Catalytic Oxidation of Cyclohexane over ZSM-5 Catalyst in N-alkyl-N-methylimidazolium Ionic Liquids*
2009-05-14HUYongqi胡永琪WANGJianying王建英ZHAORuihong赵瑞红LIUYumin刘玉敏LIURunjing刘润静andLIYongdan李永丹
HU Yongqi (胡永琪), WANG Jianying (王建英), ZHAO Ruihong (赵瑞红), LIU Yumin (刘玉敏), LIU Runjing (刘润静) and LI Yongdan (李永丹)
Catalytic Oxidation of Cyclohexane over ZSM-5 Catalyst in-alkyl--methylimidazolium Ionic Liquids*
HU Yongqi (胡永琪)1,**, WANG Jianying (王建英)1, ZHAO Ruihong (赵瑞红)1, LIU Yumin (刘玉敏)1, LIU Runjing (刘润静)1and LI Yongdan (李永丹)2
1School of Chemical and Pharmaceutical Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, China2Tianjin Key Laboratory of Applied Catalysis Science and Technology, School of Chemical Engineering, Tianjin University, Tianjin 300072, China
Heterogeneous oxidation of cyclohexane by tert-butyl hydroperoxide (TBHP) was carried out over ZSM-5 catalysts with different Si/Al ratios in ionic liquids and organic molecular solvents. Higher yield and selectivity of the desired products were found in ionic liquids than in molecular solvents. The conversion of cyclohexane exhibits a decrease from 15.8% to 10.8% with the increase of Si/Al ratio of the HZSM-5 catalyst, and all the catalysts exhibit good selectivity of monofunctional oxidation products at around 97%. The activity of catalyst is found strongly dependent on the alkyl chain length of ionic liquid.
ionic liquid, cyclohexane oxidation, ZSM-5, catalysis
1 INTRODUCTION

Recently, the use of heterogeneous catalysts such as molecular sieves or metal-containing molecular sieves has attracted a great interest due to their redox ability, shape-selectivity and recyclability [4-8]. Titanium-containing molecular sieves [9] and metalloporphyrins [3, 10] have been used to catalyze the oxidation of cyclohexane. Transition metal complex [11-14] and transition metals (Sn, Zr, Cr, Fe, Mn, Co, Au, Ce and Cu) incorporated into zeolites [15-20] were also used as catalysts for this reaction. However, most of those reported works used volatile organic solvents such as acetonitrile, acetone, acetic acid and methanol, which resulted in contamination of the products and needed complicated separation procedure, also in some cases led to serious environmental problems [4].
There has been a growing interest in the use of ionic liquids as environmentally benign solvents in chemical processes [21-23]. The ambient-temperature ionic liquids, especially those based on 1,3-dialkylimidazolium cations coupled with anions such as tetrafluoroborate and hexafluorophosphate, have been emerging as promising green solvents in recent years. There are a number of intriguing properties of ionic liquids including high thermal and chemical stability, no measurable vapor pressure, non-flammability, and friction reducibility. These properties enable them to be significantly advantageous with reusability. Moreover, with ionic liquid used in heterogeneous reactions, the stability of the molecular sieves and other heterogeneous catalysts was found higher than that in the molecular solvents [24]. Sometimes the increase in the stability is accompanied by the increases in the activity and selectivity [25]. In these reactions, ionic liquids offer the opportunity of combining the advantages of both homogeneous reaction,.. catalyst modulation, and heterogeneous reaction,.. catalyst recycling, in one system.
Recently, we reported the use of ZSM-5 and metal-loading ZSM-5 as catalysts for the cyclohexane oxidation in ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate. The conversion of cyclohexane reached about 9%-21%, with a high selectivity of oxidation of products (>97%), showing that the mixture system of ionic liquid and ZSM-5 has good properties on cyclohexane oxidation reaction [26]. In this work, ZSM-5 molecular sieves with different Si/Al ratios were applied to catalyze the oxidation of cyclohexane in ionic liquids. There catalytic behaviors in cyclohexane oxidation with the use of a series of water-miscible and immiscible ionic liquids were studied in detail using-butyl-hydroperoxide as oxidant.
2 EXPERIMENTAL
2.1 Materials
All chemicals and reagents of analytical grade were used as received without further purification. H-ZSM-5 samples with Si/Al ratio of 25, 38 and 50 were commercial products from Catalyst Plant of Naikai University and were labeled as Z1, Z2 and Z3, respectively.-butyl hydroperoxide [TBHP, 85% (by mass) in water] was a commercial reagent from Guangzhou Weibo Chemical Company.
