废旧聚酯碱性水—甲醇溶液高效解聚回收对苯二甲酸
2024-10-22旋湘桃张辉张鹏飞王毅樊争科候琳付维娟



Efficient depolymerisation of waste polyester in alkaline water-methanol solution forterephthalic acid recovery
摘要:
为了高效解聚废旧聚酯回收对苯二甲酸,本文采用水—溶剂热技术,以碱性水—甲醇溶液高温高压处理废聚酯粒料,再用硫酸析出对苯二甲酸,并优化了碱性水—甲醇溶液解聚聚酯的工艺条件。结果表明,浴比为1︰20时,质量浓度50 g/L的氢氧化钠与100 mL/L的甲醇混合溶液,160 ℃条件下处理聚酯粒料2.5 h,对苯二甲酸产回收率为94.9%;红外光谱、核磁共振谱和高效液相色谱分析表明,所得分离产物的化学性质与对苯二甲酸标准品高度吻合,其纯度为90.53%。标准化视觉和数字色彩评定仪分析表明,所得对苯二甲酸与商业品存在较大色差(16.02),含有少量淡黄色杂质。
关键词:
废旧聚酯;解聚;对苯二甲酸;碱性水—甲醇;氢氧化钠
中图分类号:
TS159; TQ340.68
文献标志码:
A
文章编号: 10017003(2024)10期数0060起始页码09篇页数
DOI: 10.3969/j.issn.1001-7003.2024.10期数.007(篇序)
收稿日期:
20240126;
修回日期:
20240913
基金项目:
国家自然科学基金面上项目(51873169)
作者简介:
旋湘桃(1996),女,硕士研究生,研究方向为纺织材料改性及功能性纺织品。通信作者:张辉,教授,hzhangw532@xpu.edu.cn。
中国是世界上最大的纺织品生产与消费国,据2013年统计,消费后纺织品废料回收利用率仅有15%[1]。至2020年,国家商务部统计中国产生的废旧纺织品已超过2 000万t,但回收利用量只有460万t,约20%。虽然可以采取能量、物理和化学方法处理废旧纺织品,但是大部分纺织固体废弃物不是被焚烧、露天倾倒或填埋,就是分解成纳/微颗粒进入到环境中[2]。这种粗放式回收技术不仅会产生二噁英等有害和难降解物质,造成二次污染,而且浪费了大量可回收资源[3]。2022年国家发展改革委与商务部、工业和信息化部联合印发526号文件《关于加快推进废旧纺织品循环利用的实施意见》,提出到2025年初步建立废旧纺织品循环利用体系,废旧纺织品循环利用率达到25%,再生纤维产量达到200万t。
聚酯纤维因其优良的热学、力学和化学稳定性成为纺织及服装工业中应用最广泛的种类[1,4],其巨大的使用量带来的废弃量引发的环境污染问题日益严重,废旧聚酯回收再生迫在眉睫[5]。聚对苯二甲酸乙二醇酯(PET)是最主要的聚酯材料,由对苯二甲酸(TPA)和乙二醇(EG)缩合形成对苯二甲酸乙酯单元后,再通过酯键形成长链。然而,大的苯环结构和强共价键酯键赋予了PET显著的刚度和强度,尤其是聚酯纤维中聚合物链的有序排列方式,使聚酯纤维具有较高的取向度和结晶度,难以在环境中被生物降解[6]。人们大多采取破碎、开松、纺纱、热处理等方式制备再生聚酯[7],或者通过焚烧废旧聚酯将化学能转变成热能[8],或者通过化学反应将废旧聚酯分解成TPA和EG单体[9]。
