苹果与胶孢炭疽菌互作研究进展
2024-06-30冀志蕊王美玉张树武杜宜南丛佳林徐秉良周宗山
冀志蕊 王美玉 张树武 杜宜南 丛佳林 徐秉良 周宗山


摘 要:胶孢炭疽菌(Colletotrichum gloeosporioides)能够引发苹果苦腐病和苹果炭疽叶枯病,危害叶片和果实,影响果品产量和品质,给苹果产业造成严重的经济损失。对苹果与病原物互作分子机制最新研究进展进行综述,包括苹果上炭疽病的病原菌组成和分类、侵染循环及其引发的果树病害种类,病原菌的致病结构和降解酶类、致病相关基因的挖掘与分析、效应蛋白的筛选与功能分析等致病相关分子机制,苹果被侵染后生理生化变化、激素信号、抗病基因挖掘、miRNA参与的免疫调控机制等抗病相关的研究内容,以期为解析病原菌致病机制及与寄主互作机制,进而为挖掘潜力候选基因,以及病害综合防控和抗病分子育种奠定理论基础。
关键词:苹果;胶孢炭疽菌;侵染机制;抗病机制
中图分类号:S661.1 文献标志码:A 文章编号:1009-9980(2024)06-1199-14
Advances in study of the interaction between apple and Colletotrichum gloeosporioides
JI Zhirui1, 2, WANG Meiyu2, ZHANG Shuwu1, DU Yinan2, CONG Jialin2, XU Bingliang1*, ZHOU Zongshan2*
(1College of Plant Protection, Gansu Agricultural University, Lanzhou 730070, Gansu, China; 2Research Institute of Pomology, Chinese Academy of Agricultural Science, Xingcheng 125100, Liaoning, China)
Abstract: Colletotrichum gloeosporioides can cause apple bitter rot, and anthracnose leaf blight, resulting in affecting fruit yield and quality, and causing serious economic losses to the apple industry. According to the latest fungal classification system, the C. gloeosporioides species complex consists of 13 different species, including C. gloeosporioides, C. aenigma and C. fructicola et al. Among them, C. fructicola and C. gloeosporioides are important pathogenic fungi on various fruit trees. Meanwhile, C. gloeosporioides can also cause diseases on other fruit trees such as cherry, passion fruit, and kiwifruit. In order to better prevent and control diseases, we need to have a comprehensive understanding of the classification, pathogenic mechanisms, and host interaction mechanisms of the pathogens on apples. In the process of colonizing host tissue, a number of C. gloeosporioides genes participate in different phases of infection procedures, which include conidiation, appressorium morphogenesis, melanization and penetration, biotrophy, necrotrophy, and various transport activities. In recent years, research on the pathogenic molecular mechanism of C. gloeosporioides on apples has mainly focused on the cloning and analysis of pathogenic related genes, screening and identification of effector proteins, pathogenic enzymes, and colletotoxins of C. gloeosporioides. Fungi secrete enzymes such as pectin, keratin and cellulase could help them successfully infect their hosts. New studies have shown that the adapter protein gene GcAP1 can regulate the expression of endopolygalacturonase genes (CgPG1 and CgPG2), pectin lyase genes (pnl-1, pnl-2), and pectate lyase genes (pelA, pelB), and GcAP1 is an important virulence factor of C. gloeosporioides. Currently, the successful application of PEG mediated genetic transformation and Agrobacterium mediated transformation in the study of C. gloeosporioides provides a basis for the development of pathogenic molecular mechanisms. It has been confirmed that the genes with different functions such as CgABCF2, CgCMK1, CgSET5, CgOpt1, CgNVF1, CgABCF2, CgChip6 are present in C. gloeosporioides, playing an important role in infecting apples. In addition, C2H2 transcription factors, cation stress response transcription factors CgSltA, CgCrzA, and CsHtf1 also play important roles in pathogen pathogenesis. During the infection process, C. gloeosporioides can also secrete a series of effectors to inhibit the host immune response, thereby promoting pathogen infection and colonization. Currently, scientists have analyzed the roles of effectors such as CfE12, CfEC92, and Sntf2 in C. gloeosporioides, laying the foundation for subsequent research on interactions of pathogen and host. In addition, C. gloeosporioides secrete toxins during the necrotrophic stage, causing necrosis of the host tissue. The research on apple disease resistance started relatively late, mainly focusing on germplasm resource identification, physiological and biochemical testing, disease resistance gene mining, plant hormone mediated disease resistance response, disease related transcription factors, and other mechanisms of action. Research has shown that after inoculation with anthrax fungus, the activities of superoxide dismutase (SOD), polyphenol oxidase (PPO), peroxidase (POD), catalase (CAT), and serotonin N-acetyltransferase (SNAT) in apple leaves increased, indicating that these enzymes are involved in the infection process of C. gloeosporioides. Plant hormones play an important role in plant defense and growth and development, and hormones related to plant immune responses include salicylic acid (SA), jasmonic acid (JA), ethylene (ET), abscisic acid (ABA), and so on. Research has shown that there are significant differences in the expression levels of SA synthesis related genes MdEDS1, MdPAD4, MdPAL and SA signal transduction related genes MdNPR1, MdPR1 and MdPR5 between resistant and susceptible varieties. There are differences in the resistance and susceptibility of different apple varieties to C. gloeosporioides. The Hanfu variety has been used to screen for resistance genes due to its high resistance to C. gloeosporioides. WRKY and NAC transcription factors play a crucial role in plant resistance to pathogen infection. In apples, transcription factors MdWRKY15, MdWRKY17, and MdWRKY100 enhance apple resistance to anthracnose by regulating SA accumulation. Here, we plotted the downstream regulatory patterns of AtwrKY33 and MdWRKYs involved in the MAPK cascade reaction, and presented some research results on MdWRKYs. At the end of the article, we summarized the research results on the regulatory mechanism of miRNA involvement in plant immunity. Clarifying the pathogenic process and molecular mechanism of the pathogen is of great significance for the comprehensive prevention and control of C. gloeosporioides. With the deepening of various studies, researchers will inevitably change their thinking on the prevention and control of C. gloeosporioides. Traditional chemical prevention and control methods, such as the extensive use of fungicides and insecticides, can achieve the effect of combating pathogens, but they also can cause serious harm to the environment and people. Breeding of resistant varieties is a fundamental means to solve the problems in preventing and controlling C. gloeosporioides. This article aimed to analyze the pathogenic mechanism of pathogens and their interaction with hosts, laying a theoretical foundation for screening potential candidate genes and breeding new varieties resistant to diseases.
