Antioxidant and Antimicrobial Activities of Echinacea (Echinacea purpurea L.) Extracts Obtained by Classical and Ultrasound Extraction*
2009-05-14IvanaStanisavljeviStojieviDraganVelikoviVladaVeljkoviandMiodragLazi
Ivana Stanisavljević, Sаša Stojičević,Dragan Veličković, Vlada Veljković and Miodrag Lazić
Antioxidant and Antimicrobial Activities of Echinacea (L.) Extracts Obtained by Classical and Ultrasound Extraction*
Ivana Stanisavljević1,**, Sаša Stojičević2,Dragan Veličković3, Vlada Veljković1and Miodrag Lazić1
1Faculty of Technology, University of Ni š, Bulevar oslobodjenja 124, 16000 Leskovac, Serbia2Chemical Industry Nevena A.D., Djordja Stamenkovića St.,16000 Leskovac, Serbia3Zdravlje-Actavis, Vlajkova 199, 16000 Leskovac, Serbia
Antioxidant and antimicrobial activities ofL. () extracts obtained by classical and ultrasound solvent extraction were compared. The dry aerial part of plant was extracted by 70% ethanol at a solid-to-liquid ratio of 1︰10 (m/v) and 25°C. The extract obtained by classical solvent extraction contained 29% larger amounts of phenolic compounds and 20% higher content of flavonoids. 2,2-diphenyl-1-picril hydrazyl radical (DPPH) scavenging reached 93.6% and the values of EC50 were (34.16±0.65) μg·ml-1and (65.48±1.12) μg·ml-1for the extracts obtained by the classical and ultrasound extractions, respectively. The extracts, independent of the extraction technique applied, showed a considerable growth inhibition onand, while no growth inhibition zones were observed for. The diameters of inhibition zone observed for all the microorganisms were larger for extracts obtained by classical extraction than those by ultrasound extraction.
antioxidant activity, antimicrobial activity,L., total phenols, flavonoids, extraction
1 INTRODUCTION
L., also known as purple coneflower, is an herbaceous perennial and a member of thefamily with a long, well-established tradition of medicinal use in North America, Europe [1] and Australia [2]. In modern cultures,.is used for medicinal purposes, in treating acute upper respiratory infections, urinary tract infections, burns and disorders such as viral infections, cutaneous affections and chronic disease due to a deficiency of immunological responses [1, 3-5]..stimulates various immune cells including macrophages and natural killer cells and has anti-inflammatory effects [4].
The roots, the leaves or the whole plant may be also used in the dietary supplement preparation. The composition of the root extracts, compared to the upper plant extracts, is very different. Root parts have more volatile oils and pyrrolizidine alkaloids, such as tussilagine and isotussilagine, than the aerial parts. The main active compounds of the aerial parts are alkamides and polyacetylenes, caffeic and ferulic acid derivatives, polysaccharides(such as 4-O-methylglucuronylarabinoxylans, rhamnoarabinogalactans and acidic arabinogalactan) and glycoproteins [5, 6]. Of the caffeic acid derivatives, only cichoric acid, which is found to be the main phenolic compound in., shows immunostimulatory properties, promoting phagocyte activityand, antihyaluronidase activity and has a protective effect on the free-radical-induced degradation of collagen [7]. Cichoric acid shows also antiviral activity and has recently been found to inhibit HIV-1 integrase and replication [7]. The quantitative analysis indicates that alkamides and caffeic acids are present at significant concentrations in ethanol/water extracts of this plant stored for longer than one year [8]. This revelation is important because alkamides and caffeic acid derivatives have been identified as possible active constituents of..
Beside cichoric acid, typical constituents of.extracts are echinacoside, chlorogenic acid, cynarine and caftaric acid. All of them are able to inhibit free radical production and lipid peroxidation, involved in the development of inflammation [1, 9]. Recent studies also suggest that melanin may contribute to the activity of.extracts [10], while echinacoside and caffeic acid derivative do not possess immunostimulant activity, but have weak antibacterial and antiviral effects and are protectants against reactive oxygen species [11].