使用化学法回收废旧聚酯价值比较高,主要有胺解法、氨解法、水解法和醇解法[10]。胺解法反应速率较慢,并伴有较多副反应发生,很难工业化生产;氨解法解聚时间较长且产品提纯步骤繁琐,需要添加催化剂来提高降解速率,普及困难[11];水解法分为酸性、碱性和中性水解法[12],其中酸性水解法在高温高压下能短时间内快速解聚聚酯,但会消耗大量的浓酸,对环境造成污染,且降解产物EG难以回收。使用1.0%的盐酸,在223~232 ℃、2.3~3.0 MPa条件下处理30 min可将聚酯完全水解[13]。碱性水解聚酯工艺较为简单,成本低廉,但碱液会对反应容器造成腐蚀。200 ℃下,在NaOH溶液中处理25 min可将聚酯水解,TPA的收率为92%[14]。中性水解是利用高温高压水蒸气将聚酯解聚成TPA和EG,环境污染小,但反应条件要求高,工程化应用有一定难度。以300 ℃的亚临界水或超临界水处理聚酯30 min,TPA收率大于85%,纯度接近100%[15]。醇解法主要有甲醇醇解法和EG醇解法[16]。其中,甲醇醇解法反应条件比较苛刻,腐蚀性强,产物分离成本高,但解聚速率较快[17]。含有ZnO的甲醇溶液在170 ℃条件下处理聚酯15 min,PET的转化率达97%,得到的对苯二甲酸二甲酯(DMT)产率为95%[18]。EG醇解法工艺条件相对温和,对设备要求较低,但解聚的单体纯度不高,质量不稳定,回收率较低[19]。含有ZnMn2O4的EG溶液,在260 ℃、0.5 MPa条件下可将聚酯解聚得到对苯二甲酸双羟乙酯(BHET),其收率为92.2%[20]。
本着节水节能、高效解聚废旧聚酯,本文采用水—溶剂热技术,使用碱性水—甲醇溶液解聚废聚酯粒料,随后向反应溶液中加入硫酸,使得对苯二甲酸二钠盐酸化,生成TPA析出,并通过称量残渣质量判定聚酯解聚程度。采用单因素分析法对NaOH质量浓度、浴比、反应温度和反应时间等反应条件进行优化,并用红外光谱、核磁共振谱和高效液相色谱测定了TPA的结构及纯度。
1 实 验
1.1 材 料
实验所用废旧聚酯(浙江佳人新材料有限公司),由收集
到的废旧涤纶织物经过破碎、造粒得到黑色颗粒物。实验用化学试剂包括氢氧化钠(NaOH)、甲醇(CH4O)、98%硫酸(H2SO4),均为分析纯。实验用水由UPH-Ⅳ-20T型制水机制得,电阻率小于5 MΩ·cm。
1.2 方 法
按照技术路线(图1),以甲醇为助催化剂,采用单因素分析法依次优化NaOH质量浓度、浴比、反应温度和反应时间,通过称量聚酯解聚的残渣质量来判断聚酯解聚程度。设定初始参考条件为:浴比1︰60,反应温度180 ℃,反应时间3 h。首
先,选用NaOH质量浓度分别为35、50、80、100 g/L的水—甲醇溶液解聚聚酯;其次,选用优化好的NaOH质量浓度,在反应温度180 ℃和反应时间3 h条件下,使用浴比分别为1︰10、1︰20、1︰30、1︰40的水—甲醇溶液解聚聚酯;接着,选用优化好的NaOH质量浓度和浴比,在反应时间3 h条件下,使用反应温度分别为120、140、160 ℃的水—甲醇溶液解聚聚酯;最后,选用优化好的NaOH质量浓度、浴比和反应温度,使用反应时间分别为1.5、2.0、2.5、3.0 h的水—甲醇溶液解聚聚酯。
具体操作如下:将一定质量的NaOH溶解在60 mL甲醇与水混合溶液中(V甲醇︰V水=1︰9),然后按照一定浴比,向混合溶液中加入一定质量的聚酯粒料,将混合物转移至100 mL内衬聚四氟乙烯的反应釜中,将反应釜置于均相反应器中加热到一定温度并恒温反应一段时间。待反应结束后将反应液冷却至室温,过滤得到聚酯残渣物后水洗、干燥和称重,按照下式计算聚酯解聚百分比P[21]和聚酯残余率C。
P/%=m1-m2m1×100(1)
C=1-P(2)
式中:m1为聚酯粒料的初始质量;m2为聚酯粒料的残渣质量。
同时,向滤液中添加一定量的硫酸与对苯二甲酸二钠盐反应,待TPA絮状物完全析出后,过滤得到的TPA用无水乙醇、水洗涤、干燥并称重,按照下式计算TPA的产率D[22]。
D/%=N1N2×100(3)
式中:N1为聚酯解聚析出的TPA摩尔数,N2为理论上聚酯100%解聚析出的TPA摩尔数。