Key words: Apples; Colletotrichum gloeosporioides; Infection mechanism; Disease resistance mechanisms
炭疽菌(Colletotrichum)属小丛壳科刺盘孢属真菌,有性型为子囊菌门盘菌亚门小丛壳属,在温暖和潮湿的条件下易暴发流行,是世界上重要的植物病原菌之一[1]。炭疽菌可分为14个复合种和部分种,胶胞炭疽菌(C. gloeosporioides)是重要的一个复合种,能侵染1000余种作物,危害枝干、叶部、果实等部位,造成果实腐烂、植株枯萎甚至死亡。
C. gloeosporioides通过“半活体营养”寄生并侵染寄主植物[2],在整个侵染周期主要有活体营养型(biotrophic)和死体营养型(necrotrophic)两种营养模式。在侵染初期活体营养阶段,菌体不会立即杀死周边寄主细胞,而是感应寄主表面的物理和化学信号(植物表面硬度、疏水性、叶片纹理、植物激素等),产生初侵染菌丝摄取寄主体内营养和能源。在侵染后期,分化出次生菌丝并迅速扩展,分泌细胞壁降解酶导致植物组织形成坏死斑,后转换为死体营养[3-5],其生活史和侵染过程如图1所示。
目前,生产上对炭疽病的防控以化学农药为主,随着人们对果品安全的逐渐重视,科研人员开展了药剂筛选和复配[6]、农药助剂应用[7]及植物免疫诱抗剂使用等[8]药剂减量增效研究。尽管对苹果炭疽病菌的侵染和致病研究取得一定进展,但因其种群多样、侵染过程复杂,对其侵染致病机制和果树抗性机制的研究仍有待深入。笔者在本文中将围绕苹果胶孢炭疽菌病原学、病原菌致病机制及果树抗性机制展开论述。
1 侵染苹果的胶孢炭疽菌复合群概述
胶孢炭疽菌(C. gloeosporioides)能够引发苹果果实炭疽病(apple bitter rot)和苹果炭疽叶枯病(Glomerella leaf spot of apple,GLSA),也能引发果实采后炭疽病。胶孢炭疽菌复合种(C. gloeosporioides complex)是苹果上主要的病原菌,包含果生刺盘孢(C. fructicola)、隐秘刺盘孢(C. aenigma)、胶孢刺盘孢(C. gloeosporioides)等13种不同炭疽菌,其中C. fructicola和C. gloeosporioides是多种果树的重要致病菌,可以在无伤条件下成功侵染寄主[9-10]。除苹果外,C. gloeosporioides还可引发樱桃、百香果、猕猴桃等果树病害[10-15](表1)。
2 胶孢炭疽菌致病分子机制
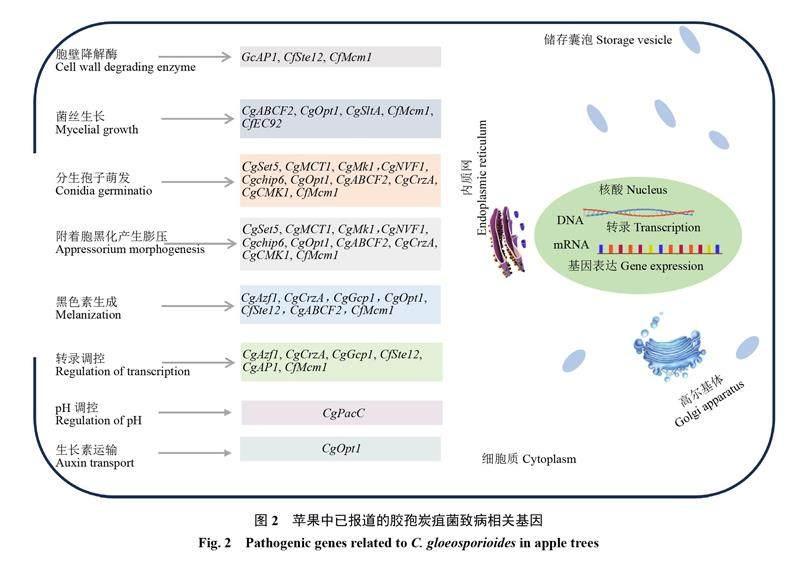
随着生物信息学和分子生物学的发展,胶孢刺盘孢(C. gloeosporioides)、希金斯刺盘孢(C. higginsianum)、禾生刺盘孢(C. graminicola)、东方刺盘孢(C. orbiculare)等全基因组测序组装完成并公布。在此基础上,PEG介导的遗传转化[25]和农杆菌介导的转化[26]在苹果炭疽菌研究中成功应用,为病原菌致病分子机制的解析提供了理论依据[22-23]。近年来苹果上胶孢炭疽菌致病分子机制的研究主要集中在致病结构和降解酶测定、致病基因的克隆与分析、效应蛋白筛选与功能研究及炭疽菌毒素等方面(图2)[27-29]。
2.1 胶孢炭疽菌致病结构和降解酶
炭疽菌要穿透寄主的表皮组织,需要对寄主组织施加机械压力,即孢子萌发时形成的附着胞及其胞内组合液产生膨压,压力施加至附着胞下部的侵染钉,当压力到达一定程度时直接穿透植物表皮而侵入,构成侵染和定殖[30]。黑化附着胞的形成可能涉及一系列复杂的生物学过程,包括分泌特定的分子、改变细胞壁结构以提供附着支持等[31]。
植物的细胞壁是抵御病原菌侵入的天然屏障,病原菌通过分泌产生果胶酶、角质酶、纤维素酶、蛋白酶等降解酶类物质破坏寄主细胞壁,辅助其侵染和定殖。薛莲[32]对苹果采后炭疽病菌细胞壁降解酶活性进行分析,明确聚甲基半乳糖醛酸酶(PMG)和羧甲基纤维素酶(Cx)在病原菌侵染中发挥重要作用。研究表明,衔接蛋白GcAP1复合体分布于细胞质中,GcAP1基因能够调控多聚半乳糖醛酸内切酶基因(CgPG1、CgPG2)、果胶裂解酶基因(pnl-1、pnl-2)及果胶酸酯裂解酶基因(pelA、pelB)的表达,从而影响炭疽菌的生长发育和毒力[33]。研究表明,胶孢炭疽菌pH依赖性转录因子CgPacC能够调节细胞壁降解酶、转运蛋白和抗氧化剂的表达,在病原菌定殖中发挥重要作用[34]。
2.2 胶孢炭疽菌侵染阶段相关致病基因挖掘与分析
C. gloeosporioides成功侵染定殖包括分生孢子萌发、附着胞形成、黑色素生成、侵染钉穿透寄主组织等不同阶段,涉及的基因及其调控机制较为复杂,目前的研究以单一基因为主。
Zhao等[35]证实苹果炭疽叶枯病菌染色质调节基因CgSET5在菌丝生长、分生孢子形成、附着胞形成、细胞壁完整性、致病性中发挥重要作用,并同时参与过氧化物酶体的生物反应,是C. gloeosporioides的核心致病调节因子。该团队在后续研究中证实单羧酸转运蛋白CgMCT1参与了C. gloeosporioides营养生长、黑色素形成、分生孢子形成,且参与寄主体内ROS降解[27]。Zhou等[28]发现当炭疽菌CgABCF2基因缺失后,菌丝生长速率和附着胞数量显著下降,导致致病性丧失。张俊祥等[36-37]研究证实CgCMK1、CgNVF1在炭疽菌分生孢子和附着胞中的表达,对分生孢子产量、附着胞形成、氧化胁迫应答反应及致病性等方面均有影响。徐杰[38]证实苹果炭疽叶枯病菌基因GTPBP1在调控附着胞的形成中发挥作用。谭清群[39]研究证实氨甲酰磷酸合成酶(carbamyl phosphate synthase,CPS)小亚基基因Cpa1通过调控精氨酸的合成从而影响病原菌致病力。研究表明,寡肽转运蛋白基因CgOpt1在菌丝中表达,参与真菌对IAA反应的调节,通过影响产孢和色素沉积来降低病菌的致病性[40]。甾醇糖基转移酶编码基因CgChip6参与分生孢子萌发和附着胞的形成,该基因缺失后病原菌毒力显著下降[41]。Liang等[42]对果生炭疽菌(C. fructicola)1104-7基因组进行了测序和组装,获得了高质量参考基因组,为C. fructicola致病相关基因的研究提供了重要的理论和数据支撑。
2.3 转录因子调控胶孢炭疽菌分子机制