The antioxidant activity of.extracts has been already shown [7, 12-15]. Generally, the tested.extracts showed medium to low activity compared to the other investigated medicinal and aromatic plants [12]. The antioxidant activity could be ascribed to the polyphenolic components [16], such as flavonoids [15, 17], phenolic acids [18] or phenolic diterpenes [15]. Moreover, some studies demonstrated that the.extracts protected immunosuppressed mice against systemic infections withandby stimulating macrophage and neutrophil function. The herb was non-toxic in mice, rats and humans even when administered intravenously at high doses [19].
In previous studies,.was reported to increase chemotoxicity in neutrophils and bactericidal activity againstand to kill tumor cells (WEHI 164 cells) and cells infected either with the parasiteor with yeast[6]. Also, it was found that hexane extracts of echinacea variably inhibited growth of yeasts,,.,.,.and.under near UV irradiation (phototoxicity) and to a lower extent without irradiation (conventional antifungal activity) [20].
So far, the classical solvent extraction with [8, 7, 21] or without [22, 23] mechanical agitation, Soxhlet [21] and Goldfisch [24] extraction techniques have been applied forsp. extraction. Recently, ultrasound extraction was used [5, 21, 25], but there is no comparative study of antioxidant and antimicrobial activities of.extracts obtained by different extraction techniques.
The purposes of this study are to compare the efficiency of extractive substances, antioxidant and antimicrobial activities of extracts obtained by ultrasound and classical extraction techniques. Also, total phenolic compunds and flavonoids in.aqueous ethanolic extracts obtained by two methods are compared. Antimicrobial and antiradical activity, antioxidant capacity, total phenolics and flavonoids of extracts are determined byassays.
2 EXPERIMENTAL
2.1 Materials
Ethanol was from Zorka-Pharma (Šabac, Serbia). Folin-Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazil (DPPH), gallic acid and rutin were obtained from Sigma (St. Louis, MO). Sodium carbonate, potasium acetate and aluminium chloride were purchased from Merck-Alkaloid (Skopje, FYR Macedonia).
Dried aerial parts of.L. were purchased from DOO. “Adonis” (Soko Banja, Serbia). Immediatly before being used, dry plant material was ground by an electrical mill with a fast-rotating knife (15000 r·min-1; 1 min). The moisture content, determined by drying at 105°C to constant mass, was 11.2% and the yield of extractive substances, obtained by the Soxhlet extraction (9 h, 13 extraction cycles) with 70% aqueous ethanol as extracting solvent, was 15.1 g per 100 g of dry plant material, which was taken to represent the content of extractive substances present in the plant material.
2.2 Extraction of plant materials
2.2.1
Ground plant material (10 g) and the predetermined volume of 70% aqueous ethanol were put in an Erlenmayer flasks (100 ml) at a ratio of plant material(g) and solvent(ml) of 1︰10. The extraction was performed at 25°C for 2.5, 5, 10, 20, 40, 60 and 90 min. The temperature was controlled and maintained at the desired level (±0.1°C). At the end of the extraction cycle the liquid extract was separated from the solid residue by vacuum filtration. The solid residue was washed twice with fresh solvent (20 ml each). The filtrates were collected and the solvent was evaporated in a rotary vacuum evaporator at 40°C.
2.2.2
The sonication was performed for 2.5, 5, 10, 20, 40 and 60 min with 70% aqueous ethanol, at a ratio of plant material (g) to solvent (ml) 1︰10 and 25°C using an ultrasonic cleaning bath (Sonic, Niš, Serbia; total nominal power: 150 W; operating at 40 kHz frequency and internal dimensions: 30 cm´15 cm´20 cm). The temperature was controlled and maintained at the desired level (±0.1°C) by water circulating from a thermostated water bath. Separation and further treatment of the filtrates were the same as described above.