The pore structure of the catalysts was characterized by N2adsorption-desorption isotherm measurements. The details of the chemical composition and the pore structure parameters of the catalysts were summarized in Table 1.

Table 1 Pore structure characteristics of the investigated catalysts
Hydrophilic room temperature ionic liquids including 1-ethyl-3-methylimidazolium tetrafluoroborate ([emim]BF4), 1-propyl-3-methylimidazolium tetrafluoroborate ([pmim]BF4), 1-butyl-3-methylimidazoliumtetrafluoroborate ([bmim]BF4), and hydrophobic 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim]PF6) were synthesized by alkylation of 1-methylimidazole with 1-bromoalkne or chloroalkne followed by substitution of bromide or chloride anion with tetrafluoroborates or hexafluorophosphate, as given in our previous work [27]. All of the ionic liquids were finally dehydrated under vacuum at 80°C for 12 h and were stored in an inert nitrogen atmosphere. The synthesized ionic liquids were characterized with1H-NMR and no impurities were detected [27]. Water mass content of all the ionic liquid was lower than 0.03%, determined by Karl-Fischer analysis.
2.2 Catalytic reaction
Cyclohexane oxidation reaction was performed in a teflon-lined 50 ml stainless-steel autoclave equipped with a magnetic stirrer [27]. Typically, 27.8 mmol cyclohexane, 5 ml (6.25 g) ionic liquid, 0.15 g catalyst, and 55.6 mmol-butyl-hydroperoxide (TBHP, 85% in H2O) were introduced into the reactor. The autoclave was closed and submerged in a thermostatic oil bath at 90°C. The reaction mixture was vigorously stirred at 1000 r·min-1for 12 h. After the reaction, the autoclave was removed from the oil bath and stood in ambient air for about 2 h to cool down. Then, the autoclave was opened and the upper phase of the reaction mixture was collected by using a separating funnel. An Agilent-6890 GC with a flame ionization detector (FID) and a capillary column (PEG-20M, 30 m×0.25 mm) was used for quantitative analysis of the oxidation products with nitrogen as the carrier gas. The conversion was calculated based on the starting cyclohexane. Cyclohexyl hydroperoxide (CHHP) content was determined by decomposition with PPh3followed by quantification of the formed cyclohexanol by gas chromatography (GC) [12, 15, 28].
To prepare a solution of anhydrous TBHP in cyclohexane as an oxidant, TBHP (85% in H2O) was mixed with cyclohexane, then anhydrous MgSO4was introduced into the solution and magnetically stirred for 3 h. The organic phase was separated and the concentration of TBHP in cyclohexane was determined by titration according to Ref. [20].
The amount of TBHP consumed, denoted as TBHP cons., was determined by iodometric titration of the unreacted part. The selectivities with respect to THBP were calculated based on the stoichiometry that 1 mol of TBHP is needed to produce 1 mol of cyclohexanol, 1 mol of CHHP or 0.5 mol of cyclohexanone.
3 RESULTS AND DISCUSSION
3.1 Catalytic activity of ZSM-5 with different Si/Al ratio
The oxidation of cyclohexane was carried out at 90°C for 12 h over Z1, Z2 and Z3 catalysts in ionic liquid [emim]BF4with TBHP/cyclohexane molar ratio of 2. The monofunctional oxidation products, including cyclohexanone, cyclohexanol and cyclohexyl hydroperoxide, were formed and the results are given in Table 2. The conversion of cyclohexane exhibits a decrease from 15.8% to 10.8% with the increase of Si/Al ratio of the HZSM-5 catalyst, and the same trend on the yields of cyclohexanone, cyclohexanol and CHHP was observed. All the catalysts exhibit good selectivity of monofunctional oxidation products at around 97%. The results obtained here with HZSM-5 catalysts were much higher than those reported in the commercial process. Cyclohexanone was a favored product on the three catalysts. For Z1, the ratio of cyclohexanone to cyclohexanol was approximately 2.59, whereas for Z2 and Z3, cyclohexanone formation was more favored.