转录因子(transcription factor,TF)能够与基因启动子区域的顺式作用元件进行特异性互作,从而调控目的基因的表达强度,可分为4类,即锌指蛋白(包括3类:C2H2、C4和C6)、碱性亮氨酸拉链、碱性螺旋环螺旋和同源异形盒类转录因子[43]。已有研究表明,胶孢炭疽菌的转录因子在表达调控中能起到协调作用,能够促进附着胞黑化和定殖。C2H2锌指蛋白型转录因子CgAzf1、CgCrzA及CgGcp1能够调控黑色素生物合成途径相关基因的表达,参与分生孢子的萌发和侵染过程[31,44-45]。CfSte12能够调控与附着胞功能相关的四次穿模蛋白PLS1(tetraspanin PLS1)、Gas1样蛋白(Gas1-like proteins)、角质酶和黑色素合成的基因表达[46]。阳离子胁迫反应转录因子CgSltA、CgCrzA及CsHtf1在炭疽菌营养生长、分生孢子产生、附着胞形成和致病性等方面均发挥重要作用[45,47]。碱性亮氨酸拉链(basic leucine zipper,bZIP)转录因子CgAP1在C. gloeosporioides中起氧化还原传感器的作用[48-49]。转录因子CfMcm1是C. fructicola的关键调节因子,在病原菌无性繁殖、黑色素形成、致病性、果胶酶降解等过程中发挥作用[50]。
2.4 效应蛋白筛选及功能研究
在侵染过程中,炭疽菌通过分泌一系列效应蛋白抑制寄主免疫反应,从而促进病原菌的侵染和定殖[51-52],不同侵染阶段所分泌的效应因子功能不同[3,53]。随着基因组测序的应用,炭疽菌中多个候选的效应因子被成功筛选鉴定[54-55]。真菌胞外膜蛋白CFEM(common in several fungal extracellular membrane)是真菌所独有的蛋白结构域,与病原菌致病性密切相关。Shang等[56]研究证实,刺盘孢属真菌CFEM型效应因子CfEC12能够与苹果中MdNIMIN2互作,与水杨酸受体NPR1竞争MdNIMIN2蛋白的结合位点,从而抑制苹果抗性基因的表达和免疫反应。LysM型效应蛋白可以保护真菌细胞壁免受植物几丁质酶的作用或隔离释放的壳寡糖,从而避免被植物的防御系统识别,其具有几丁质结合活性,可以结合几丁质从而抑制植物的PTI(pattern-triggered immunity),促进病原菌的侵染[57]。Shang等[58]研究证实C. fructicola中效应蛋白CfEC92在早期附着胞生成和附着胞介导的渗透阶段上调表达,抑制苹果的PTI和相关防御基因表达,促进病原菌侵染。王美玉[59]开展效应蛋白Sntf2功能研究,证实其能够与叶绿体组装因子Mdycf39互作干扰叶绿体功能,从而抑制寄主植物的免疫反应,促进C. gloeosporioides的侵染和定殖。
2.5 胶孢炭疽菌毒素
炭疽菌在死体营养阶段通过分泌毒素造成寄主组织坏死。目前,对于炭疽菌毒素的研究多集中在毒素生物学测定、成分鉴定纯化阶段。C. gloeosporioides产生的毒素为非寄主专化性毒素,能够侵染多种寄主。Khodadadi等[60]分离鉴定了苹果苦腐病病原菌,明确其毒素能够对3种不同树种造成危害,关于炭疽菌的毒素和作用机制仍然有待进一步深入研究。
3 苹果抗胶胞炭疽菌侵染的分子机制
果树在自然环境中会受到各类病原物的侵染,为了抵御病原菌的侵染,植物进化出识别和抵御病原菌的PTI和ETI两层免疫系统[61]。第一层免疫系统是由植物细胞质膜上的模式识别受体感知微生物相关分子模式(microbe-associated molecular pattern,MAMPs)或损伤相关分子模式(damage-associated molecular pattern,DAMPs)而触发一系列的免疫反应,称为“模式触发免疫”(PTI),该免疫反应包括活性氧(reactive oxygen species,ROS)的激活及抗病基因表达量上调等[62-63]。病原菌为了应对植物的PTI免疫反应进化出毒力蛋白(效应因子),抑制植物PTI反应,从而成功侵入,这一中间过程被称为“效应因子触发的易感性”,即EST(effector-triggered susceptibility)。最后,植物进化出识别和抵御这些效应子的胞内NLR来诱导更为强大的抗性反应,即第二层免疫“效应因子触发的免疫”,ETI(effector-triggered immunnity)[64-65]。ETI的免疫反应主要包括程序性细胞死亡的过敏性反应(hypersensitive responses,HR)、Ca2+内流、胼胝质的沉积等。植物在PTI和ETI期间,产生的免疫反应幅度和时间有所不同,但所触发的免疫信号网络和下游反应有所重叠[66-68]。
苹果抗炭疽病的研究起步相对较晚,主要开展了种质资源鉴定[69]、生理生化检测、抗病基因挖掘、植物激素介导的抗病反应及抗病相关转录因子[70]作用机制等研究。
3.1 生理生化变化
研究表明接种炭疽菌后,嘎拉和富士叶片内超氧化物歧化酶(superoxide dismutase,SOD)、多酚氧化酶(polyphenoloxidas,PPO)、过氧化物酶(peroxidase,POD)、过氧化氢酶(catalase,CAT)、5-羟色胺-N-乙酰基转移酶(SNAT)的活性增强,其相关基因表达量呈先升后降的趋势,表明以上酶类参与了炭疽叶枯病菌的侵染过程[71-72]。苹果不同组织被炭疽菌侵染后,PPO、POD、苯丙氨酸解氨酶(phenylalanine ammonia-lyase,PAL)等7种酶活性均有不同程度的提高[73-75]。通过分析不同抗感品种感染炭疽菌后细胞壁降解酶活性的变化,证实甲基半乳糖醛酸酶(PMG)和羧甲基纤维素酶(Cx)在病菌侵染过程中发挥作用,且抗病品种中细胞壁降解酶活性高峰的出现早于感病品种[76]。白静科[30]比较了C. fructicola侵染后抗感品种中过氧化氢(H2O2)和乳突产生的差异,发现炭疽菌的侵染诱导了苹果细胞中H2O2的积累和乳突的产生,并随着侵染时间延长不断积累。此外,生防菌也可以通过提高感病品种嘎拉叶片中POD、CAT、SOD等防御酶活性,减少活性氧的积累,从而诱导苹果对炭疽菌的抗性[77]。
3.2 植物激素
植物激素在植物防御和生长发育中发挥重要作用,与植物免疫反应相关的激素包括水杨酸(SA)、茉莉酸(JA)、乙烯(ET)、脱落酸(ABA)等。SA和JA-ET激素作为重要的调控因子,在苹果生物和非生物胁迫反应中发挥重要作用[78-79]。SA是通过异分支酸合成酶(ICS)和苯丙氨酸解氨酶(PAL)途径合成。在应激条件下,超过90%的受刺激SA是通过ICS合成的[80]。当没有遇到病原体或逆境时,植物细胞积累相对较低浓度的SA,外源喷施SA可增强抗病相关酶的活性,诱导高感苹果品种对C. gloeosporioides产生抗性[81-82]。在苹果中,藤牧1号、40-9及16-16等抗性品种(系)中SA合成相关基因MdEDS1、MdPAD4和MdPAL被C. gloeosporioides诱导表达,SA信号转导相关基因MdNPR1、MdPR1、MdPR5的表达量显著高于嘎拉等感病品种(系)[83]。水杨酸合成途径中的关键酶MdICS1可以被G. cingulata诱导上调表达,而JA、ABA和ETH三种外源信号可抑制其表达。
3.3 苹果抗病基因挖掘