另外,本文尝试采用蒸馏方法回收EG,理论上当温度达到64.7 ℃时,首先蒸馏出甲醇,继续升温至100 ℃,水分蒸发使得硫酸钠结晶析出。但在具体实验中发现,随着温度的升高,甲醇、EG和水分都会蒸发,因而无法完全将EG分离出来,EG分离提纯有待以后解决。
1.3 表 征
采用KBr压片法,用Nicolet 6700型傅里叶变换红外光谱仪分析TPA和EG的分子结构,扫描范围400~4 000 cm-1。用Agilent 1290 Infinity Ⅱ型高效液相色谱仪分析TPA的纯度,色谱柱选用GL Inertsil ODS-3,流动相为0.2%磷酸,流速为1.0 mL/min,紫外检测器检测波长为210 nm。采用德国Bruker 400 MHz核磁共振(NMR)波谱仪测定TPA的质子核磁共振谱氢谱(1H-NMR)和碳谱(13C-NMR),氘代溶剂为氘代二甲基亚砜(DMSO-d6)。用甲醇溶液配制成1 000 μg/mL的色谱级TPA标准品储备液,用甲醇溶液稀释得到质量浓度分别为25、50、100、250、500 μg/mL的TPA标准工作液,绘制出TPA的标准工作曲线。将制备得到的TPA样品配制成100 μg/mL的工作液,测定TPA的纯度。使用标准化视觉和数字色彩评定仪VeriVide DigiEye(Roachelab)测定商业TPA和实验所得TPA的色彩L*、a*、b*值,计算两样品之间的色差。其中,L*指亮度,变化范围为0~100(0为黑色、100为白色),a*代表红绿色(正值为红色、负值为绿色),b*代表黄蓝色(正值为黄色、负值为蓝色)。根据下式计算两种样品之间的色差[23]。
ΔE*ab=[(L*1-L*0)2+(a*1-a*0)2+(b*1-b*0)2]12(4)
式中:下标0表示商业TPA测试数据,下标1表示实验所得TPA测试数据。
2 结果与分析
2.1 聚酯解聚工艺优化分析
研究表明,使用氢氧化钠亲核试剂攻击聚酯中的羰基,会使得酯键断裂生成对苯二甲酸二钠盐和EG,在浓硫酸作用下对苯二甲酸二钠盐转化成为TPA[10];而使用甲醇作为亲核试剂,会导致酯键断裂生成DMT和EG[11-12]。碱性水—甲醇混合体系能够利用水解或醇解两者反应的优势,即碱性条件快速激活酯键,降低反应活化能,甲醇有效捕获解聚产生的羧酸盐,进而生成的DMT继续与甲醇反应,进一步水解生成TPA和甲醇,从而推动反应进行。这种协同效应可以提高聚酯解聚的速率和TPA收率。另外,混合体系提供了更强的亲核攻击性,甲醇参与可以提高聚酯解聚产物的挥发性,有助于产物分离和纯化。图2(a)为浴比1︰60、反应温度180 ℃、反应时间3 h条件下,不同质量浓度的氢氧化钠解聚聚酯的残余率。当氢氧化钠质量浓度为35 g/L时,聚酯残余率为3.7%;氢氧化钠质量浓度增加到50 g/L时,聚酯残余率降至0.2%,进一步增加氢氧化钠质量浓度至80 g/L,聚酯残余率为0.1%;氢氧化钠质量浓度增加至100 g/L时,聚酯残余率反而有所增大,其原因可能是氢氧化钠质量浓度过高,导致其他副反应发生,从而抑制聚酯的解聚[24]。考虑到成本和环保,本文选用50 g/L的氢氧化钠进行后续优化。图2(b)为氢氧化钠50 g/L、反应温度180 ℃、反应时间3 h条件下,不同浴比解聚聚酯的残余率。某种程度上加大水的用量有利于促进聚酯解