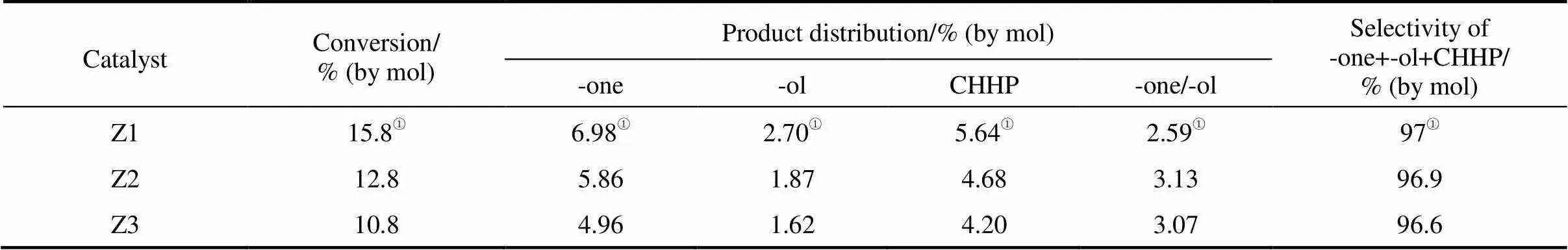
Table 2 The oxidation of cyclohexane over ZSM-5 catalysts with different Si/Al ratios
①Data cited from Ref. [26].
Note: Reaction conditions (0.15 g catalyst, 27.8 mmol cyclohexane, 55.6 mmol TBHP (85% in H2O), 5 ml ionic liquid, 12 h and 90°C); -one, cyclohexanone; -ol, cyclohexanol; CHHP, cyclohexyl hydroperoxide.
3.2 Effect of solvents on the reaction
A comparison of the cyclohexane conversion and the selectivity to monofunctional oxidation products among the reactions in ionic liquids and in molecular organic solvents is given in Table 3. Clearly, higher activities were observed for all catalysts in the ionic liquids than those either in acetone or in the absence of solvent. The activity of catalysts is strongly dependent on the types of the cation of the ionic liquid, and the order of reactivity has been found to be [emim]+>[pmim]+>[bmim]+. This phenomenon is analogous to the oxidation of pyrimidine thioether in ionic liquids reported by Hardacre. [29], where the activity decreases with the increase of the alkyl chain length. In all of the ionic liquids, the selectivity for monofunctional oxidation products remained higher than 96%, even for the conversion higher than 15% for all of the catalysts. However, for the reaction in acetone, the selectivity was sharply decreased and fell in the range of 70.2%-74.7%.
Compared to conventional solvents, enhanced reaction rates and improved yields were obtained in ionic liquids. The reaction in [emim]BF4afforded 15.3% yield of monofunctional oxidation products, whereas the same reaction in acetone gave a yield of 2.46%.
In contrast to the reaction in water-miscible ionic liquids,.. [bmim]BF4, cyclohexane oxidation in water-immiscible ionic liquids,. [bmim]PF6, showed lower activity, indicating that the anion has an effect on the activity of the catalysts. Although cyclohexane oxidation was found to proceed for all the catalysts in [bmim]PF6, the lower ionic liquid phase became less viscous after reaction than the fresh [bmim]PF6ionic liquid, and a little amount of white solid was observed on the wall of the reactor. This phenomenon was not observed in the reaction with the other ionic liquids and implies the formation of HF during the reaction through the hydrolysis of the anion [30].
The cation effect observed with [emim]BF4, [pmim]BF4and [bmim]BF4may be associated with the increasing solvent viscosity as the alkyl chain length increases. The effect of viscosity of ionic liquids on the activity of oxidation reaction has been observed previously in the oxidation of pyrimidine thioether with a mesoporous catalyst [31]. It is also likely that the decrease of activity with the cation size is associated with the reduction of the accessibility of the catalyst pores [29, 31].