2.3 Determination of free radical scavenging activity
The stable 1,1-diphenyl-2-picryl hydrazyl radical (DPPH) was used for determination of free radical- scavenging activity of the extracts [26]. Different concentrations of extracts (10, 20, 50, 100, 200, 500 and 1000 μg·ml-1, in 70% ethanol) were added, at an equal volume (2.5 ml) to an ethanolic solution of DPPH (0.3 mmol·L-1, 1 ml). After 30 min at room temperature, the absorbance of the plant extract with DPPH was measured at 517 nm on a spectrophotometer (VARIAN Cary-100) and converted into the percentage antioxidant activity using the following equation:
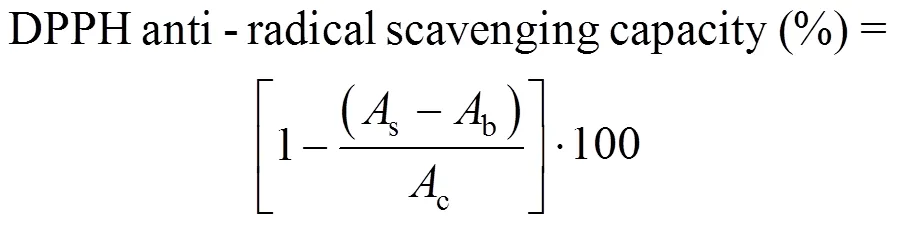
wheresis the absorbance of the plant extract containing DPPH,bis the absorbance of ethanol (1.0 ml) plus plant extract solution (2.5 ml) andcis the absorbance of DPPH solution (1.0 ml) plus ethanol (2.5 ml). The EC50values were calculated by sigmoid non-linear regression model using plots, where the abscissa and the ordinate represented the concentration of tested plant extracts the average percent of scavenging capacity from three replicates, respectively.
2.4 Determination of total phenols
2.5 Determination of total flavonoids
2.6 Antimicrobial activity
An agar well-diffusion method was employed for the determination of antimicrobial activities of extracts [29]. Seven microorganisms were selected to test the antimicrobial activity:ATCC 25922,ATCC 9027,ATCC 6633,ATCC 6538,ATCC 10231,ATCC 9763 andATCC 16404 (Oxoid, England).
For the yeast and mould, sabouraud dextrose agar (SDA) (Merck) was used; for cultures of bacteria, trypton soya agar (TSA) (Merck) was used, and plate count agar (Merck) was used for determination of the total number of microorganisms (CFU·ml-1). 0.1 ml of microorganism suspension, formed by 24 h culture on obliquely agar with 10 ml sterile 0.9% NaCl, was suspended into 10 ml of the nutritive medium (ca. 106CFU·ml-1). Petri dish (86 mm internal diameter) was filled with this system. The wells (10 mm in diameter) were cut from the agar and 30 µl of extract solution (concentration 20 mg·ml-1in methanol) was delivered into them. As a control, methanol (30 µl) was delivered into a well for each Petri dish. Erythromycin (997 µg·mg-1; [114-07-8]; Approx. 98%; H2O content 4%; Sigma) and Tylosin Tartarat (950 µg·mg-1; [74610-55-2]; Sigma) were used as a positive control (concentration in methanol solution, 0.05 mg·ml-1). All dilutions were filtrated using a 0.45 µm membrane filter (Sartorius, Germany). After incubation at 37°C for 24 h, agar plates were examined for any zones of inhibition. Diameters of zones of inhibition (mm) were measured by Fisher Lilly Antibiotic Zone Reader (Fisher Scientific Co., USA) and each test was run in triplicate.
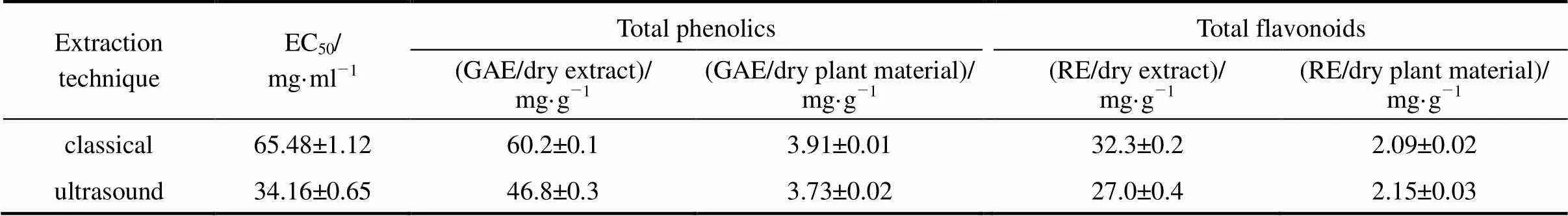
Table 1 Total antioxidant capacity, amount of phenolic and flavonoids compounds of E. purpurea extracts (25°C, 40 min)
Note: Data were expressed as the mean of three replicates ± standard deviation.