聚[25]。当浴比为1︰10时,聚酯残余率为0.47%;浴比增加到1︰20时,聚酯残余率显著减少,降至0.13%,进一步增大浴比1︰30、1︰40时,聚酯残余率分别为0.05%和0.07%。浴比1︰40和1︰30得到的聚酯残渣质量很少,均为0.001 g。较浴比1︰30,浴比1︰40所得聚酯残余率略有所上升的原因可能是用水量增大会在一定程度上抑制聚酯解聚的速率。为节约水资源,本文选用浴比1︰20进行后续优化。图2(c)为氢氧化钠质量浓度50 g/L、浴比1︰20、反应时间3 h条件下,不同反应温度解聚聚酯的残余率。聚酯解聚反应属于吸热过程,升高温度将会增强聚酯解聚反应的内聚能,加大聚酯与溶液的接触效率[26],加速聚酯链段的迁移能力[27],提高聚酯解聚效率和TPA的产率[28]。当反应温度为120 ℃时,聚酯残余率为39.47%;增加反应温度到140 ℃时,聚酯解聚率残余率降至4.5%;继续升高温度至160 ℃,残余率为0.57%。故本文选用160 ℃优化反应时间。图2(d)为氢氧化钠质量浓度50 g/L、浴比1︰20、反应温度160 ℃条件下,不同反应时间解聚聚酯的残余率。当反应时间为1.5 h时,聚酯残余率为5%;延长反应时间至2 h,聚酯残余率降至1.27%;反应时间2.5 h时,聚酯残余率为0.87%;反应时间为3 h时,聚酯残余率变化不大为0.57%。其原因可能是随着反应时间的延长,虽然会发生副反应,但溶液中的碱和甲醇促使聚酯解聚并使对苯二甲酸二钠盐析出[23]。反应时间为2.5、3 h时,聚酯残余率均小于1%,为节约时间选用2.5 h。
综上所述,聚酯粒料碱性水—甲醇溶液解聚的最佳工艺条件为:氢氧化钠质量浓度50 g/L、浴比1︰20、反应温度160 ℃、反应时间2.5 h,聚酯残余率为0.87%。由式(1)计算得到聚酯解聚百分比P为99.13%。聚酯结构单元摩尔质量为192 g/mol,TPA的摩尔质量为166.13 g/mol,理论上将3 g聚酯100%转化为TPA可以得到2.591 6 g(0.015 6 mol)。本实验废聚酯粒料解聚得到2.458 7 g(0.014 8 mol)的TPA,由式(3)计算出TPA产率D为94.9%。
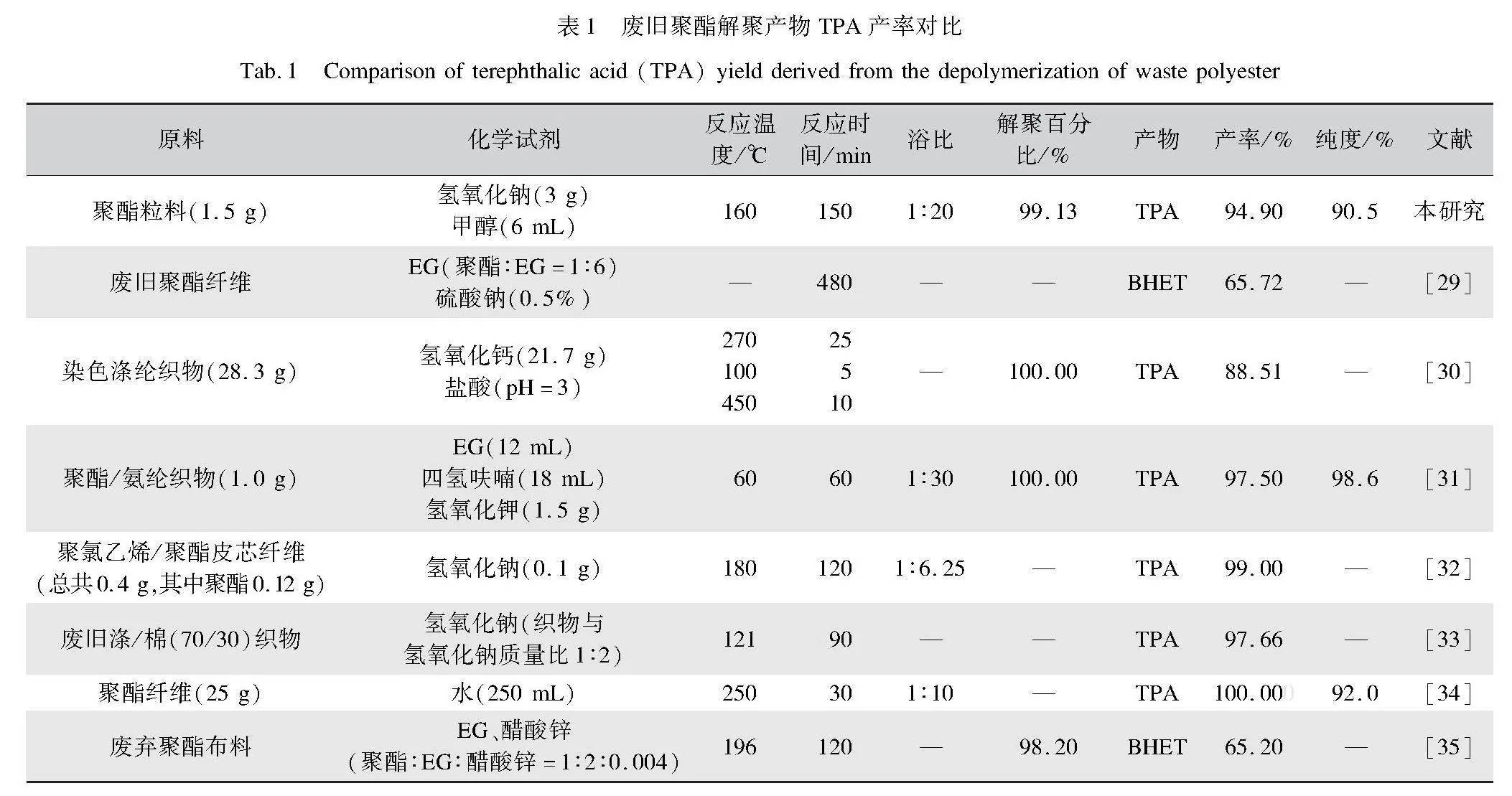
本文将废聚酯粒料解聚回收TPA与文献进行了比较,如表1所示。文献中使用的原料大多为废旧聚酯纤维或涤棉织物,化学试剂多为氢氧化钠、氢氧化锂、氢氧化钙、乙二醇和四氢呋喃等,反应温度范围在60~270 ℃,反应时间在0.42~8 h,对苯二甲酸产率为65~100%。由表1可以看出,本实验用碱性水—甲醇溶液160 ℃处理聚酯粒料2.5 h,TPA产率94.9%,在节能和效率方面具有一定优势。
2.2 对苯二甲酸、乙二醇分子结构分析
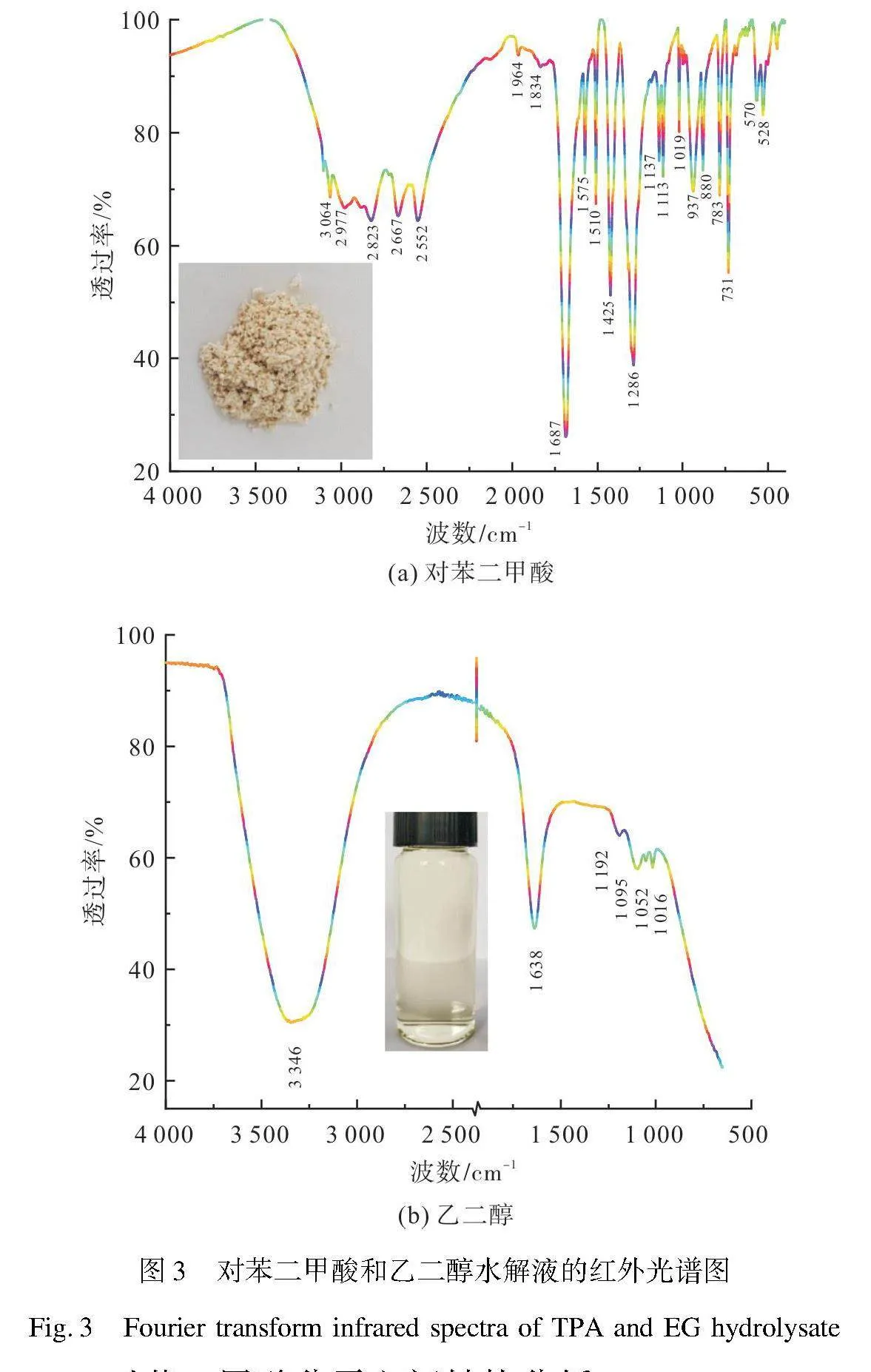
图3分别为解聚产物所得粉末和水解液的傅里叶变换红外光谱图。由图3(a)所得粉末产物与文献[26]中标准品对苯二甲酸红外光谱曲线非常吻合,其中,3 064 cm-1处为聚酯芳香环上的羟基—OH非对称伸缩振动吸收峰;2 667、2 552 cm-1处为芳香环羟基—OH对称伸缩振动吸收峰[36];2 977、2 823 cm-1处对应着C—H对称、不对称伸缩振动吸收峰;1 964、1 834、880 cm-1处为对称p-取代芳香环的特征吸收峰;1 687 cm-1处为碳基CO特征吸收峰;1 575、1 510 cm-1处由苯环CC伸缩振动吸收峰引起[25];1 425、1 286、1 137、1 113、1 019、937 cm-1处多个峰均由C—O酯键吸收峰引起[36-37];783、731、570、528 cm-1处可归属为芳香环C—H伸缩振动吸收
峰[38],表明回收得到粉末应为TPA。由图3(b)可知,3 346 cm-1处宽峰为羟基—OH伸缩振动吸收峰[9],1 638 cm-1处峰由—CH2弯曲振动与C—OH面内弯曲振动吸收引起[39],1 192、1 095、1 016 cm-1处峰由C—OH伸缩振动与CH2面内摇摆振动吸收引起[38],1 052 cm-1处峰为羟基—OH弯曲振动吸收峰[40]。然而,在3 000~2 800 cm-1未检测到乙二醇—CH2伸缩振动吸收峰[39],860 cm-1处也未检测出—CH2面内弯曲振动吸收峰[41],这可能是由于蒸馏溶液中虽然含有EG成分,但含量非常低。