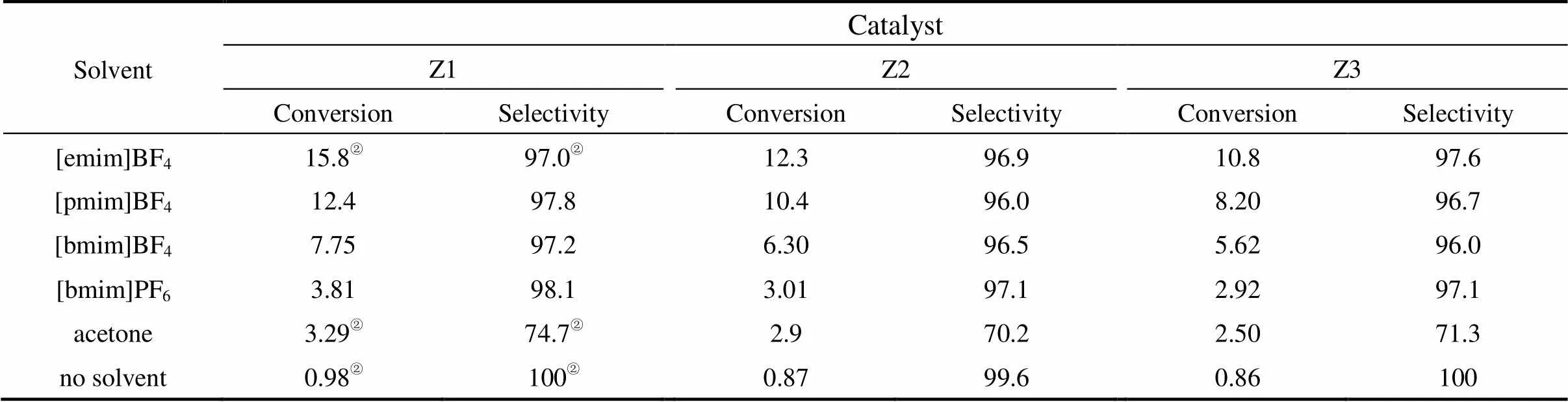
Table 3 Conversion and selectivity to monofunctional oxidation products as a function of solvent and catalyst used after 12 h reaction at 90°C①
① Monofunctional oxidation products are cyclohexanone, cyclohexanol and cyclohexyl hydroperoxide; reaction conditions: 0.15 g catalyst, 27.8 mmol cyclohexane, 55.6 mmol TBHP (85% in H2O), 5 ml solvent, 12h and 90°C.
② Data cited from Ref. [26].
3.3 Effect of water on the reaction
In order to investigate whether the water has an influence on the catalytic activity, another series of experiments on cyclohexane oxidation were performedwith anhydrous TBHP, a solution in cyclohexane, using Z1 catalyst in ionic liquids [emim]BF4, [pmim]BF4and [bmim]BF4under the same experimental conditions. The results are presented in Table 4. It can be seen that a same trend of decreasing activity with the increase of the alkyl chain length of ionic liquid was observed. Compared with the reaction using aqueous TBHP, the cyclohexane oxidation using anhydrous TBHP showed a much lower yield of monofunctional oxidation products in all of the ionic liquids used. These results indicate that little amount of water played an important role on the higher reactivity of cyclohexane. This phenomenon might be explained that the presence of water facilitates the transfer of hydrophilic TBHP to the ionic liquid phase containing HZSM-5, thus increasing the accessibility of oxidant to molecular sieve and improving consequently the catalytic activity.
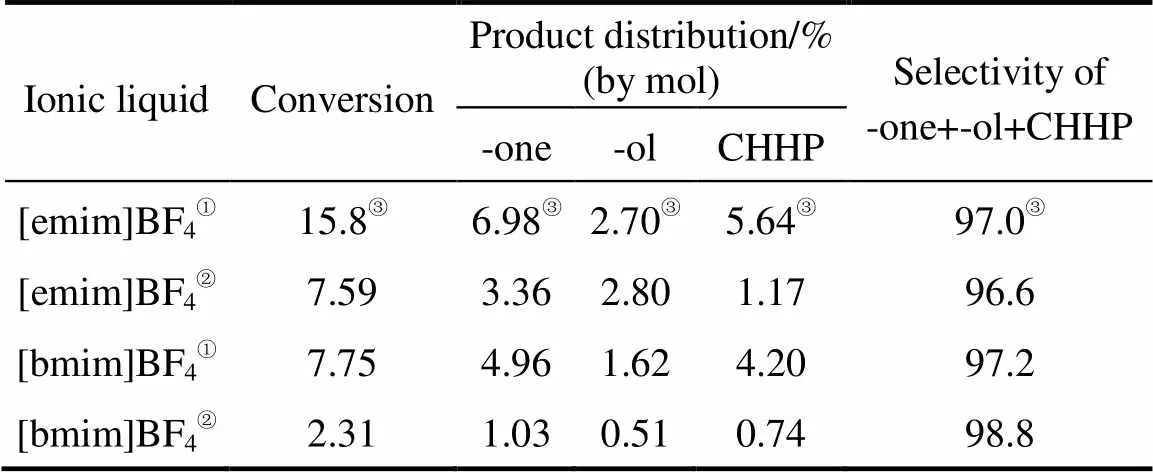
Table 4 Comparisons of yields of monofunctional oxidation products on cyclohexane oxidation with aqueous TBHP and anhydrous TBHP as oxidant using Z1 catalyst
① Reaction with aqueous TBHP (85% in H2O).
