Overbased Calcium Sulfonate Detergent Technology Overview
2009-04-30MAQing-gaoMUIRRonaldJ.
MA Qing-gao MUIR Ronald J.
Abstract:Overbased calcium sulfonate is used widely as detergent in automotive and marine lubricants, as well as various industrial oil applications. In this paper, the process to produce overbased calcium sulfonate is overviewed.The sulfonate structure and molecular weight and its molecular weight distribution, the enclosed calcium carbonate nanoparticle size and crystalline structure, properties of the carrier oil, all influence its properties, such as stability, viscosity, and detergency of the system.
Key words:calcium sulfonate; detergent; overbased
中图分类号:TE624.82 文献标识码:A
0 Introduction
Overbased calcium sulfonate is one of the largest commercially produced nanomaterials (>400,000 ton/year globally). In overbased calcium sulfonate, amphiphilic alkylbenzene sulfonate self-assemble to form core-shell structured inverse/reverse micelles and trap nanoparticular inorganic calcium carbonate colloids and suspend them in carrier oil, forming the “overbased” alkaline reserve.The inverse micelles have a size ranging from 10 to 50 nm for amorphous type core and 50 nm to 1000 nm for crystalline type core.The sulfonate polar head is attracted to polar surfaces or polar species, while the non-polar alkyl chain tail with an appropriate number of carbons to ensure good oil solubility and ability to form high total base number (TBN) sulfonates prefers non-polar species[1], thus the sulfonate functions very similarly to well known laundry detergents, and is also commonly referred as “soap”.
Overbased calcium sulfonates are widely used in the automotive industry and play an important role among the commonly used detergent-dispersant systems[2]. During the course of internal combustion engine operation, residual sulfur and nitrogen from the fuel and lubricant oil are oxidized and converted to H2SO4, and HNO3acidic species[3]. These acidic products corrode engine parts and catalyze the formation of sludge, thereby reducing lubricity and accelerating wear of moving parts in contact with the lubricating oil.Overbased calcium sulfonate can neutralize the acid formed in the engine before they reach concentrations sufficient to cause corrosion or to catalyze the sludge reaction. Overbased calcium sulfonates can be used to prevent wear occurring during friction phenomenon by forming a boundary film on friction surfaces[4]. The sulfonates also tend to form a protective layer on the metal surface to control rust, and corrosion, thus it is used widely as corrosion inhibitor[5].
The nature of the sulfonates, either petroleum sulfonates or synthetic sulfonates, whether the sulfonate is linear or branched with varying chain length and molecular weight distribution are important factors impacting the preparation, process and performance of overbased sulfonates.
One drawback for overbased calcium sulfonate use in automotive and heavy duty engine oils is that it generates sulfated ash after combustion. The sulfated ash is undesired because it may cause deposits in the after-treatment system and also be emitted to the environment as particulates.The market has a demand for ashless detergents, but no robust technology has been identified so far.
In this paper, we will discuss the process to make overbased calcium sulfonates, their application and performance.
1 Overbased Calcium Sulfonate Process
The process to make overbased calcium sulfonates (Figure 1) requires firstly alkylate selection, followed by sulfonation of the alkylates to make alkylbenzene sulfonic acid, then neutralization of the sulfonic acid with calcium hydroxide and subsequent overbased with CO2.

High quality alkylate is key to manufacture a good overbased calcium sulfonate product, while low quality alkylate may cause difficulties at various stages of the process and even affect the performance of the final product. High quality means that the alkylate has a certain molecular weight range and distribution and that they can be readily sulfonated in good yield to provide low H2SO4containing sulfonic acid.