2.3 对苯二甲酸分子空间结构分析
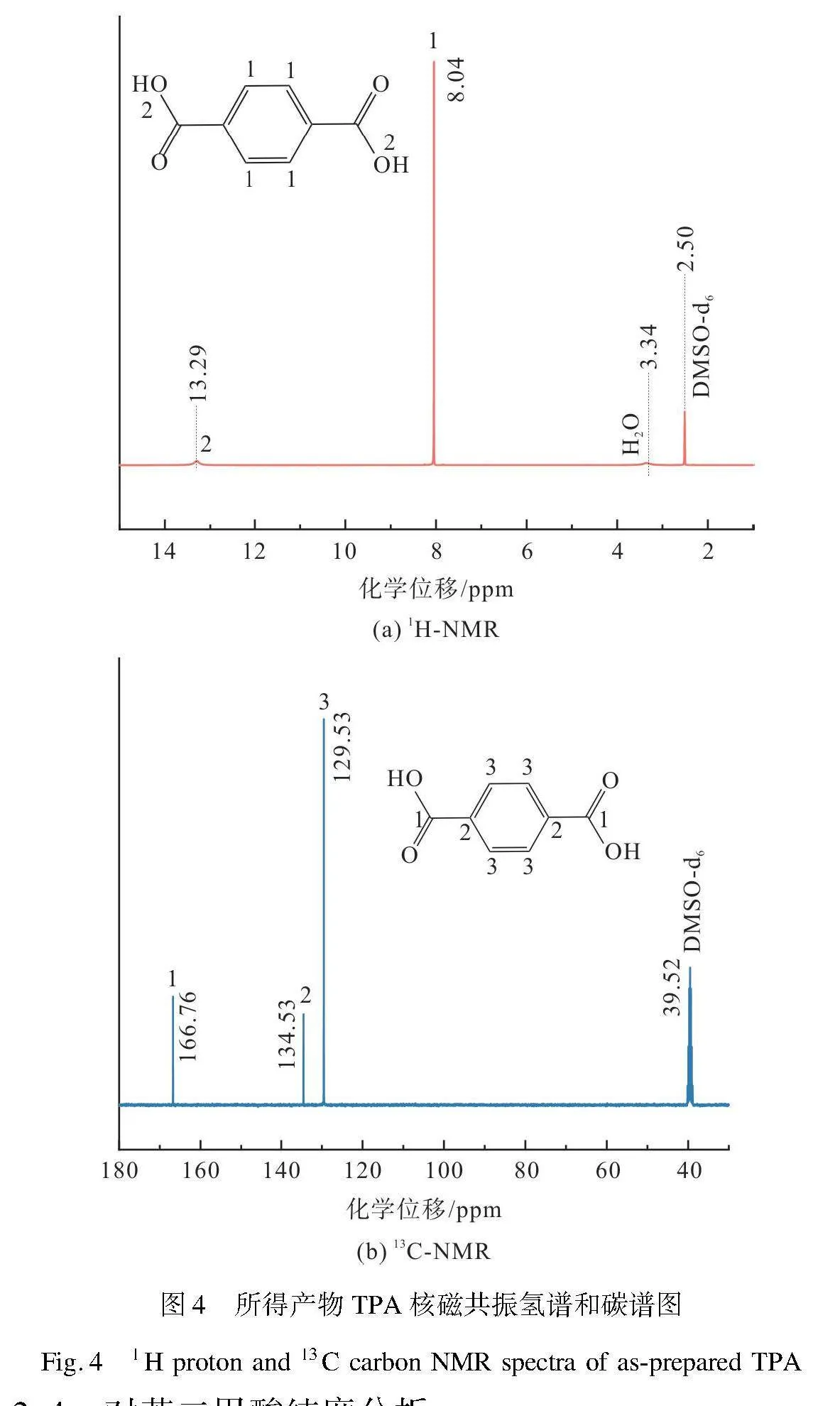
为进一步验证TPA结构,本文采用核磁共振氢谱和碳谱对TPA中的元素H和C空间结构位置进行了分析,结果如图4所示。本实验所得产物对苯二甲酸与文献[25]中标准品TPA的核磁共振氢谱和碳谱基本一致。由图4(a)氢谱可知,化学位移2.50 ppm为氘代溶剂DMSO的峰,相应水的峰对应化学位移3.34 ppm。化学位移8.04、13.29 ppm分别对应着芳香苯环(记为1)和羟基(记为2)的H质子[30]。由图4(b)碳谱可知,化学位移39.52 ppm是氘代溶剂DMSO的峰,化学位移129.53、134.53、166.76 ppm分别对应着芳香环(记为3)、季芳环(记为2)、羰基碳原子(记为1)[25]。结合红外光谱结果可确定,聚酯解聚产物主要成分为TPA,核磁共振谱图中没有其他杂峰,表明解聚产物TPA纯度较高。
2.4 对苯二甲酸纯度分析
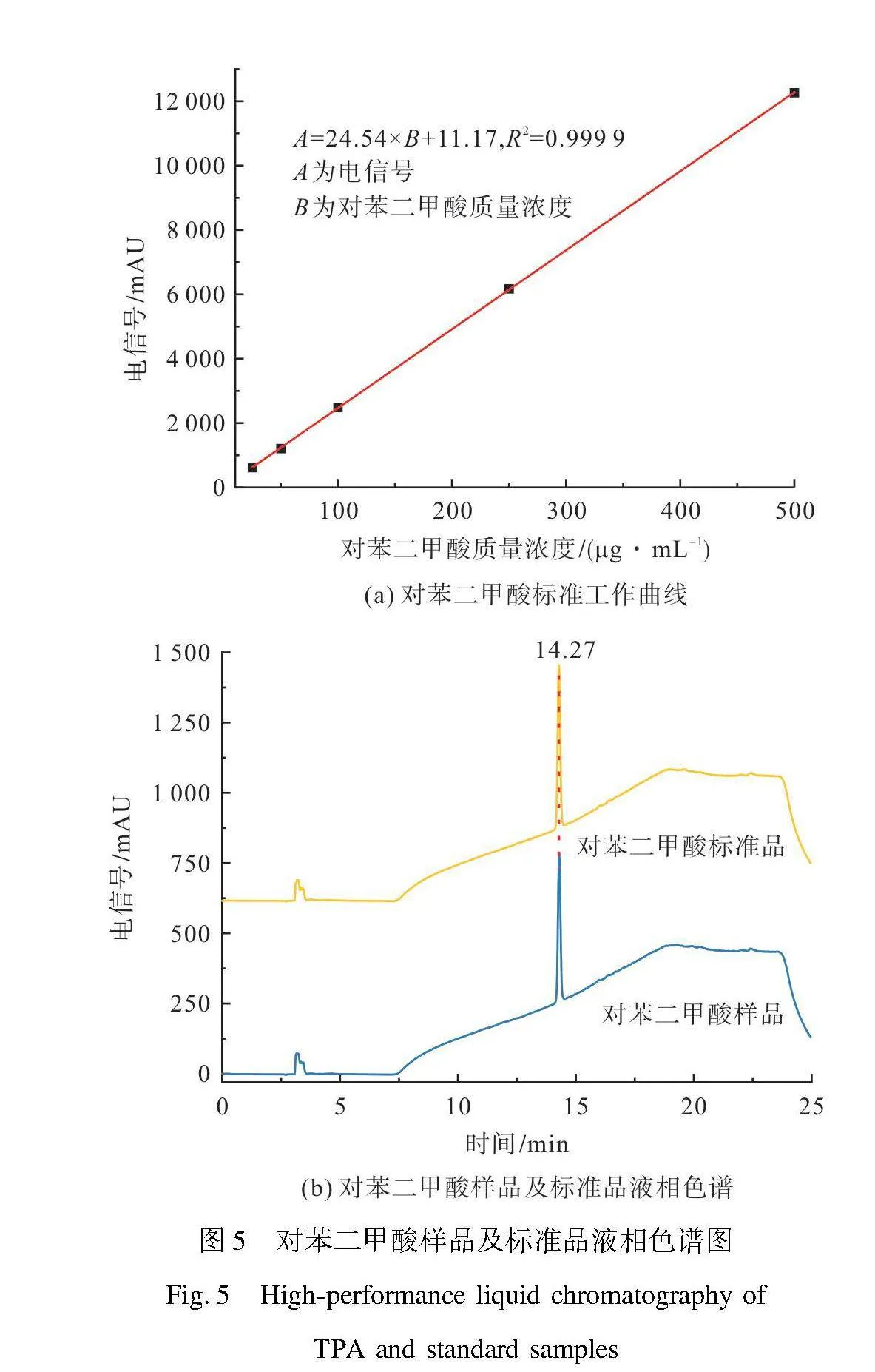
图5为TPA标准品的标准工作曲线和TPA样品及标准品的液相色谱图。由图5(a)TPA标准品标准工作曲线可知,TPA质量浓度电信号与其质量浓度满足方程:A=24.54×B+11.17,R2=0.999 9,当TPA质量浓度在25~500 μg/mL内,具有良好的线性相关。使用质量浓度100 μg/mL的色谱级TPA标准品与TPA样品进行对比分析,由图5(b)液相色谱图可知,TPA样品与标准品的保留时间所对应的峰值一致,在保留时间14.27 min出现TPA的特征峰。TPA样品的峰面积为2 231.75 nriu,标准品的峰面积为2 465.17 nriu,根据峰面积可计算得到TPA的纯度为90.53%,这可能是由于所得产物TPA中含有微量的有机染料、甲醇或者聚酯解聚过程中产生的其他低聚物[42]。
2.5 对苯二甲酸色差分析
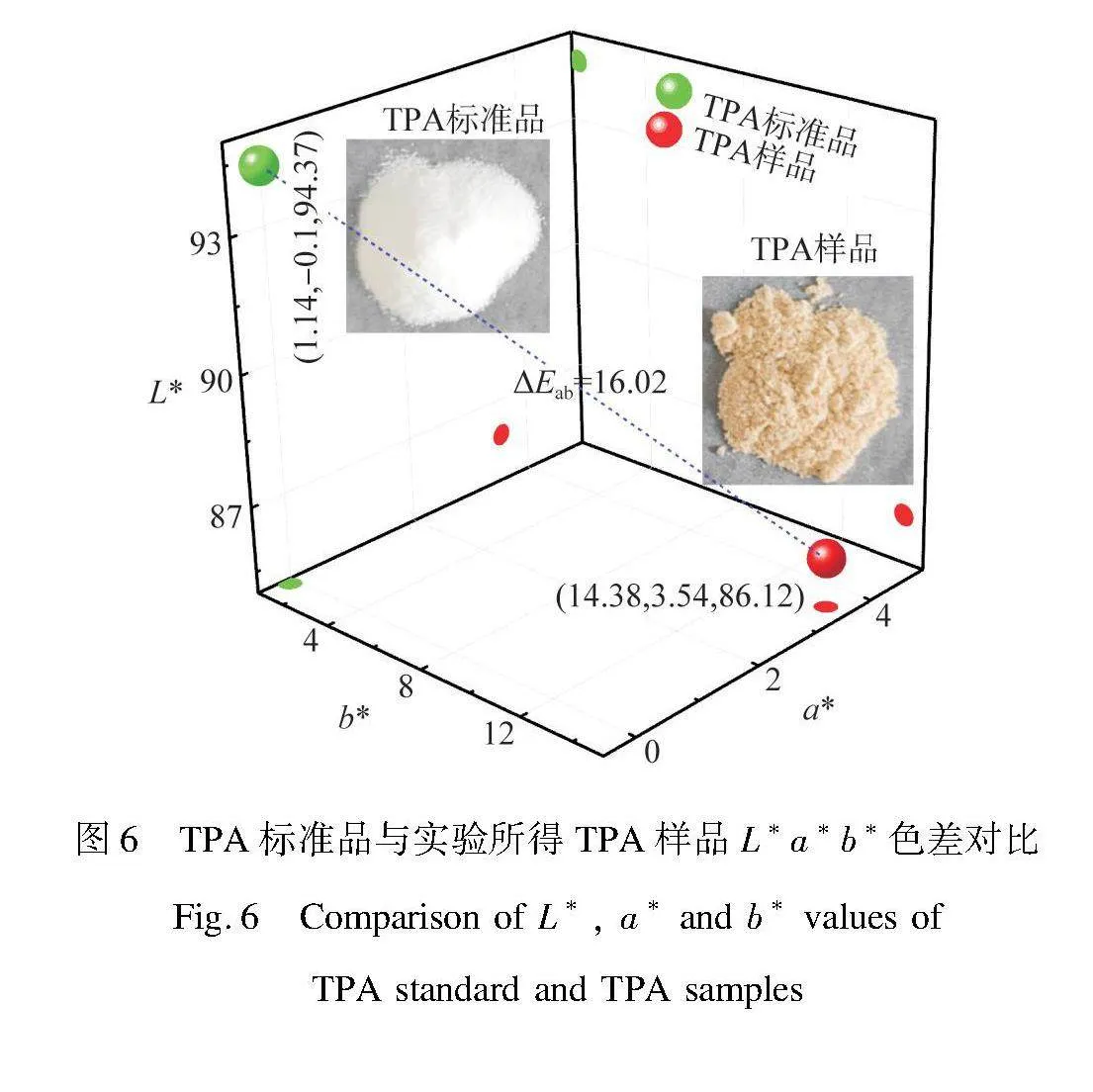
图6为TPA标准品与本实验所得TPA样品的L*、a*、b*色度坐标。与TPA标准品相比,本实验所得TPA样品的L*值由94.37减小至86.12,表明样品亮度变暗,灰度增加;a*值由-0.1增加至3.54,表明样品由绿变得更红;b*值由1.14增加至14.38,表明样品变得更黄。由式(4)计算出两者
的色差ΔE*ab为16.02,远大于1,肉眼可以分辨出两者的颜色差异[23],从光学照片也可看出两者颜色有较大差异,可能是由于废聚酯中染料经高温高压水热处理及酸氧化而引起的颜色变化。
3 结 论
本文采用水—溶剂热技术,以碱性水—甲醇溶液解聚废聚酯粒料,用硫酸析出TPA,通过称量聚酯解聚的残渣质量,优化了废聚酯解聚条件参数:氢氧化钠质量浓度50 g/L、浴比1︰20、反应时间2.5 h和反应温度160 ℃,聚酯解聚百分比为99.13%,TPA产率为94.9%。采用傅里叶变换红外光谱、核磁共振氢谱和碳谱及高效液相色谱对比了TPA与标准品的分子结构,并计算了其纯度(90.53%);采用标准化视觉和数字色彩评定仪分析了TPA标准品与实验所得TPA样品的色差(16.02)。本文研究废聚酯优化条件可为工业规模生产提供重要参考,有助于提高生产效率、降低成本,具有重要的实际应用价值。本研究提供了一种高效解聚废聚酯回收对苯二甲酸的方法,有助于减少废聚酯纤维对环境的影响,推动了循环经济的发展。然而,也存在不足之处,所得TPA与标准品存在较大色差,并含有其他杂质,需要进一步研究提纯方法。另外,所得水解液含有EG,但含量过少故无法与溶液分离,需要进一步解决。