② Reaction with anhydrous TBHP.
③ Data cited from Ref. [26].
Note: Reaction conditions (0.15 g catalyst, 27.8 mmol cyclohexane, 55.6 mmol TBHP, 5 ml ionic liquid, 12 h and 90°C).
3.4 Conversion and selectivity of TBHP
The conversion and selectivity of TBHP were also examined in cyclohexane oxidation using ZSM-5 catalysts with different Si/Al ratios both in ionic and molecular media. For all the solvents used, the TBHP conversion was high while the efficiency of the oxidant was very low as shown in Table 5. The initial amount of hydroperoxide was one time more than the stoiciometric need, however, about 80%-97% of the oxidant was unselectively decomposed after 12 h. The non-productive conversion of the hydroperoxide may be the reason for the cyclohexane oxidation process was limited to a yield below 15% [32]. The Si/Al ratio of the catalyst influenced the extent of the peroxide decomposition, as shown in Table 5. This might be explained by the different hydrophilicity of the zeolite. The zeolite with high aluminium content,.. low Si/Al ratio, is more hydrophilic. The results reported here are consistent with the Ref. [33]. For both molecular and ionic solvents, the catalyst with higher Si/Al ratios showed a lower TBHP selectivity.
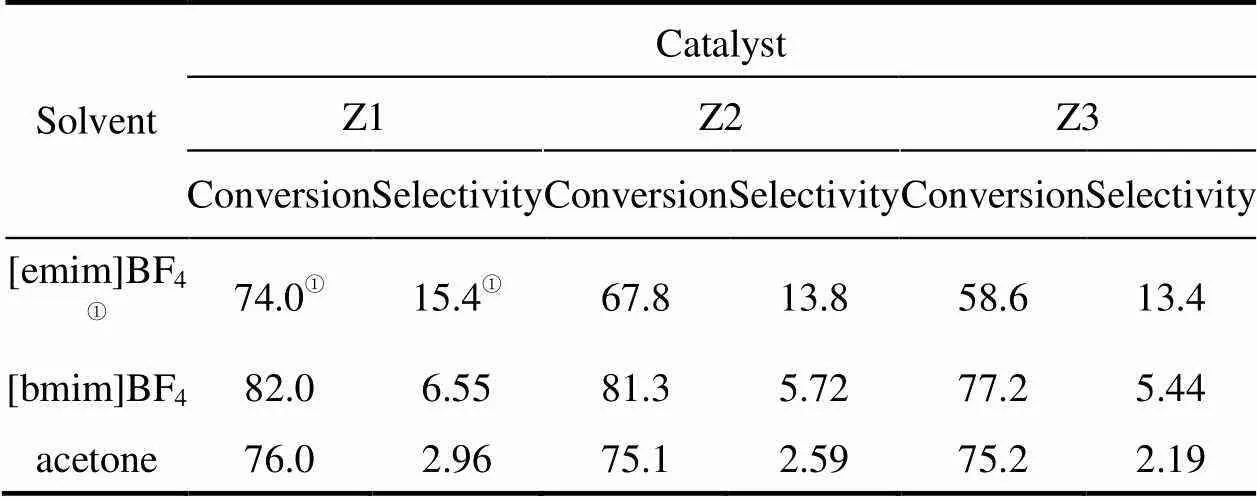
Table 5 TBHP conversion and selectivity as a function of solvent and catalyst after 12 h reaction
① Data cited from Ref. [26].
Note: Reaction conditions (0.15 g catalyst, 27.8 mmol cyclohexane, 55.6 mmol TBHP (85% in H2O), 5 ml ionic liquid or acetone, 12 h and 90°C).