不同苹果品种对炭疽菌抗感表现存在差异,在田间苹果炭疽叶枯病的表现尤为突出[84]。马玉鑫[85]研究表明,寒富品种CDPK基因家族成员MdCDPK24基因在炭疽菌侵染后显著上调表达。对寒富苹果同源四倍体进行转录组测序,发现MdCaMBP6、MdIPT8在苹果炭疽叶枯病菌侵染后显著上调表达,能够提高品种抗性[86-87]。Guo等[88]报道湖北苹果M. hupehensis YT521-B同源结构域包含蛋白2(MhYTP2),其与MdRGA2L mRNA结合并降低其稳定性,在调节对炭疽叶枯病的抗性中发挥重要作用,可用于开发具有GLS抗性的苹果品种。刘源霞等[89]采用分离群体分组分析(BSA)方法,筛选获得了一个与抗病性状相关的分子标记S0506206-24,在此基础上,采用全基因组重测序和BSA相结合的方法,在该杂交群体中定位了1个苹果抗炭疽叶枯病基因位点Rgls,并将其精细定位于标记InDel4199和SNP4299之间[90],室内接种验证与Rgls位点紧密连锁的4个分子标记S0405127(SSR)、S0304673(SSR)、SNP4236和InDel4254,准确率均高于90%[91]。
3.4 抗病相关转录因子参与的防御反应
植物被病原物感染后,当病原体相关的分子模式(PAMP)或效应器被植物识别时,细胞内的信号可以被激活,导致活性氧簇(ROS)的产生、丝裂原激活的蛋白激酶(MAPK)激活和防御基因的表达[62]。MAPKs能够靶向并磷酸化调节下游基因转录的转录因子,最终响应病原菌的侵入。已报道的与炭疽菌侵染响应相关的转录因子有AP2/ERF、TGACG基序结合因子(BZIP)、MYC2(BHLH)、ARF、MYB、WRKY和NAC等7种,后两者是高等植物特有的转录因子家族[92]。WRKYs转录因子作为MAPK级联反应的重要靶标,在植物对病原菌的抗性中起关键作用。当病原菌侵入后,SA依赖的WRKY基因会迅速表达并积累,与抗病基因启动子上的W盒[W-box,TTGAC(C/T)]特异性结合,启动防御反应,从而形成复杂的WRKY调控网络。在苹果中,MKK4-MPK3下游转录因子MdWRKY15、MdWRKY17及MdWRKY100通过调控SA积累增强苹果对炭疽菌的抗性[70,93]。其中,MdWRKY100正向调节苹果对C. gloeosporioides的抗性;C. fructicola可提高感病品种中MdWRKY17蛋白积累,诱导MdMEK4-MdMPK3-MdWRKY17-MdDMR6-SA途径,加速SA降解,从而降低果树抗性[94]。此外,有研究表明,MdWRKY15通过激活SA合成酶MdICS1的表达增强对轮纹病的抗性[95]。酯酶/脂肪酶GELP1是MPK3/MPK6及其下游转录因子MdWRKY100的靶标,在苹果抵御病原菌侵染中发挥重要作用[96]。Li等[97]和Lippok等[98]研究证实,γ-氨基丁酸(GABA)关键合成基因MdGAD1能够与MdWRKY33互作,增强转基因苹果愈伤组织形成和叶片的抗氧化能力,正向调控苹果对C. gloeosporioides的抗性。此外,MdWRKY31能够与苹果超敏反应蛋白MdHIR4(hypersensitive-induced reaction protein,HIR)相互作用,影响SA信号通路中基因的转录从而调节苹果对葡萄座腔菌B.dothidea的抗性[99]。MdWRKY75能够与MdRAC7启动子结合,调节漆酶的生物合成,并在苹果斑点落叶病菌Alternaria alternata感染期间促进了木质素的合成[100]。最新研究表明,MdVQ10能够与MdWRKY75互作,增强衰老相关基因MdSAG12和MdSAG18的转录,促进叶片损伤引发的衰老进程[101]。然而,以上几个转录因子是否在苹果对炭疽菌抗性中也发挥着相同或类似的作用仍有待进一步验证(图3)。
3.5 miRNA参与植物免疫的调控机制
非编码的RNA分为微小RNA(microRNAs,miRNAs)和小干扰RNA(small interfering RNAs,siRNA)两大类,miRNA是植物生长发育和胁迫应答中重要的调控因子[102]。miRNA可能参与调节病原菌感染中胼胝质沉积过程,在模式植物拟南芥中,miR773靶向抑制甲基转移酶2(MET2),影响胼胝质沉积和ROS累积,负调控C. higginianum的抗病性[103]。Zhang等[104]研究发现,Md-miRln20靶向Md-TN1-GLS负调控苹果对胶孢炭疽菌的侵染。此外,Zhang等[105]研究证实两种CCR-NB-LRR蛋白MdRNL2和MdRNL6能够形成复合物,抑制病原菌生长,提高了苹果树对苹果斑点落叶病菌A. alternata的抗性,进一步研究证实其同样能够提高果树对C. gloeosporioides的抗性[106]。张亚楠等[107]分析了抗感品种中抗病相关miRNA的表达量差异,预测miR390a、miR482b及miR396b/c/f 在苹果被炭疽菌侵染中发挥重要作用。Shen等[108]研究发现,Mdm-miR160-MdARF17-MdWRKY33模块能够通过调节活性氧(ROS)提高苹果耐寒性能,但其对病原菌致病力的影响仍未证实。上述结果对果树抗病育种起重要的推动作用。
4 问题与展望
近年来,在分子生物学和生物信息学的推动下,苹果和炭疽菌互作方面的研究取得了巨大进展。笔者详细阐述了苹果炭疽菌组成、病原菌侵染相关基因及其与寄主互作的研究进展。明确病原菌致病过程及其分子机制,对苹果炭疽叶枯病的综合防控具有重要意义,随着各项研究的日益深入,研究者对炭疽病的防控思路必将有所转变。
炭疽菌对苹果产业造成巨大危害,由于炭疽菌具有潜伏侵染的特性,果树病害监测不仅耗时费力,还存在一定的技术难题。利用传统的化学防控手段,通过大量使用杀菌剂和杀虫剂等虽能达到对抗病原菌的效果,但对环境和人体也会造成严重的危害。随着生活水平的不断提高,健康问题已经逐渐成为关注的焦点。目前,研发生态友好型生物防治策略已经被人们广泛接受和认可,前景一片光明。开展抗性品种选育是从根本上解决果树炭疽菌防控难问题的有效手段,符合现代农业的生产需求[109-110]。解析抗病机制能够为苹果抗病性改良提供重要的理论依据,是果业科研的重点研究领域。
生物信息学和分子生物学的各种先进技术为作物改良提供了其他途径,能够极大地缩短果树育种周期,解决传统实生苗选育耗时长的难题。通过构建苹果高效遗传转化体系进一步开展基因编辑、RNA干扰等生物育种技术研究,培育具有优良性状的苹果新种质或新品种是果树科研工作者努力的方向[110]。
总之,了解苹果与炭疽菌互作的分子机制,能够为培育抗病品种和创新病害防控策略提供新的见解,对果树产业健康发展具有重要的指导意义。
参考文献 References:
[1] DEAN R,VAN KAN J A L,PRETORIUS Z A,HAMMOND-KOSACK K E,DI PIETRO A,SPANU P D,RUDD J J,DICKMAN M,KAHMANN R,ELLIS J,FOSTER G D. The Top 10 fungal pathogens in molecular plant pathology[J]. Molecular Plant Pathology,2012,13(4):414-430.