The sulfonates can be classified as “natural” or “synthetic” sulfonates.If the alkylate source is from a suitable fraction of refined petroleum oil, it is known as natural sulfonates, also called petroleum sulfonates or mahogany soaps[6]. Natural sulfonates have very complex structures and a broad molecular weight distribution as seen from the mass spectra of the sulfonic acids (Figure 2).Natural sulfonates are a by-product of white oil refining[7], however white oil production processes now involve hydrogenation and acid treating of mineral oils is increasingly uneconomical and thus natural sulfonate production has declined substantially resulting in a significant shortage of natural sulfonates.Natural sulfonates′ market share is being replaced more and more by synthetic sulfonates.However, due to some of the unique properties of natural sulfonates, such as robustness of the formulated system based on natural sulfonate, it is still been preferred over synthetic sulfonates in some applications.
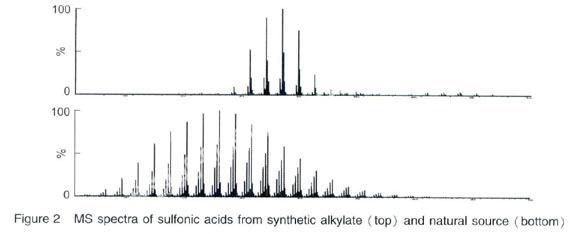
Synthetic alkylate used for overbased calcium sulfonate is different from typical laundry sulfonate detergent in that it has a higher molecular weight and is more oil soluble.For example, linear alkylbenzene (LAB) is the most common raw material in the manufacture of biodegradable household detergents, a typical synthetic alkylate with a C12chain alkylbenzene for laundry sulfonates detergent has a molecular weight of 246, the resulting sulfonate anion from it has a molecular weight of 325; a synthetic alkylate with a C16~C28 chain alkylbenzene for overbased calcium sulfonates has a molecular weight 302~470, the resulting sulfonate anions from them have a molecular weight of 382~586.The preferred molecular weight of the sulfonic acid portion used for automobile lubricant is from 350 to 600.
The synthetic alkylate used for overbased calcium sulfonates are typically linear alkylbenzene from alkylation of benzene, with a C14and above olefin (typically ethylene oligomers, ethylene-propylene oligomers, propylene oligomers, and isobutylene oligomers) to achieve sufficient oil solubility; or from the heavy bottom of alkylation of benzene, using the olefin from PACOL (paraffin conversion to olefin) process, the heavy bottoms are mostly dialkylbenzenes (DAB).Prefractionated n-paraffins with limited to four carbon numbers of n-paraffin due to fractionation limit, typically either C10to C13paraffin or C11to C14n-paraffin from straight run kerosene is a raw material for PACOL process. It should also be pointed out that during the PACOL process some unwanted diolefins are normally produced and presence of diolefins within the alkylation reaction media usually led to formation of undesirable diphenylalkane (DPA) and consequently, reduces the quality of the final alkylate product.
Blends of petroleum sulfonates and synthetic sulfonates are also often used for overbased calcium sulfonates. Depending on the natural sulfonate content in the natural/synthetic blends, such a system could mimic the properties of natural sulfonates by providing a broad molecular weight distribution thus extending the usage of petroleum sulfonates.
The common reagents used to sulfonate alkylates include sulfur trioxide, fuming sulfuric acid (Oleum), and chlorosulfonic acid[8]. In general, the alkylate is dissolved in a hydrocarbon solvent, such as hexane or heptane, and reacted with the sulfonating agent.Different sulfonation processes may affect the quality of the finished sulfonic acid.Depending on the source, sulfonation yield from crude oil to make natural sulfonic acids could be 5%~25%[6], while sulfonation yield from synthetic source is usually more than 85%.There is typically some residual sulfuric acid solubilized in the alkylbenzene sulfonic acid and if carried through the overbasing process ends up as calcium sulfate inorganic salt in the final overbased calcium sulfonate.