3.5 Preliminary study of mechanism of cyclohexane oxidation catalyzed by HZSM-5 in ionic liquids
Schuchardt. [34] proposed a mechanism for the oxidation reaction. TBHP decomposes with the presence of zeolite, generating-butoxy radicals that abstract a hydrogen atom from cyclohexane forming a cyclohexyl radical, which is the initiating step. The cyclohexyl radical then reacts rapidly with molecular oxygen in air, generating the cyclohexylperoxyl radical which can undergo two different pathways. (1) Reaction with another cyclohexylperoxyl radical to form molecular oxygen and the non radical products, cyclohexnol and cyclohexanone, and (2) reaction with a cyclohexane molecule (substrate) abstracting a hydrogen atom to form cyclohexylhydroperoxide (CHHP) and regenerating its precursor, the cyclohexyl radical [28, 33, 35]. The intermediate CHHP is further decomposed on HZSM-5 to produce cyclohexanol and cyclohexanone [36]. The abstraction of hydrogen from cyclohexane by cyclohexylperoxy accounts for the formation of 40% CHHP in the overall oxidation products.
The ratio of cyclohexanone to cyclohexanol formed in the reaction with HZSM-5 catalysts in [emim]BF4was around 3.0 (Table 2), which indicates higher cyclohexnone selectivity. It seems likely that the catalyst promote the reaction from cyclohexanol to cyclohexanone. This hypothesis was then tested in the experiments using cyclohexanol as a reactant. The results are shown in Table 6. Cyclohexanol is more active than cyclohexane under the reaction conditions and can be converted to cyclohexanone easily.
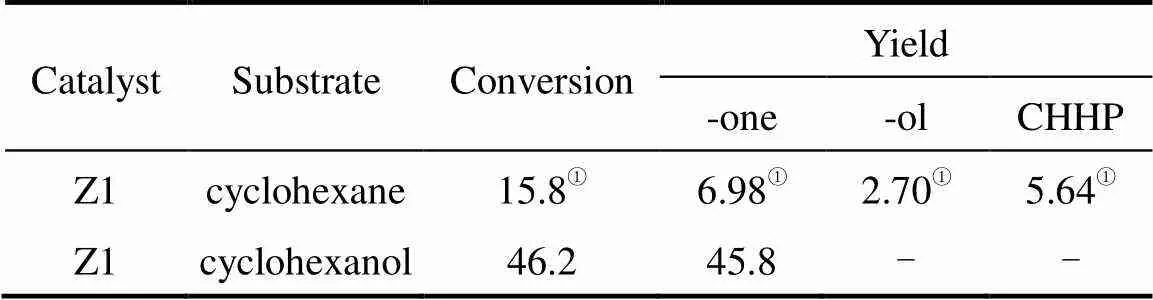
Table 6 Comparison of cyclohexane and cyclohexanol oxidation in [emim]BF4
① Data cited from Ref. [26].
Note: Reaction conditions (0.15 g catalyst, 27.8 mmol substrate, 55.6 mmol TBHP (85% in H2O), 5 ml ionic liquid, 12 h and 90°C).
4 CONCLUSIONS
The effect of several ionic liquids solvent on the liquid phase oxidation of cyclohexane over ZSM-5 catalysts with different Si/Al ratios was performed in ionic liquids using-butyl-hydroperoxide as oxidant. Among all the ZSM-5 catalysts, Z1 (low Si/Al ratio of 25) exhibited the highest catalytic activity under the experimental conditions in ionic liquids and achieved 15.8% conversion of cyclohexane and 97.0% overall selectivity of cyclohexanone, cyclohexanol and cyclohexyl hydroperoxide. The comparison with typical molecular solvent acetone indicated that much higher activities were obtained in ionic liquids. Different activities were also found among the investigated ionic liquids. Both cation and anion of ionic liquid contributed to the effects of the ionic liquids. The best ionic solvent was found to be [emim]BF4. Some amount of water in-butyl-hydroperoxide was helpful to the oxidation of cyclohexane. The reaction products can be easily isolated from the ionic liquid phase containing catalyst by decantation.
1 Shylesh, S., Samuel, P.P., Singh, A.P., “Chromium-containing small pore mesoporous silicas: Synthesis, characterization and catalytic behavior in the liquid phase oxidation of cyclohexane”,.., 318, 128-136 (2007).
2 Pillai, U.R., Sahle-Demessie, E., “A highly efficient oxidation of cyclohexane over VPO catalysts using hydrogen peroxide”,.., 2142-2143 (2002).
3 Guo, C.C., Huang, G.., Zhang, X.B., Guo, D.C., “Catalysis of chitosan-supported iron tetraphenylporphyrin for aerobic oxidation of cyclohexane in absence of reductants and solvents”,.., 247, 261-267 (2003).