2.7 Statistical analysis
All of the measurements were carried out in triplicate and the results were expressed as mean ± standard deviation, except extract yields which were in duplicate. Comparison of means was analyzed by Student’stest and differences were considered significant when<0.05.
3 RESULTS AND DISCUSSION
3.1 Antioxidant capacity
The percentage of DPPH radical-scavenging activity was plotted against the plant extract concentration (Fig. 1) to determine the concentration of extract necessary to decrease DPPH radical concentration by 50% (so called EC50). The EC50value was used to measure the antioxidant activity of extracts: the lower EC50, the higher the value of the antioxidant activity.
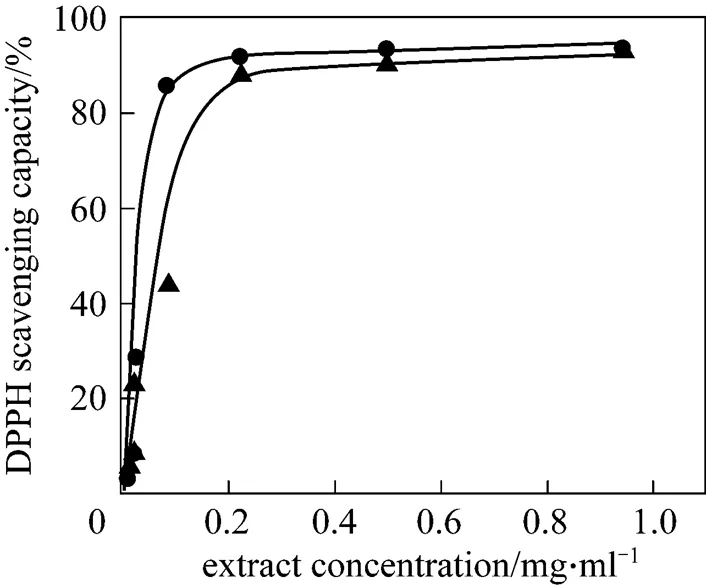
Figure 1 Antioxidant activity for.extracts obtained by classical and ultrasound extraction● classical extraction;▲ ultrasound extraction
As can be seen in Table 1, the extract obtained by the classical extraction shows higher antioxidant activity, where the differences observed are statistically significant with 95% confidence interval. This extract also contains larger amount of total phenolic compounds per gram of either dry extract or dry plant material. The total flavonoides content of the extract obtained by classical extraction is higher if calculated per gram of the extract but it is lower if calculated per gram of the dry plant material. Thus, ultrasonic extraction gave higher yield but lower purity of total flavonoides. The total amount of these compounds is accepted as an indication of antioxidant potential because they act in plants as antioxidants, antimicrobials and photoreceptors [30].
The extract obtained by classical solvent extraction contained 29% larger amount of phenolic compounds and 20% higher content of flavonoids. The differences observed were statistically significant with 95% confidence interval. We believed that the observed reduction of phenolic compounds and flavonoids in the extract obtained by ultrasound extraction was the result of their degradation by interaction with highly reactive hydroxyl radicals formed during sonication. Sonication of water results in the formation of hydroxyl radicals, which can combine to form hydrogen peroxide that may or may not be beneficial to the extraction process itself [31]. Organic compounds in aqueous solution exposed to an ultrasonic irradiation behave differently according to their physical and chemical properties [32]. In the cases of some compounds with antioxidant activity, aqueous solvents appeared to be unsuitable for ultrasonic extractions due to the formation of free radicals from the insonation of the solvent [31].
3.2 Antimicrobial activity
The antimicrobial activity of.extracts obtained by different extraction techniques were tested against the seven microorganisms mentioned above by the agar well-diffusion method.
As shown in Table 2, independent of the extraction technique, the ethanolic.extracts show activity against almost all of the tested microorganisms, exception is only mould.. The control treatment (methanol) has no inhibitory effect on any of the test microorganisms. Sensitivities of.,., and.are higher for both echinacea extracts than the case of tested antibiotic (Erytromycin and Tylosin tartarat).