[2] DE SILVA D D,CROUS P W,ADES P K,HYDE K D,TAYLOR P W J. Life styles of Colletotrichum species and implications for plant biosecurity[J]. Fungal Biology Reviews,2017,31(3):155-168.
[3] OCONNELL R,HERBERT C,SREENIVASAPRASAD S,KHATIB M,ESQUERR?-TUGAY? M T,DUMAS B. A novel Arabidopsis-Colletotrichum pathosystem for the molecular dissection of plant-fungal interactions[J]. Molecular Plant-Microbe Interactions,2004,17(3):272-282.
[4] TUCKER S L,TALBOT N J. Surface attachment and pre-penetration stage development by plant pathogenic fungi[J]. Annual Review of Phytopathology,2001,39:385-417.
[5] M?NCH S,LINGNER U,FLOSS D S,LUDWIG N,SAUER N,DEISING H B. The hemibiotrophic lifestyle of Colletotrichum species[J]. Journal of Plant Physiology,2008,165(1):41-51.
[6] 梁晓飞,侯彦忠,白云芳,李苏莉,孙广宇,朱明旗. 防控苹果炭疽叶枯病的化学农药减量增效研究[J]. 陕西农业科学,2022,68(1):39-43.
LIANG Xiaofei,HOU Yanzhong,BAI Yunfang,LI Suli,SUN Guangyu,ZHU Mingqi. Studies on improvement of chemical fungicide efficiency in control of apple Glomerella leaf spot disease[J]. Shaanxi Journal of Agricultural Sciences,2022,68(1):39-43.
[7] 刘安泰,张朝敏,李紫腾,曹克强,孟祥龙,胡同乐. 有机硅助剂在苹果炭疽叶枯病化学防控中的减药增效作用评价[J]. 植物保护,2022,48(1):284-290.
LIU Antai,ZHANG Chaomin,LI Ziteng,CAO Keqiang,MENG Xianglong,HU Tongle. Evaluation of increasing control effect and reducing fungicide effect of organosilicon adjuvant on chemical control of Glomerella apple leaf spot[J]. Plant Protection,2022,48(1):284-290.
[8] 刘禹彤,徐瑞旋,王洪涛,时彦娇,李翠英,马锋旺,梁微. 外源甜菜碱提高苹果对炭疽叶枯病的抗病性[J]. 果树学报,2022,39(7):1252-1261.
LIU Yutong,XU Ruixuan,WANG Hongtao,SHI Yanjiao,LI Cuiying,MA Fengwang,LIANG Wei. Exogenous glycine betaine improved the resistance of apple to Glomerella leaf spot[J]. Journal of Fruit Science,2022,39(7):1252-1261.
[9] WENNEKER M,PHAM K,KERKHOF E,HARTEVELD D O C. First report of preharvest fruit rot of ‘Pink Lady apples caused by Colletotrichum fructicola in Italy[J]. Plant Disease,2021,105(5):1561.
[10] XU C N,ZHOU Z S,WU Y X,CHI F M,JI Z R,ZHANG H J. First report of stem and leaf anthracnose on blueberry caused by Colletotrichum gloeosporioides in China[J]. Plant Disease,2013,97(6):845.
[11] 刘丽萍,高洁,李玉. 植物炭疽菌属Colletotrichum真菌研究进展[J]. 菌物研究,2020,18(4):266-281.
LIU Liping,GAO Jie,LI Yu. Advances in knowledge of the fungi referred to the genus Colletotrichum[J]. Journal of Fungal Research,2020,18(4):266-281.
[12] 王薇,符丹丹,张荣,孙广宇. 苹果炭疽叶枯病病原学研究[J]. 菌物学报,2015,34(1):13-25.
WANG Wei,FU Dandan,ZHANG Rong,SUN Guangyu. Etiology of apple leaf spot caused by Colletotrichum spp.[J]. Mycosystema,2015,34(1):13-25.
[13] LI Q L,BU J Y,SHU J,YU Z H,TANG L H,HUANG S P,GUO T X,MO J Y,LUO S M,SOLANGI G S,HSIANG T. Colletotrichum species associated with mango in Southern China[J]. Scientific Reports,2019,9(1):18891.
[14] ZHANG R,WANG S F,CUI J Q,SUN G Y,GLEASON M L. First report of bitter rot caused by Colletotrichum acutatum on apple in China[J]. Plant Disease,2008,92(10):1474.
[15] CHEN Y,FU D D,WANG W,GLEASON M L,ZHANG R,LIANG X F,SUN G Y. Diversity of Colletotrichum species causing apple bitter rot and Glomerella leaf spot in China[J]. Journal of Fungi,2022,8(7):740.
[16] YANG Z N,MO J Y,GUO T X,LI Q L,TANG L H,HUANG S P,WEI J G,HSIANG T. First report of Colletotrichum fructicola causing anthracnose on Pouteria campechiana in China[J]. Plant Disease,2021,105(3):708.
[17] TANG Z Y,LOU J,HE L Q,WANG Q D,CHEN L H,ZHONG X T,WU C F,ZHANG L Q,WANG Z Q. First report of Colletotrichum fructicola causing anthracnose on cherry (Prunus avium) in China[J]. Plant Disease,2021,106(1):317.
[18] HU Y F,LUO X X,XU Z H,ZHANG L H,WANG Y Q,CUI R Q,KUANG W G,XIA Y Y,MA J. First report of Colletotrichum fructicola causing anthracnose on Punica granatum in China[J]. Plant Disease,2023,107(9):2863.
[19] LI W Z,RAN F,LONG Y H,MO F X,SHU R,YIN X H. Evidences of Colletotrichum fructicola causing anthracnose on Passiflora edulis Sims in China[J]. Pathogens,2021,11(1):6.
[20] ZHANG Z H,YAN M J,LI W W,GUO Y Z,LIANG X F. First report of Colletotrichum aenigma causing apple Glomerella leaf spot on the Granny Smith cultivar in China[J]. Plant Disease,2021,105(5):1563.
[21] ZHANG Y J,SUN W X,NING P,GUO T X,HUANG S P,TANG L H,LI Q L,MO J Y. First report of anthracnose of Papaya (Carica papaya L.) caused by Colletotrichum siamense in China[J]. Plant Disease,2021,105(8):2252.
[22] FAN Y C,GUO F Y,WU R H,CHEN Z Q,LI Z. First report of Colletotrichum gloeosporioides causing anthracnose on grapevine (Vitis vinifera) in Shaanxi Province,China[J]. Plant Disease,2023,107(7):2249.
[23] AHMAD T,WANG J J,ZHENG Y Q,MUGIZI A E,MOOSA A,NIE C R,LIU Y. First record of Colletotrichum alienum causing postharvest anthracnose disease of mango fruit in China[J]. Plant Disease,2021,105(6):1852.
[24] WANG N,XU J,ZHAO X Z,WANG M Y,ZHANG J X. First report of Glomerella leaf spot of apple caused by Colletotrichum asianum[J]. Plant Disease,2020,104(10):2734.