Not all alkylates can be sulfonated.For alkylbenzene, multi-alkylated benzenes, such as 1,3,5-trialkylbenzenes could not be sulfonated; alkyl chains with high degree of branching in 1,4-alkylbenzenes could not be sulfonated either; these are due to steric hindrance effect on the sulfonatable positions.Multi-sulfonation for regular alkylbenzene is not observed, since after the first sulfonate group is attached to the benzene ring, the electron density on the ring is significantly reduced preventing the second sulfonation from occurring.Di-sulfonation during natural sulfonates production is observed, and most of the di-sulfonated species are precipitated as sludge due to its high polarity and poorer oil solubility.
Overbased calcium sulfonate can be prepared by a one-step process or a two-step process from alkylbenzene sulfonic acid.In the two-step process, the alkylbenzene sulfonic acid is neutralized with excess amount of calcium oxide or calcium hydroxide base first, which is then subsequently overbased by bubbling CO2. In the one-step process, excess amount of base is charged to the reactor, and once the neutralization is complete, CO2is bubbled into the reaction, thereby incorporating an excess of calcium carbonate into the calcium sulfonate, the so-called “overbasing”.The completion of the reaction could be monitored through the stop of CO2uptake and drop of reaction temperature. The reaction mixture is then filtered to remove excess amount of solid base and other particulate materials. Finished overbased calcium sulfonate is obtained through stripping of the solvent.
The contact between the sulfonic acid and the inorganic base is poor, the higher the molecular weight of the alkylate chain in the sulfonic acid, the poorer the contact between the sulfonic acid and the base, thus slower and poorer reactivity. A “promoter” is generally used to facilitate neutralization and overbasing. Promoters are surfactants, typically low molecular weight carboxylic acids, such as acetic acid, propionic acid, etc.; low molecular weight alkyl phenols; alcohols, such as methanol, ethanol, iso-propanol, etc.The promoters function as phase transfer agents to increase the contact of the sulfonic acid and the base in the water phase.Depending on the molecular weight, molecular weight distribution of the alkylate used for the overbased calcium sulfonates, one needs to identify experimentally a suitable promoter for neutralization and overbasing process[9].
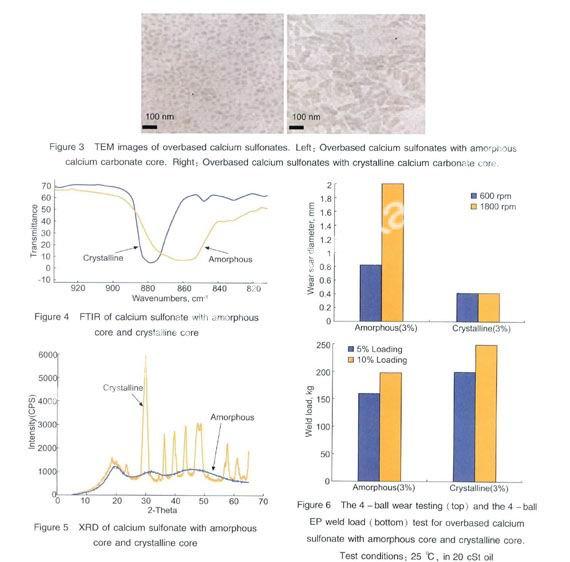
It is common practice to stop bubbling CO2at a point corresponding to 50%~80% carbonation of available lime during the synthesis of overbased calcium sulfonates.The reason is that an excess amount of CO2and longer exposure time to high temperature and polar solvent, can convert the amorphous calcium carbonate core in the inverse micelle into crystalline calcite structure. As shown in the TEM, the amorphous core in overbased calcium sulfonates are quite uniform, and the sizes are from 10~50 nm, while the crystalline core are small crystals of calcite (Figure 3). The difference between amorphous and crystalline calcium carbonate core in overbased calcium sulfonate is also very obvious from IR, crystalline calcite has a sharp transmittance peak at 881 cm-1, while the amorphous has a broad transmittance peak at 860 cm-1 (Figure 4). XRD also clearly points out the difference between amorphous and crystalline carbonate core (Figure 5). An inverse micelle with a crystalline core is larger, less stable, and prone to gel upon storage. Although this is not desirable for auto engine oil, “gelled” or crystalline calcium carbonate in overbased calcium sulfonate has excellent EP and antiwear properties (Figure 6), and this gelling tendency can be controlled by the addition of viscosity drift control agents[10].