4 Yuan, H.X., Xia, Q.H., Zhan, H.J., Lu, X.H., Xu, K.X., “Catalytic oxidation of cyclohexane to cyclohexanone and cyclohexanol by oxygen in a solvent-free system over metal-containing ZSM-5 catalysts”,.., 304, 178-184 (2006).
5 Zhang, H.J., Li, Y.D., “Preparation and characterization of Beta/MCM-41composite zeolite with a stepwise-distributed pore structure”,., 183, 73-78 (2008).
6 Liu, H.R., Meng, X.C., Zhao, D.S., Li, Y.D., “The effect of sulfur compound on the hydrogenation of tetralin over a Pd-Pt/HDAY catalyst”,..., 140, 424-431 (2008).
7 Liu, Y.M., Yang, H.Q., Jin, F., Zhang, Y., Li, Y.D., “Synthesis of pyridine and picolines over Co-modified HZSM-5 catalyst”,..., 136, 282-287 (2007).
8 Zhang, H.J., Meng, X.C., Li, Y.D., Lin, Y.S., “MCM-41 overgrown on Y composite zeolite as support of Pd-Pt catalyst for hydrogenation of polyaromatic compounds”,...., 46, 4186-4192 (2007)
9 Spinacé, E.V., Pastore, H.O., Schuchardt, U., “Cyclohexane oxidation catalyzed by titanium silicalite (TS-1): Overoxidation and comparison with other oxidation systems”,.., 157, 631-635 (1995).
10 Guo, C.C., Chu, M.F., Liu, Q., Liu, Y., Guo, D.C., Liu, X.Q., “Effective catalysis of simple metalloporphyrins for cyclohexane oxidation with air in the absence of additives and solvents”,.., 246, 303-309 (2003).
11 Morvillo, A., Romanelio, G., “Ruthenium-catalyzed oxygenation of saturated hydrocarbons by-butylhydroperoxide”,..., 77, 283-288 (1992).
12 Schuchardt, U., Pereira, R., Rufo, M., “Iron (III) and copper (II) catalysed cyclohexane oxidation by molecular oxygen in the presence of tert-butyl hydroperoxide”,..., 135, 257-262 (1998).
13 Bellifa, A., Lahcene, D., Tchenar, Y.N., Choukchou-Braham, A., Bachir, R., Bedrane, S., Kappenstein, C., “Preparation and characterization of 20 wt.% V2O5–TiO2catalyst oxidation of cyclohexane”,.., 305, 1-6 (2006).
14 Simoões, M.M.Q., Conceicão, C.M.M., Gamelas, J.A.F., Domingues, P.M.D.N., Cavaleiro, A.M.V.J., Cavaleiro, A.S., Ferrer-Correia, A.J.V., Johnbstone, R.A.W., “Keggin-type polyoxotungstates as catalysts in the oxidation of cyclohexane by dilute aqueous hydrogen peroxide”,..., 144, 461-468 (1999).
15 Tian, P., Liu, Z.M., Wu, Z.B., Xu, L., He, Y.L., “Characterization of metal-containing molecular sieves and their catalytic properties in the selective oxidation of cyclohexane”,., 93-95, 735-742 (2004).
16 Pires, E.L., Arnold, U., Schuchardt, U., “Amorphous silicates containing cerium: Selective catalysts for the oxidation of cyclohexane”,..., 169, 157-161 (2001).
17 Sökmen, L., Sevin, F., “Oxidation of cyclohexane catalyzed by metal-ion-exchanged zeolites”,..., 264, 208-211 (2003).
18 Sooknoi, T., Limtrakul, J., “Activity enhancement by acetic acid in cyclohexane oxidation using Ti-containing zeolite catalyst”,..., 233, 227-237 (2002).
19 Zhao, R., Ji, D., Lv, G. M., Qian, G., Yan, L., Wang, X. L., Suo, J. S., “A highly efficient oxidation of cyclohexane over Au/ZSM-5 molecular sieve catalyst with oxygen as oxidant”,.., 904-905 (2004).
20 Johnson, R.M., Siddiqi, I.W., The Determination of Organic Peroxides, Pergamon Press, Oxford (1970).
21 Law, M.C., Wong, K.Y., Chan, T.H., “Solvent-free route to ionic liquid precursors using a water-moderated microwave process”,.., 4, 328-330 (2002).
22 Yang, X., Fei, Z.F., Zhao, D.B., Ang, W.H., Li, Y.D., Dyson, P.J., “Palladium nanoparticles stabilized by an ionic polymer and ionic liquid: A versatile system for C-C cross coupling reactions”,.., 47, 3292-3297 (2008).