《丝绸》官网下载
中国知网下载
参考文献:
[1]ATHANASOPOULOS P, ZABANIOTOU A. Post-consumer textile thermochemical recycling to fuels and biocarbon: A critical review[J]. Science of the Total Environment, 2022(834): 155387.
[2]TRAN N P, GUNASEKARA C, LAW D W, et al. Comprehensive review on sustainable fiber reinforced concrete incorporating recycled textile waste[J]. Journal of Sustainable Cement-Based Materials, 2022, 11(1): 28-42.
[3]STANESCU M D. State of the art of post-consumer textile waste upcycling to reach the zero waste milestone[J]. Environmental Science and Pollution Research, 2021, 28(12): 14253-14270.
[4]钱蔚然, 徐平华, 王来力. 聚酯纤维回收再利用及环境影响评价进展综述[J]. 现代纺织技术, 2021, 29(1): 22-26.
QIAN W R, XU P H, WANG L L. Review on polyester fiber recycling and progress of its environmental impact assessment[J]. Advanced Textile Technology, 2021, 29(1): 22-26.
[5]姜涛, 蔡宇凌, 周安展. 聚酯回收再生产业绿色发展模型构建及实践[J]. 现代化工, 2022, 42(2): 45-50.
JIANG T, CAI Y L, ZHOU A Z. Construction and practice of green development innovation model for spent polyester recycling industry[J]. Modern Chemical Industry, 2022, 42(2): 45-50.
[6]PALACIOS M C, VAN D M Y, SEIDE G. Analysis of the polyester clothing value chain to identify key intervention points for sustainability[J]. Environmental Sciences Europe, 2021, 33(1): 1-25.
[7]MOHAMED E W, MOHAMED E F, ABDESLAM E B, et al. Thermo physical characterization of sustainable insulation materials made from textile waste[J]. Journal of Building Engineering, 2017(12): 196-201.