The diameters of inhibition zone observed for all microorganisms were larger for extracts obtained by classical extraction than those by ultrasound extraction. The differences observed were statistically significant (with 95% confidence interval) in the case of.,.,.and.. No growth inhibition zones were observed for.for both tested extracts.
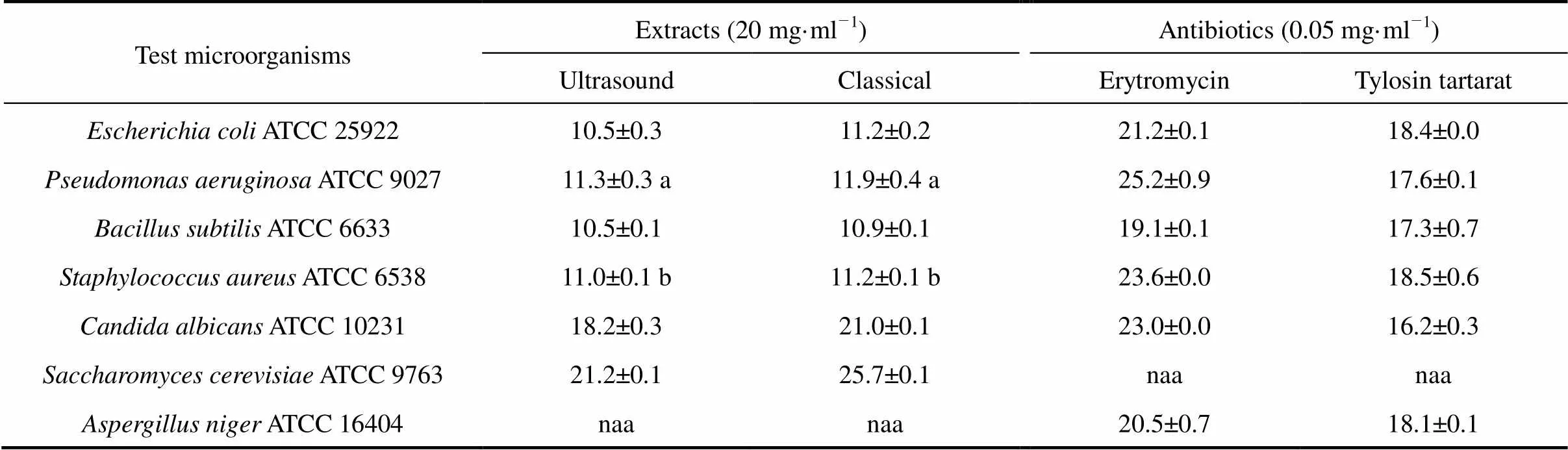
Table 2 Antimicrobial activity of E. purpurea extracts (40 min, 25ºC) and antibiotic sensitivity of microorganisms (zone size, mm)
Note: Control treatment (methanol) had no inhibitory effect on any of the test microorganisms. Differences between values for parameters designated with the same letters (a and b) were not statistically significant with 95% confidence interval (Student’stest). “naa” means no antimicrobial activity.
3.3 Kinetics of extraction
The changes of the extract yield from the aerial parts of.L. during the classical and ultrasound extraction are shown in Fig. 2. Independent of the extraction method, the extraction occurs in two main stages: first, dissolution of material near the surface characterized by a rapid increase in the extractive substance yield in the beginning of the process (washing or fast extraction), and second, diffusion of the solute from the porous plant residue into the solution (slow extraction). The optimum time for both extraction techniques was approximately 40 min, ensuring nearly the maximum oil yield.
As can be seen in Fig. 2, the main benefits of ultrasound included the increase of extractive substance yield and faster extraction. In recovering the extractive substances from.L. the ultrasound extraction was more efficient than the classical solvent extraction, but less efficient than the Soxhlet extraction. The total extract content obtained by ultrasound extraction after 40 min was 22.8% higher than that obtained by the classical extraction. At the same time, it was 52.8% of the yield obtained by the Soxhlet extraction, which was 15.1 g·(100 g)-1. However, a shortcoming of the Soxhlet extraction is higher operating temperature and in this case more than tenfold longer extraction time.