The total alkaline reserve determined by method ASTM D2896 for overbased sulfonates are derived from three chemical sources: a) the most significant alkalinity is derived from the calcium carbonate nanoparticle encapsulated in the inverse micelle; b) some are from promoters used, such as acetic acid if it is being used; c) and a small amount is from residue calcium hydroxide left over from incomplete carbonation, also known as “free-alkalinity”.Typical commercial overbased sulfonates and neutral calcium sulfonates have a free alkalinity from 5 to 30 mg KOH/g, as measured by dilute acid titration with phenolphthalein indicator.Since calcium hydroxide is more basic, and more reactive than calcium carbonate, overbased calcium sulfonates with high free alkalinity react with acid, esters, phenols, etc faster than sulfonates with low free alkalinity, and sometime may cause compatibility issues for final oil formulation.
The detergency of the sulfonates depend more on the sulfonate content, the structure of the sulfonate, and not with the TBN of the sulfonates. LAB sulfonate from linear olefin alkylates has better detergency in comparison to branched-alkylbenzene sulfonates as shown for regular laundry detergent[11], and it is believed that inverse micelles formed from linear alkylate based sulfonates have better packing, thus better stability and water tolerance than that of DAB. However, depending on the final oil formulation, branched sulfonates may be more effective in certain applications.Generally inverse micelles from LAB are more stable.Depending on the catalyst of the olefin alkylation, for example, AlCl3 vs HF catalyst, sulfonates from the same olefin can have different performance, due to the catalyst activity difference and the resulting structure and structure distribution differences.AlCl3 is a strong Lewis acid catalyst and alkylates made from AlCl3 catalyst and 1-olefin have higher 2-phenyl alkane content, due to the high reactivity of 2-cation, while the HF catalyst is less reactive, and the cation formed on the 2- position on the 1-olefin rearranges to more stable internal cation, and the phenyl group is located at a more internal position in the resulting alkylates. Differences in overbased sulfonate performance can be attributed to structural changes observed as a result of the alkylation catalyst used to make the alkyl benzene substrate.
2 Summary
Overbased calcium sulfonates are finding continued uses in automotive applications, marine applications including in marine cylinder oils[12], industrial applications, such as various machine oil and rust preventatives, and metal working fluids. Overbased calcium sulfonate is also the raw material to prepare calcium sulfonate greases[13], while overbased calcium sulfonates with dispersed calcite find application as automotive and industrial antiwear additives[14].
References:
[1] C C Colyer, W C Gergel. Chemistry and Technology of Lubricants[M]. New York: CH Publishers Inc, 1992: 62-82.
[2] J Galsworthy, S Hammond, D Hone. Oil-Soluble Colloidal Additives[J]. Curr Opin Colloidal Interface Sci, 2000(5): 274-279.
[3] a)K U Ingold. Inhibition of the Autoxidation of Organic Substances in the Liquid Phase[J]. Chemical Rev, 1961, 61: 563-589. b) R E Kornbrekke, P Patrzyk-Semanik, T Kirchner-Jean, et al. Understanding Soot-Mediated Oil Thickening-Part 6:Base Oil Effects[C]. SAE Technical Paper 982665,1998.
[4] a) S Giasson, T Palermo,T Buffeteau, et al. Study of Boundary Film Formation with Overbased Calcium Sulfonate by PM-IRRAS Spectroscopy[J]. Thin Solid Films, 1994, 252: 111-119. b) N Han, L Shui, W Liu, et al. Study of the Lubrication Mechanism of Overbased Ca Sulfonate on Additives Containing S or P[J]. Tribol Lett, 2003,14(4): 269-274. c) A T Riga, H Hong,R E Kornbrekke, et al. Reactions of Overbased Sulfonates and Sulfurized Compounds with Ferric Oxide[J]. Lubric Eng, 1993,49(1): 65-71. d) M J Morizur, O Teysset. Antiwear Actions of Additives in Solid Dispersion[J]. Lubric Sci, 1992,4(4): 277-299.