[8]PARK S H, KIM S H. Poly (ethylene terephthalate) recycling for high value added textiles[J]. Fashion and Textiles, 2014, 1(1): 1-17.
[9]KRATOFIL K L, ZLATA H M, JASENKA J, et al. Evaluation of poly (ethylene-terephthalate) products of chemical recycling by differential scanning calorimetry[J]. Journal of Polymers and the Environment, 2009(17): 20-27.
[10]KOSLOSKI O S C, WOOD Z A, MANJARREZ Y, et al. Catalytic methods for chemical recycling or upcycling of commercial polymers[J]. Materials Horizons, 2021, 8(4): 1084-1129.
[11]GHASEMI M H, NEEKZAD N, AJDARI F B, et al. Mechanistic aspects of poly (ethylene terephthalate) recycling-toward enabling high quality sustainability decisions in waste management[J]. Environmental Science and Pollution Research, 2021, 28(32): 43074-43101.
[12]SINHA V, PATEL M R, PATEL J V. PET waste management by chemical recycling: A review[J]. Journal of Polymers and the Environment, 2010, 18(1): 8-25.
[13]IKENAGA K, INOUE T, KUSAKABE K. Hydrolysis of PET by combining direct microwave heating with high pressure[J]. Procedia Engineering, 2016(148): 314-318.
[14]SINGH S, SHARMA S, UMAR A, et al. Recycling of waste poly (ethylene terephthalate) bottles by alkaline hydrolysis and recovery of pure nanospindle-shaped terephthalic acid[J]. Journal of Nanoscience and Nanotechnology, 2018, 18(8): 5804-5809.
[15]MAJA C, ZELJKO K, MOJCA S. Sub-and supercritical water for chemical recycling of polyethylene terephthalate waste[J]. Chemical Engineering Science, 2021(233): 116389.
[16]SIDDIQUI M N, REDHWI H H, AL-ARFAJ A A, et al. Chemical recycling of PET in the presence of the bio-based polymers, PLA, PHB and PEF: A review[J]. Sustainability, 2021, 13(19): 10528.
[17]雷丹丹, 肖荔人, 陈庆华, 等. 废聚酯混纺纤维的化学回收[J]. 精细石油化工进展, 2020, 21(4): 53-57.
LEI D D, XIAO L R, CHEN Q H, et al. Chemical recovery of waste mixed polyester fiber[J]. Advances in Fine Petrochemicals, 2020, 21(4): 53-57.
[18]DU J T, SUN Q, ZENG X F, et al. ZnO nanodispersion as pseudohomogeneous catalyst for alcoholysis of polyethylene terephthalate[J]. Chemical Engineering Science, 2020(220): 115642.
[19]刘洋, 姚响, 杨革生, 等. 中国废旧军服面料回收利用的研究现状及发展前景[J]. 现代纺织技术, 2024, 32(1): 119-129.
LIU Y, YAO X, YANG G S, et al. Research progress and development prospects of the fabric recycling of waste military uniforms in China[J]. Advanced Textile Technology, 2024, 32(1): 119-129.
[20]IMRAN M, KIM D H, AL-MASRY W A, et al. Manganese-, cobalt-, and zinc-based mixed-oxide spinels as novel catalysts for the chemical recycling of poly (ethylene terephthalate) via glycolysis[J]. Polymer Degradation and Stability, 2013, 98(4): 904-915.
[21]MOHSIN M A, ALNAQBI M A, BUSHEER R M, et al. Sodium methoxide catalyzed depolymerization of waste polyethylene terephthalate under microwave irradiation[J]. Catalysis in Industry, 2018(10): 41-48.
[22]PALIWAL N R, MUNGRAY A K. Ultrasound assisted alkaline hydrolysis of poly (ethylene terephthalate) in presence of phase transfer catalyst[J]. Polymer Degradation and Stability, 2013, 98(10): 2094-2101.
[23]VISINTIN P M, MIMS R W. Di (methylsulphonyl) quinacridone: Synthesis, characterisation and application in nylon[J]. Coloration Technology, 2010, 126(1): 11-17.
[24]江涛, 杨光, 武丽梅. 聚酯废料化学解聚新工艺[J]. 重庆大学学报(自然科学版), 2000, 23(5): 70-73.
JIANG T, YANG G, WU L M. New technology of depolymerising waste PET[J]. Journal of Chongqing University (Nature Science Edition), 2000, 23(5): 70-73.
[25]YANG W S, LIU R, LI C, et al. Hydrolysis of waste polyethylene terephthalate catalyzed by easily recyclable terephthalic acid[J]. Waste Management, 2021(135): 267-274.
[26]孙琮皓, 陈湘萍, 卓强, 等. 醇碱水解作用下废旧聚酯瓶的解聚回收利用[J]. 中南大学学报, 2018, 25(3): 543-549.
SUN C H, CHEN X P, ZHUO Q, et al. Recycling and depolymerization of waste polyethylene terephthalate bottles by alcohol alkali hydrolysis[J]. Journal of Central South University, 2018, 25(3): 543-549.
[27]GAMERITH C, ZARTL B, PELLIS A, et al. Enzymatic recovery of polyester building blocks from polymer blends[J]. Process Biochemistry, 2017(59): 58-64.
[28]DAS J, HALGERI A B, SAHU V, et al. Alkaline hydrolysis of poly (ethylene terephthalate) in presence of a phase transfer catalyst[J]. Indian Journal of Chemical Technology, 2007, 14(2): 173-177.
[29]SHUKLA S R, HARAD A M. Glycolysis of polyethylene terephthalate waste fibers[J]. Journal of Applied Polymer Science, 2005, 97(2): 513-517.
[30]LI Y t5jnv/p3DAuAeq/0oRgqFa7nZlStXCXjEfWFly5RJHE=Y, YI H L, LI M J, et al. Synchronous degradation and decolorization of colored poly (ethylene terephthalate) fabrics for the synthesis of high purity terephthalic acid[J]. Journal of Cleaner Production, 2022(366): 132985.
[31]ZHANG S, XU W H, DU R C, et al. Cosolvent-promoted selective non-aqueous hydrolysis of PET wastes and facile product separation[J]. Green Chemistry, 2022, 24(8): 3284-3292.
[32]KUMAGAI S, HIRAHASHI S, GRAUSE G, et al. Alkaline hydrolysis of PVC-coated PET fibers for simultaneous recycling of PET and PVC[J]. Journal of Material Cycles and Waste Management, 2018, 20(1): 439-449.
[33]NAHAR S S, RAHAMAN M S, SAMADDER R, et al. A modified hydrolysis method of decolorizing reactive-dyed polycotton waste fabric and extraction of terephthalic acid: A perspective to reduce textile solid waste[J]. Advances in Polymer Technology, 2022(1): 1-15.
[34]VALH J V, VONCINA B, LOBNIK A, et al. Conversion of polyethylene terephthalate to high-quality terephthalic acid by hydrothermal hydrolysis: The study of process parameters[J]. Textile Research Journal, 2020, 90(13/14): 1446-1461.