Figure 2 Variation of the extractive substance yield (based on dry plant meterial) from the.L.△ ultrasound extraction;▲ classical extraction
4 CONCLUSIONS
The present study suggests that 70% aqueous ethanolic extracts of.is a potential source of active natural and non-toxic substances, which have functions as antioxidants, antimicrobials and antibiotics. The extract obtained by the classical extraction from aerial parts of.L. contained larger amount of bioactive compound (total phenols and favonoid). Also, it showed stronger antioxidant and antimicrobial activities than the extract obtained by ultrasound extraction. Independent of the extraction technique, the ethanolic.extracts showed antimicrobial activity against all tested microorganisms except in the case of.. Ultrasound had a positive effect on the extractive substance yield from.L., but negative effects on the content of total phenolic compounds and flavonoids. Some bioactive compounds were probably degradated by interaction with highly reactive hydroxyl radicals formed during sonication.
1 Speroni, E., Govonib, P., Guizzardib, S., Renzullia, C., Guerra, M.C., “Anti-inflammatory and cicatrizing activity ofNutt. root extract”,.., 79, 265-272 (2002).
2 Wills, R.B.H., Stuart, D.L., “Alkylamide and cichoric acid levels ingrown in Australia”,., 67, 385-388 (1999).
3 NCCAM Publication No. D271 July 2005, http://nccam.nih.gov/ health/echinacea/, (17.05.2007).
4 Barrett, B., “Medicinal properties of Echinacea: A critical review”,, 10, 66-86 (2003).
5 Luo, X.B., Chen, B., Yao, S.Z., Zeng, J.G., “Simultaneous analysis of caffeic acid derivatives and alkamides in roots and extracts ofby high-performance liquid chromatography-photodiode array detection-electrospray mass spectrometry”,.., 986, 73-81 (2003).
6 Percival, S.S., “Use of Echinacea in medicine”,., 60, 155-158 (2000).
7 Pellati, F., Benvenuti, S., Magro, L., Melegari, M., Soragni, F., “Analysis of phenolic compounds and radical scavenging activity ofspp.”,...., 35, 289-301 (2004).
8 Cech, N.B., Eleazer, M.S., Shoffner, L.T., Crosswhite, M.R., Davis, A.C., Mortenson, A.M., “High performance liquid chromatography/electrospray ionization mass spectrometry for simultaneous analysis of alkamides and caffeic acid derivatives fromextracts”,.., 1103, 219-228 (2006).
9 NSF International (2004), INA Methods, Phenolics in Echinacea by HPLC, ttp://www.nsf.org/business/ina/echinacea.asp?program=INA, (26.09.2007).
10 Pugh, N.D., Balachandran, P., Lata H., Dayan, F.E., Joshi, V., Bedir, E., Makino, T., Moraes, R., Khan, I., Pasco, D.S., “Melanin: dietary mucosal immune modulator from Echinacea and other botanical supplements”,.., 5, 637-647 (2005).
11 Bergeron, C., Livesey, J.F., Awang, D.V.C., Arnason, J.T., Rana, J.B., Baum, R., Letchamo, W., “A quantitative HPLC method for the quality assurance of Echinacea products on the North American Market”,.., 11, 207-215 (2000).
12 Dalby-Brown, L., Barsett, H., Landbo, A.R., Meyer, A.S., Molgaard, P., “Synergistic antioxidative effects of alkamides, caffeic acid derivatives, and polysaccharide fractions fromonoxidation of human low-density lipoproteins”,..., 53, 9413-9423 (2005).
13 Hu, C., Kitts, D.D., “Studies on the antioxidant activity of Echinacea root extract”,..., 48, 1466-1472 (2000).
14 Duff Sloley, B., Urichuk, L.J., Tywin, C., Coutts, R.T., Pang, P.K.T., Shan, J.J., “Comparison of chemical components and antioxidant capacity of different Echinacea species”,..., 53, 849-857 (2001).
