the polymerization of alpha-olefins”, US Pat., 4399054 (1981).
7 Hoechst, A.G., “Process for preparing a polyolefin”, DE Pat., 3620060 (1987).
8 Hoechst, A.G., “Process for producing a poly-1-olefin”, EU Pat., 0613909 (1994).
9 Hoechst, A.G., “Verfahren zur herstellung eines poly-1-olefins”, DE Pat., 4017661 (1990).
10 Mitsui Petrochemical Industries Ltd., “Process for polymerization or copolymerization of olefin and catalyst compositions used therefore”, US Pat., 4071674 (1978).
11 Yashiki, T., Minami, S.,“Solid titanium catalyst component, ethylene polymerization catalyst containing the same, and ethylene polymerization process”, US Pat., 6806222 (2002).
12 China Petrochem Corp., “Catalyst system for use in olefinic polymerization”,US Pat., 4784983 (1988).
2008-12-25,
2009-04-16.
* To whom correspondence should be addressed. E-mail: guozf@brici.ac.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- On-line Monitoring for Phosphorus Removal Process and Bacterial Community in Sequencing Batch Reactor*
- Mechanism Study of Rice Straw Pyrolysis by Fourier Transform Infrared Technique*
- Simultaneously Designing and Targeting for Networks with Multiple Resources of Different Qualities*
- Modeling and Control of Nonlinear Discrete-time Systems Based on Compound Neural Networks*
- Effect of Doping Cerium in the Support of Catalyst Pd-Co/Cu-Co-Mn Mixed Oxides on the Oxidative Carbonylation of Phenol
- Biodegradation of Aniline by a Newly Isolated Delftia sp. XYJ6*