23 Zhao, H., “Innovative applications of ionic liquids as 'Green' engineering liquids”,..., 193, 1660-1677 (2006).
24 Chhikara, B.S., Chandra, R., Tandon, V., “Oxidation of alcohols with hydrogen peroxide catalyzed by a new imidazolium ion based phosphotungstate complex in ionic liquid”,.., 230, 436-439 (2005).
25 Cimpeanu, V., Pârvulescu, V., Pârvulescu, V. I., Thompson, J.M., Hardacre, C., “Thioethers oxidation on dispersed Ta-silica mesoporous catalysts in ionic liquids”,., 117, 126-132 (2006).
26 Wang, J.Y., Zhao, F.Y., Liu, R.J., Hu, Y.Q., “Oxidation of cyclohexane catalyzed by metal-containing ZSM-5 in ionic liquid”,..., 279, 153-158 (2007).
27 Wang, J.Y., Zhao, F.Y., Liu, Y.M., Hu, Y.Q., “Study on surface tension of a series of-alkyl--methyimidazolium room temperature ionic liquids”,.., 65, 1443-1448 (2007).
28 Pires, E.L., Arnold, U., Schuchardt, U., “Amorphous silicates containing cerium: selective catalysts for the oxidation of cyclohexane”,..., 169, 157-161 (2001).
29 Hardacre, C., Katdare, S. P., Milroy, D., Nancarrow, P., Rooney, D.W., Thompson, J. M., “A catalytic and mechanistic study of the Friedel-Crafts benzoylation of anisole using zeolites in ionic liquids”,.., 227, 44-52 (2004).
30 Swatloski, R.P., Holbrey, J.D., Rogers, R.D., “Ionic liquids are not always green: Hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate”,.., 5, 361-363 (2003).
31 Cimpeanu, V., Pârulescu, A.N., Pârulescu, V. I., On, D.T., Kaliaguine, S., Thompson, J. M., Hardacre, C., “Liquid-phase oxidation of a pyrimidine thioether on Ti-SBA-15 and UL-TS-1 catalysts in ionic liquids”,.., 232, 60-67 (2005).
32 Luts, T., Frank, R., Suprun, W., Fritzsche, S., Hey-Hawkins, E., Papp, H., “Epoxidation of olefins catalyzed by novel Mn(III) and Mo(IV)-Salen complexes immobilized on mesoporous silica gel (2) Study of the catalytic epoxidation of olefins”,..., 273, 250-258 (2007).
33 Pires, E.L., Wallau, M., Schuchardt, U., “Cyclohexane oxidation over rare earth exchanged zeolite Y”,..., 136, 69-74 (1998).
34 Schuchardt, U., Cardoso, D., Sercheli, R., Pereira, R., da Cruz, R.S., Guerreiro, M.C., Mandelli, D., Spinacé, E.V., Pires, E.L., “Cyclohexane oxidation continues to be a challenge”,.., 211, 1-17 (2001).
35 Pires, E.L., Magalhães, J.C., Schuchardt, U., “Effects of oxidant and solvent on the liquid-phase cyclohexane oxidation catalyzed by Ce-exchanged zeolite Y”,.., 203, 231-237 (2000).
36 Sun, Z.Q., Xu, J., Du, Z.T., Zhang, W., “Decomposition of cyclohexyl hydroperoxide over transition metal-free zeolite H-beta”,.., 323, 119-126 (2007).
2008-10-20,
2009-02-27.
the National Natural Science Foundation of China (20776037, 20425619), the Program for Changjiang Scholars and Innovative Research Teams in Universities (IRT0641), and the Research Foundation of Hebei University of Science and Technology (XL200716).
** To whom correspondence should be addressed. E-mail: yongqi_h@yahoo.com.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- On-line Monitoring for Phosphorus Removal Process and Bacterial Community in Sequencing Batch Reactor*
- Mechanism Study of Rice Straw Pyrolysis by Fourier Transform Infrared Technique*
- Simultaneously Designing and Targeting for Networks with Multiple Resources of Different Qualities*
- Modeling and Control of Nonlinear Discrete-time Systems Based on Compound Neural Networks*
- Effect of Doping Cerium in the Support of Catalyst Pd-Co/Cu-Co-Mn Mixed Oxides on the Oxidative Carbonylation of Phenol
- Biodegradation of Aniline by a Newly Isolated Delftia sp. XYJ6*