15 Pietta, P., Simonetti, P., Mauri, P., “Antioxidant activity of selected medicinal plants”,..., 46, 4487-4490 (1998).
16 Cervellati, R., Renzulli, C., Guerra, M.C., Speroni, E., “Evaluation of antioxidant activity of some natural polyphenolic compounds using the Briggs-Rauscher reaction method”,..., 50, 7504-7509 (2002).
17 Burda, S., Oleszek, W., “Antioxidant and antiradical activities of flavonoids”,..., 49, 2774-2779 (2001).
18 Velioglu, Y.S., Mazza, G., Gao, L., Oomah, B.D., “Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products”,..., 46, 4113-4117 (1998).
19 See, D.M., Broumand, N., Sahl, L., Tilles, J.G., “effects of echinacea and ginseng on natural killer and antibody-dependent cell cytotoxicity in healthy subjects and chronic fatigue syndrome or acquired immunodeficiency syndrome patients”,, 35, 229-235 (1997).
20 Binns, S.E., Purgina, B., Bergeron, C., Smith, M.L., Ball, L., Baum, B.R., Arnason, J.T., “Light-mediated antifungal activity of echinacea Extracts”,., 66, 241-244 (2000).
21 Perry, N.B., Burgess, E.J., Glennie, V.A., “standardization: Analytical methods for phenolic compounds and typical levels in medicinal species”,..., 49, 1702-1706 (2001).
22 Wanga, C., Chiaoa, M., Yena, P., Huanga, W., Houa, C., Chiena, S., Yeha, K., Yanga, W., Shyur, L., Yang, N., “Modulatory effects ofextracts on human dendritic cells: A cell- and gene-based study”,, 88, 801-808 (2006).
23 Binns, S.E., Livesey, J.F., Arnason, J.T., Baum, B.R., “Phytochemical variation in echinacea from roots and flowerheads of wild and cultivated populations”,..., 50, 3673-3687 (2002).
24 Kim, H., Durance, T.D., Scaman, C.H., Kitts, D.D., “Retention of alkamides in dried”,..., 48, 4182-4192 (2000).
25 Thygesen, L., Thulin, J., Mortensen, A., Skibsted, L.H., Molgaard, P., “Antioxidant activity of cichoric acid and alkamides fromalone and in combination”,., 101, 74-81 (2007).
26 Choi, C.W., Kim, S.C., Hwang, S.S., Choi, B.K., Ahn, H.J., Lee, M.Y., Park, S.H., Kim, S.K., “Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison”,., 163, 1161-1168 (2002).
27 Singleton, V.L., Rossi, J.A., “Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents”,...., 16, 144-158 (1965).
28 Chang, C., Yang, M., Wen, H., Chern, J., “Estimation of total flavonoid content in propolis by two complementary colorimetric methods”,.., 10, 178-182 (2002).
29 NCCLS (National Committee for Clinical Laboratory Standards), Performance Standards for Antimicrobial Susceptibility Testing 9th International Supplement, Wayne, PA, M100-S9 (1999).
30 Pietta, P., “Flavonoids as antioxidants”,..., 63, 1035-1042 (2000).
31 Paniwnyk, L., Beaufoy, E., Lorimer, J.P., Mason, T.J., “The extraction of rutin from flower buds of”,.., 8, 299-301 (2001).
32 Pétrier, C., Francony, A., “Ultrasonic waste-water treatment: incidence of ultrasonic frequency on the rate of phenol and carbon tetrachloride degradation”,., 4, 295-300 (1997).
2008-06-25,
2009-03-19.
the Ministry of Science and Environmental Protection, Republic of Serbia (142073B).
** To whom correspondence should be addressed. E-mail: icaneca@gmail.com
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- On-line Monitoring for Phosphorus Removal Process and Bacterial Community in Sequencing Batch Reactor*
- Mechanism Study of Rice Straw Pyrolysis by Fourier Transform Infrared Technique*
- Simultaneously Designing and Targeting for Networks with Multiple Resources of Different Qualities*
- Modeling and Control of Nonlinear Discrete-time Systems Based on Compound Neural Networks*
- Effect of Doping Cerium in the Support of Catalyst Pd-Co/Cu-Co-Mn Mixed Oxides on the Oxidative Carbonylation of Phenol
- Biodegradation of Aniline by a Newly Isolated Delftia sp. XYJ6*