[25] 韩小路,白静科,张玮,张荣,孙广宇. PEG介导的苹果果生刺盘孢Colletotrichum fructicola原生质体转化[J]. 西北农业学报,2016,25(3):442-449.
HAN Xiaolu,BAI Jingke,ZHANG Wei,ZHANG Rong,SUN Guangyu. PEG-mediated transformation of Colletotrichum fructicola[J]. Acta Agriculturae Boreali-Occidentalis Sinica,2016,25(3):442-449.
[26] 徐杰,王美玉,王娜,周宗山,张俊祥. 适用于ATMT介导的RNA干扰载体的构建及在苹果炭疽叶枯病菌中的应用[J]. 基因组学与应用生物学,2020,39(9):4053-4057.
XU Jie,WANG Meiyu,WANG Na,ZHOU Zongshan,ZHANG Junxiang. Construction of RNAi vector and application of ATMT-mediated genetic transformation in Colletotrichum gloeosporioides of apple[J]. Genomics and Applied Biology,2020,39(9):4053-4057.
[27] WU J Y,JI Z R,WANG N,CHI F M,XU C N,ZHOU Z S,ZHANG J X. Identification of conidiogenesis-associated genes in Colletotrichum gloeosporioides by Agrobacterium tumefaciens-mediated transformation[J]. Current Microbiology,2016,73(6):802-810.
[28] ZHOU Z S,WU J Y,WANG M Y,ZHANG J X. ABC protein CgABCF2 is required for asexual and sexual development,appressorial formation and plant infection in Colletotrichum gloeosporioides[J]. Microbial Pathogenesis,2017,110:85-92.
[29] WANG M Y,JI Z R,YAN H F,XU J,ZHAO X Z,ZHOU Z S. Effector Sntf2 interacted with chloroplast-related protein Mdycf39 promoting the colonization of Colletotrichum gloeosporioides in apple leaf[J]. International Journal of Molecular Sciences,2022,23(12):6379.
[30] 白静科. 果生炭疽菌Colletotrichum fructicola与苹果不同抗性品种互作研究[D]. 杨凌:西北农林科技大学,2016.
BAI Jingke. Interaction between resistant and susceptible apple cultivars and Colletotrichum fructicola[D]. Yangling:Northwest A & F University,2016.
[31] 张兴媛,王地广,高菁,唐雯,李晓宇. 胶孢炭疽菌C2H2型转录因子CgGcp1的生物学功能[J]. 微生物学通报,2022,49(7):2587-2598.
ZHANG Xingyuan,WANG Diguang,GAO Jing,TANG Wen,LI Xiaoyu. Biological functions of a C2H2 transcription factor CgGcp1 in Colletotrichum gloeosporioides[J]. Microbiology China,2022,49(7):2587-2598.
[32] 薛莲. 采后苹果与炭疽菌互作的生理生化机制的研究[D]. 合肥:安徽农业大学,2005.
XUE Lian. Studies on physiological and biochemical mechanism of host-pathogen reaction between postharvest apple and Colletotrichum gloeosporioides[D]. Hefei:Anhui Agricultural University,2005.
[33] 张俊祥,冀志蕊,王娜,徐成楠,迟福梅,周宗山. 苹果炭疽叶枯病菌GcAP1复合体β亚基基因的克隆及功能分析[J]. 中国农业科学,2017,50(8):1430-1439.
ZHANG Junxiang,JI Zhirui,WANG Na,XU Chengnan,CHI Fumei,ZHOU Zongshan. Gene cloning and functional analysis of GcAP1 complex beta subunit in Glomerella cingulata[J]. Scientia Agricultura Sinica,2017,50(8):1430-1439.
[34] ALKAN N,MENG X C,FRIEDLANDER G,REUVENI E,SUKNO S,SHERMAN A,THON M,FLUHR R,PRUSKY D. Global aspects of pacC regulation of pathogenicity genes in Colletotrichum gloeosporioides as revealed by transcriptome analysis[J]. Molecular Plant-Microbe Interactions,2013,26(11):1345-1358.
[35] ZHAO X Z,TANG B Z,XU J,WANG N,ZHOU Z S,ZHANG J X. A SET domain-containing protein involved in cell wall integrity signaling and peroxisome biogenesis is essential for appressorium formation and pathogenicity of Colletotrichum gloeosporioides[J]. Fungal Genetics and Biology,2020,145:103474.
[36] 张俊祥,王美玉,迟福梅,徐杰,周宗山. 苹果炭疽叶枯病菌CgCMK1基因的克隆与功能分析[J]. 植物病理学报,2020,50(1):40-48.
ZHANG Junxiang,WANG Meiyu,CHI Fumei,XU Jie,ZHOU Zongshan. Cloning and functional analysis of CgCMK1 in Colletotrichum gloeosporioides[J]. Acta Phytopathologica Sinica,2020,50(1):40-48.
[37] 张俊祥,王美玉,徐成楠,周宗山. 苹果炭疽叶枯病菌致病相关基因CgNVF1的功能初步分析[J]. 植物病理学报,2018,48(6):810-816.
ZHANG Junxiang,WANG Meiyu,XU Chengnan,ZHOU Zongshan. Functional analysis of the pathogenicity-related gene CgNVF1 in Colletotrichum gloeosporioides[J]. Acta Phytopathologica Sinica,2018,48(6):810-816.
[38] 徐杰. 苹果炭疽叶枯病菌GTP结合蛋白GTPBP1的功能分析[D]. 北京:中国农业科学院,2020.
XU Jie. Function analysis of GTP-binding protein GTPBP1 in Colletotrichum gloeosporioides[D]. Beijing:Chinese Academy of Agricultural Sciences,2020.
[39] 谭清群. 苹果炭疽叶枯病菌CPA1基因的鉴定与功能分析[D]. 长沙:湖南农业大学,2021.
TAN Qingqun. Identification and functional analysis of CPA1 in Colletotrichum gloeosporioides[D]. Changsha:Hunan Agricultural University,2021.
[40] CHAGU? V,MAOR R,SHARON A. CgOpt1,a putative oligopeptide transporter from Colletotrichum gloeosporioides that is involved in responses to auxin and pathogenicity[J]. BMC Microbiology,2009,9:173.
[41] KIM Y K,WANG Y H,LIU Z M,KOLATTUKUDY P E. Identification of a hard surface contact-induced gene in Colletotrichum gloeosporioides conidia as a sterol glycosyl transferase,a novel fungal virulence factor[J]. The Plant Journal,2002,30(2):177-187.
[42] LIANG X F,CAO M Y,LI S,KONG Y Y,ROLLINS J A,ZHANG R,SUN G Y. Highly contiguous genome resource of Colletotrichum fructicola generated using long-read sequencing[J]. Molecular Plant-Microbe Interactions,2020,33(6):790-793.
[43] LIU Q G,WANG Z C,XU X M,ZHANG H Z,LI C H. Genome-wide analysis of C2H2 zinc-finger family transcription factors and their responses to abiotic stresses in poplar (Populus trichocarpa)[J]. PLoS One,2015,10(8):e0134753.
[44] LI X Y,KE Z J,YU X J,LIU Z Q,ZHANG C H. Transcription factor CgAzf1 regulates melanin production,conidial development and infection in Colletotrichum gloeosporioides[J]. Antonie Van Leeuwenhoek,2019,112(7):1095-1104.