[5] Z S Erukhimovich, M A Zubareva, V M Shkol′nikov, et al. Sulfonate-Type Corrosion Inhibitor from Petroleum and Synthetic Raw Material[J]. Khimiya i Tekhnologiya Topliv i Masel, 1985(10):10-12.
[6] a) O N Anand, V P Malik, P K Goel. Oil-Soluble Metallic Petroleum Sulfonates[J]. Petroleum & Hydrocarbons, 1972, 7(2): 59-67. b) A Brown, J Knobloch.Composition of Petroleum Distillates as Revealed by their Sulfonates[J]. ASTM Special Tech Publ, 1957,224:213-226,discussion 227-229.
[7] K S Ramayya. Art of Purifying Petroleum Sulphonic Acids Derived from the Treatment of Mineral Oils with Sulphuric Acid[P]. US: 1930488, 1933-10-17.
[8] A Abrams, E J Carlson, E E Gilbert, et al. Sulfonationwith Sulfur Trioxide:Detergent Alkylate in a Scraping-Blade Heat Exchanger[J]. Journal of the American Oil Chemists′ Society, 1960, 37 (2): 63-68.
[9] B Besergil, A Akm, S Celik. Determination of Synthesis Conditions of Medium, High, and Overbased Alkali Calcium Sulfonate[J]. Ind Eng Chem Res, 2007,46(7):1867-1873.
[10] R Muir, L Mathews, T I Eliades. Viscosity Drift Control in Overbased Detergents[P]. US: 6239084, 2001-05-29.
[11] a) K Lee Matheson. Detergency Performance Comparison between LAS and ABS Using Calcium Sulfonate Precipitation Boundary Diagrams[J]. Journal of the American Oil Chemists′ Society, 1985,62(8):1269-1274. b) Cox M F, T P Matson, J L Berna, et al. Worldwide Studies of Mixed Active Laundry Detergency[J]. Journal of the American Oil Chemists′ Society, 1984,61(2): 330-339.
[12] R Muir, L Mathews, T I Eliades. High Viscosity Overbased Sulfonate Detergent and Marine Cylinder Oils Containing Same[P]. US: 6444625, 2002-09-02.
[13] a) R Muir, W Blokhuis. High Performance Calcium Borate Modified Overbased Calcium Sulfonate Complex Greases[P]. US: 4560489, 1985-12-24. b) R Muir, K Mulrooney. One-Step Process for Preparation of Thixotropic Overbased Calcium Sulfonate Complex Thickened Compositions[P]. US: 4824584, 1989-04-25. c) W D Olson, R Muir,T Steib. Sulfonate Greases[P]. US: 5308514, 1994-05-03. d) W D Olson, R Muir, T I Eliades. Sulfonate Grease Improvement[P]. US: 5338467, 1994-08-16. e) W D Olson, R Muir,W Mackwood. Overbased Calcium Salicylate Greases[P]. US: 7407920, 2008-08-05.
[14] R Muir,T I Eliades, K Niece,et al. Oil Soluble Calcite Overbased Detergents and Engine Oils Containing Same[P]. US: 6107259, 2000-08-22.
收稿日期:2008-08-19。
作者简介:MA Qing-gao(1970-), male, Ph.D., joined Chemtura Corporation as research scientist in 2002.He is currently the technical manger in charge of Chemtura Corporation's global sulfonates product lines. He is a member of ACS, AAAS, STLE, SPE, and Sigma XI. He has published more than 30 peer-reviewed papers and has more than 15 patents credited to him.