[35]刘志阳, 官军, 顾日强, 等. 密实化方式对废弃聚酯纺织品的醇解及酯交换产物的影响[J]. 现代纺织技术, 2023, 31(1): 123-129.
LIU Z Y, GUAN J, GU R Q, et al. Effects of densification methods on glycolysis and transesterification products of waste polyester textiles[J]. Advanced Textile Technology, 2023, 31(1): 123-129.
[36]ISSAM A M. A new approach to obtain kevlar-49 from PET waste bottles[J]. Research on Chemical Intermediates, 2014, 40(8): 3033-3044.
[37]STOSKI A, VIANTE M F, NUNES C S, et al. Oligomer production through glycolysis of poly (ethylene terephthalate): Effects of temperature and water content on reaction extent[J]. Polymer International, 2016, 65(9): 1024-1030.
[38]MOGHBELI M R, NAMAYANDEH S, HASHEMABADI S H. Wet hydrolysis of waste polyethylene terephthalate thermoplastic resin with sulfuric acid and cfd simulation for high viscous liquid mixing[J]. International Journal of Chemical Reactor Engineering, 2011, 8(1): 1-15.
[39]于宏伟, 李研, 田野, 等. 乙二醇氢键作用二维红外光谱研究[J]. 精细石油化工进展, 2017, 18(2): 50-53.
YU H W, LI Y, TIAN Y, et al. Study on hydrogen bonding of ethylene glycol by two-dimensional infrared spectroscopy[J]. Advances in Fine Petrochemicals, 2017, 18(2): 50-53.
[40]吴凤芹, 聂天琛, 姚超, 等. 乙二醇溶剂热法制备纳米TiO2[J]. 化工新型材料, 2008, 431(11): 39-41.
WU F Q, NIE T C, YAO C, et al. Preparation of nano TiO2 by glycol solvothermal method[J]. New Chemical Materials, 2008, 431(11): 39-41.
[41]王兴原, 费美丽, 陈纪忠. 原位红外光谱研究聚对苯二甲酸乙二醇酯(PET)醇解反应过程[J]. 高校化学工程学报, 2013, 27(1): 58-64.
WANG X Y, FEI M L, CHEN J Z. Study of process of polyethylene terephthalate (PET) methanolysis by FTIR in situ[J]. Journal of Chemical Engineering of Chinese, 2013, 27(1): 58-64.
[42]ISLAM M S, ISLAM Z, HASAN R, et al. Acidic hydrolysis of recycled polyethylene terephthalate plastic for the production of its monomer terephthalic acid[J]. Progress in Rubber Plastics and Recycling Technology, 2023, 39(1): 12-25.
Efficient depolymerisation of waste polyester in alkaline water-methanol solution forterephthalic acid recovery
ZHANG Chi, WANG Xiangrong
XUAN Xiangtao1, ZHANG Hui1, ZHANG Pengfei1, WANG Yi2, FAN Zhengke3, HOU Lin3, FU Weijuan2
(1a. School of Textile Science and Engineering 1b. Key Laboratory of Functional Textile Material and Product Ministry of Education Xi'an Polytechnic University Xi an 710048 China 2. Xi'an Supervision and Inspection Institute of Fiber Textile Xi'an 710068 China 3. Shaanxi Yuanfeng Prosafe Co. Ltd. Xi'an 710025 China
Abstract:
Polyester, especially polyethylene terephthalate (PET), is widely used in the textile and apparel industries due to its excellent thermal, mechanical, and chemical stability. However, with increasing consumption, the environmental impact of waste polyester fibers has become increasingly pronounced. The problem in biodegrading PET poses technical challenges for the recycling and reuse of waste polyester, particularly in terms of efficient depolymerization and the recovery of valuable chemical monomers. Traditional methods for dealing with waste polyester are plagued by low efficiency, high costs, cumbersome processes, and significant environmental pollution. Therefore, developing efficient, economical, and energy-conserving recycling technology for waste polyester is significant for alleviating environmental pressure and achieving resource recycling. This study aims to explore a novel water-solvent thermal technology for the efficient depolymerization of waste polyester and the recovery of terephthalic acid (TPA), providing a new approach for the high-value recycling of waste polyester.
To efficiently depolymerize waste polyester and recover TPA, this study employed water-solvent thermal technology. Waste polyester pellets were treated with an alkaline water-methanol solution under high temperature and pressure, followed by the precipitation of TPA using sulfuric acid. The process conditions for the depolymerization of polyester in the alkaline water-methanol solution were optimized. Various analytical techniques, including Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR) spectroscopy, and high-performance liquid chromatography (HPLC), were used to characterize the chemical properties and purity of the obtained TPA. Additionally, the VeriVide DigiEye (Roachelab) system was used to evaluate the color characteristics of the product. The use of hydro-solvent thermal technology in this study achieved efficient depolymerization of waste polyester, and the use of sulfuric acid facilitated the efficient precipitation of TPA. The systematic optimization of depolymerization process parameters provided a basis for further improving the recovery rate and reducing costs. The experimental results showed that when waste polyester granules were treated in a mixture containing 50 g/L of sodium hydroxide and 100 mL/L methanol at 160 ℃ for 2.5 hours, with a bath ratio of 1︰20, the recovery rate of TPA was calculated to be 94.9%. The chemical properties of the separated product, as indicated by FTIR, NMR, and HPLC analysis, were highly consistent with the TPA standard, showing a purity of 90.53%. However, the color difference between commercial TPA and the obtained TPA was 16.02 according to the VeriVide DigiEye (Roachelab) system, indicating the presence of a small amount of light yellow impurities.
This study has significant value in practical application and environmental protection. The optimization of depolymerization conditions provides key reference information for the industrial-scale recycling of waste polyester, which can help improve production efficiency and effectively reduce economic costs. Moreover, this study proposes a new method for the efficient recovery of TPA from waste polyester, which not only reduces the potential environmental impact of waste polyester fibers but also promotes the further development of the circular economy. However, there are some limitations in the research process that point the way for future work. For example, the TPA product obtained in the experiment shows a significant color difference compared to the standard one, indicating that the product contains a certain amount of impurities. This necessitates the exploration of more effective purification technologies in future research to improve the purity and quality of the product. Additionally, during the experimental process, ethylene glycol (EG) was observed in the hydrolysate, but due to its low content, it is difficult to achieve effective separation. This issue also needs to be resolved in future research to fully recover and utilize all valuable components in waste polyester. In summary, although this study has made positive progress in the depolymerization of waste polyester and the recovery of TPA, further research and improvement are still needed to overcome current challenges and achieve a more efficient and environmentally friendly recycling process.
Key words:
waste polyester; depolymerization; terephthalic acid (TPA); alkaline water-methanol; sodium hydroxide
作者单位1. 西安工程大学a. 纺织科学与工程学院; b. 功能性纺织材料及制品教育部重点实验室,西安710048;
2. 西安纤维纺织品监督检验所,西安710068; 3. 陕西元丰新材料科技有限公司,西安710025