[45] WANG P,LI B,PAN Y T,ZHANG Y Z,LI D W,HUANG L. Calcineurin-responsive transcription factor CgCrzA is required for cell wall integrity and infection-related morphogenesis in Colletotrichum gloeosporioides[J]. The Plant Pathology Journal,2020,36(5):385-397.
[46] LIU W K,LIANG X F,GLEASON M L,CAO M Y,ZHANG R,SUN G Y. Transcription factor CfSte12 of Colletotrichum fructicola is a key regulator of early apple Glomerella leaf spot pathogenesis[J]. Applied and Environmental Microbiology,2020,87(1):e02212-e02220.
[47] 刘欢庆,周双针,高菁,王金泓,唐雯,柳志强,李晓宇. 暹罗炭疽菌同源异型盒转录因子CsHtf1的生物学功能[J]. 微生物学通报,2023,50(12):5350-5362.
LIU Huanqing,ZHOU Shuangzhen,GAO Jing,WANG Jinhong,TANG Wen,LIU Zhiqiang,LI Xiaoyu. Biological function of a homeobox transcription factor CsHtf1 in Colletotrichum siamense[J]. Microbiology China,2023,50(12):5350-5362.
[48] SUN Y J,WANG Y L,TIAN C M. bZIP transcription factor CgAP1 is essential for oxidative stress tolerance and full virulence of the poplar anthracnose fungus Colletotrichum gloeosporioides[J]. Fungal Genetics and Biology,2016,95:58-66.
[49] LI X Y,WU Y T,LIU Z Q,ZHANG C H. The function and transcriptome analysis of a bZIP transcription factor CgAP1 in Colletotrichum gloeosporioides[J]. Microbiological Research,2017,197:39-48.
[50] LIU W K,HAN L,CHEN J Z,LIANG X F,WANG B,GLEASON M L,ZHANG R,SUN G Y. The CfMcm1 regulates pathogenicity,conidium germination,and sexual development in Colletotrichum fructicola[J]. Phytopathology,2022,112(10):2159-2173.
[51] ELLIS J G,RAFIQI M,GAN P,CHAKRABARTI A,DODDS P N. Recent progress in discovery and functional analysis of effector proteins of fungal and oomycete plant pathogens[J]. Current Opinion in Plant Biology,2009,12(4):399-405.
[52] RAFIQI M,ELLIS J G,LUDOWICI V A,HARDHAM A R,DODDS P N. Challenges and progress towards understanding the role of effectors in plant-fungal interactions[J]. Current Opinion in Plant Biology,2012,15(4):477-482.
[53] SHIMADA C,LIPKA V,OCONNELL R,OKUNO T,SCHULZE-LEFERT P,TAKANO Y. Nonhost resistance in Arabidopsis-Colletotrichum interactions acts at the cell periphery and requires actin filament function[J]. Molecular Plant-Microbe Interactions,2006,19(3):270-279.
[54] GAN P,IKEDA K,IRIEDA H,NARUSAKA M,OCONNELL R J,NARUSAKA Y,TAKANO Y,KUBO Y,SHIRASU K. Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi[J]. New Phytologist,2013,197(4):1236-1249.
[55] OCONNELL R J,THON M R,HACQUARD S,AMYOTTE S G,KLEEMANN J,TORRES M F,DAMM U,BUIATE E A,EPSTEIN L,ALKAN N,ALTM?LLER J,ALVARADO-BALDERRAMA L,BAUSER C A,BECKER C,BIRREN B W,CHEN Z H,CHOI J,CROUCH J A,DUVICK J P,FARMAN M A,GAN P,HEIMAN D,HENRISSAT B,HOWARD R J,KABBAGE M,KOCH C,KRACHER B,KUBO Y,LAW A D,LEBRUN M H,LEE Y H,MIYARA I,MOORE N,NEUMANN U,NORDSTR?M K,PANACCIONE D G,PANSTRUGA R,PLACE M,PROCTOR R H,PRUSKY D,RECH G,REINHARDT R,ROLLINS J A,ROUNSLEY S,SCHARDL C L,SCHWARTZ D C,SHENOY N,SHIRASU K,SIKHAKOLLI U R,ST?BER K,SUKNO S A,SWEIGARD J A,TAKANO Y,TAKAHARA H,TRAIL F,VAN DER DOES H C,VOLL L M,WILL I,YOUNG S,ZENG Q D,ZHANG J Z,ZHOU S G,DICKMAN M B,SCHULZE-LEFERT P,VAN THEMAAT E V L,MA L J,VAILLANCOURT L J. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses[J]. Nature Genetics,2012,44(9):1060-1065.
[56] SHANG S P,LIU G L,ZHANG S,LIANG X F,ZHANG R,SUN G Y. A fungal CFEM-containing effector targets NPR1 regulator NIMIN2 to suppress plant immunity[J]. Plant Biotechnology Journal,2024,22(1):82-97.
[57] LIU L P,XU L,JIA Q,PAN R,OELM?LLER R,ZHANG W Y,WU C. Arms race:Diverse effector proteins with conserved motifs[J]. Plant Signaling & Behavior,2019,14(2):1557008.
[58] SHANG S P,WANG B,ZHANG S,LIU G L,LIANG X F,ZHANG R,GLEASON M L,SUN G Y. A novel effector CfEC92 of Colletotrichum fructicola contributes to glomerella leaf spot virulence by suppressing plant defences at the early infection phase[J]. Molecular Plant Pathology,2020,21(7):936-950.
[59] 王美玉. 苹果炭疽叶枯病菌效应蛋白Sntf2抑制植物免疫的分子机制研究[D]. 北京:中国农业科学院,2022.
WANG Meiyu. Molecular mechanism of inhibition of plant immunity by effector Sntf2 from Colletotrichum gloeosporioides[D]. Beijing:Chinese Academy of Agricultural Sciences,2022.
[60] KHODADADI F,GONZ?LEZ J B,MARTIN P L,GIROUX E,BILODEAU G J,PETER K A,DOYLE V P,A?IMOVI? S G. Identification and characterization of Colletotrichum species causing apple bitter rot in New York and description of C. noveboracense sp. nov.[J]. Scientific Reports,2020,10(1):11043.
[61] PENG Y J,VAN WERSCH R,ZHANG Y L. Convergent and divergent signaling in PAMP-triggered immunity and effector-triggered immunity[J]. Molecular Plant-Microbe Interactions,2018,31(4):403-409.
[62] COUTO D,ZIPFEL C. Regulation of pattern recognition receptor signalling in plants[J]. Nature Reviews Immunology,2016,16(9):537-552.
[63] TANG D Z,WANG G X,ZHOU J M. Receptor kinases in plant-pathogen interactions:More than pattern recognition[J]. The Plant Cell,2017,29(4):618-637.
[64] BIA?AS A,ZESS E K,DE LA CONCEPCION J C,FRANCESCHETTI M,PENNINGTON H G,YOSHIDA K,UPSON J L,CHANCLUD E,WU C H,LANGNER T,MAQBOOL A,VARDEN F A,DEREVNINA L,BELHAJ K,FUJISAKI K,SAITOH H,TERAUCHI R,BANFIELD M J,KAMOUN S. Lessons in effector and NLR biology of plant-microbe systems[J]. Molecular Plant-Microbe Interactions,2018,31(1):34-45.
[65] JONES J D G,DANGL J L. The plant immune system[J]. Nature,2006,444(7117):323-329.
[66] TSUDA K,SATO M,STODDARD T,GLAZEBROOK J,KATAGIRI F. Network properties of robust immunity in plants[J]. PLoS Genetics,2009,5(12):e1000772.
[67] DODDS P N,RATHJEN J P. Plant immunity:Towards an integrated view of plant-pathogen interactions[J]. Nature Reviews Genetics,2010,11(8):539-548.
[68] QI Y P,TSUDA K,GLAZEBROOK J,KATAGIRI F. Physical association of pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) immune receptors in Arabidopsis[J]. Molecular Plant Pathology,2011,12(7):702-708.
[69] 吴建圆,王娜,冀志蕊,迟福梅,周宗山,张俊祥. 苹果炭疽叶枯病菌致病力分析及苹果种质抗病性鉴定[J]. 植物遗传资源学报,2017,18(2):210-216.
WU Jianyuan,WANG Na,JI Zhirui,CHI Fumei,ZHOU Zongshan,ZHANG Junxiang. Pathogenicity differentiation of pathogen causing Glomerella leaf spot of apple (GLSA) and evaluation of resistance to GLSA in apple germplasms[J]. Journal of Plant Genetic Resources,2017,18(2):210-216.
[70] ZHANG F,WANG F,YANG S,ZHANG Y Y,XUE H,WANG Y S,YAN S P,WANG Y,ZHANG Z H,MA Y. MdWRKY100 encodes a group Ⅰ WRKY transcription factor in Malus domestica that positively regulates resistance to Colletotrichum gloeosporioides infection[J]. Plant Science,2019,286:68-77.
[71] 刘瑛双,房中文,TURAKULOV K S,刘春晓,董超华,张玉刚. 接种苹果炭疽叶枯病菌对苹果叶片相关酶活性及基因表达的影响[J]. 青岛农业大学学报(自然科学版),2021,38(3):157-163.
LIU Yingshuang,FANG Zhongwen,TURAKULOV K S,LIU Chunxiao,DONG Chaohua,ZHANG Yugang. Effects on related enzymes activity and gene expression analysis of inoculate apple leaves under Glomerella leaf spot stress[J]. Journal of Qingdao Agricultural University (Natural Science),2021,38(3):157-163.
[72] 吴成成,李婷,赵芮嘉,李保华,梁文星,王彩霞. 苹果MpSNAT1的克隆与表达特性分析[J]. 植物生理学报,2018,54(5):872-878.
WU Chengcheng,LI Ting,ZHAO Ruijia,LI Baohua,LIANG Wenxing,WANG Caixia. Cloning and expression characterization of MpSNAT1 in Malus pumila[J]. Plant Physiology Journal,2018,54(5):872-878.
[73] 吴芳芳,檀根甲. 苹果感染炭疽病菌后6种酶活性的变化[J]. 安徽农业大学学报,2004,31(1):46-50.
WU Fangfang,TAN Genjia. Changes in six kinds of enzyme activities in apple fruit inoculatedwith Colletotrichum gloeosporioides[J]. Journal of Anhui Agricultural University,2004,31(1):46-50.
[74] 吴芳芳,郑有飞,胡正华,陈魁. UV-B辐射增强对苹果炭疽菌生长特性及其过氧化氢酶活性的影响[J]. 生态环境,2008,17(1):158-162.
WU Fangfang,ZHENG Youfei,HU Zhenghua,CHEN Kui. Effects of enhancing ultraviolet-B radiation on grown character and catalase activity in Colletitrichum gloeosporiodes[J]. Ecology and Environment,2008,17(1):158-162.
[75] 孔祥华,侯董亮,张伟,田义轲,刘源霞,王彩虹. 炭疽叶枯病菌诱导的不同苹果种质中防御酶活性及丙二醛含量比较[J]. 青岛农业大学学报(自然科学版),2017,34(1):5-8.
KONG Xianghua,HOU Dongliang,ZHANG Wei,TIAN Yike,LIU Yuanxia,WANG Caihong. Comparison of defensive enzymes activities and malonaldehyde content induced by Glomerella cingulata among different apple resources[J]. Journal of Qingdao Agricultural University (Natural Science),2017,34(1):5-8.
[76] 薛莲,檀根甲,徐先松,李丽,韩翔. 苹果炭疽病菌对苹果果实致病机制初探[J]. 安徽农业大学学报,2006,33(4):522-525.
XUE Lian,TAN Genjia,XU Xiansong,LI Li,HAN Xiang. Pathogenesis of the cell wall degrading enzymes produced by Colletotrichum gloeosporioides[J]. Journal of Anhui Agricultural University,2006,33(4):522-525.
[77] 朱学明,史祥鹏,雍道敬,张颖,李保华,梁文星,王彩霞. 内生放线菌A-1诱导苹果对炭疽叶枯病的抗性[J]. 植物生理学报,2015,51(6):949-954.
ZHU Xueming,SHI Xiangpeng,YONG Daojing,ZHANG Ying,LI Baohua,LIANG Wenxing,WANG Caixia. Induction of resistance against Glomerella cingulata in apple by endophytic actinomycetes strain A-1[J]. Plant Physiology Journal,2015,51(6):949-954.
[78] BARI R,JONES J D G. Role of plant hormones in plant defence responses[J]. Plant Molecular Biology,2009,69(4):473-488.
[79] TANG L G,YANG G G,MA M,LIU X F,LI B,XIE J T,FU Y P,CHEN T,YU Y,CHEN W D,JIANG D H,CHENG J S. An effector of a necrotrophic fungal pathogen targets the calcium-sensing receptor in chloroplasts to inhibit host resistance[J]. Molecular Plant Pathology,2020,21(5):686-701.
[80] DEMPSEY D A,VLOT A C,WILDERMUTH M C,KLESSIG D F. Salicylic acid biosynthesis and metabolism[J]. The Arabidopsis Book,2011,9:e0156.
[81] ZHANG Y,SHI X P,LI B H,ZHANG Q M,LIANG W X,WANG C X. Salicylic acid confers enhanced resistance to Glomerella leaf spot in apple[J]. Plant Physiology and Biochemistry,2016,106:64-72.
[82] 赵妍,王晨. 水杨酸处理对苹果采后品质及炭疽病害的影响[J]. 食品工业,2015,36(9):195-198.
ZHAO Yan,WANG Chen. Influence of salicylic acid on quality and anthracnose in postharvest apple fruit[J]. The Food Industry,2015,36(9):195-198.
[83] 何晓文,孟慧,张家虎,范昆,李林光. ‘嘎拉苹果及其杂交后代对炭疽叶枯病的抗性机制分析[J]. 西北植物学报,2022,42(6):983-993.
HE Xiaowen,MENG Hui,ZHANG Jiahu,FAN Kun,LI Linguang. Resistance analysis of ‘Gala and F1 individuals to Glomerella leaf spot[J]. Acta Botanica Boreali-Occidentalia Sinica,2022,42(6):983-993.
[84] 张朝红,陈东玫,杨凤秋,赵同生,赵国栋,李扬,赵永波. 苹果种质及杂种对炭疽菌叶枯病的田间抗性分析[J]. 河北农业科学,2018,22(3):42-46.
ZHANG Chaohong,CHEN Dongmei,YANG Fengqiu,ZHAO Tongsheng,ZHAO Guodong,LI Yang,ZHAO Yongbo. Field resistance of apple germplasm and hybrids to Glomerella leaf spot[J]. Journal of Hebei Agricultural Sciences,2018,22(3):42-46.
[85] 马玉鑫. ‘寒富苹果CDPK基因家族分析及MdCDPK24对苹果炭疽病抗性功能鉴定[D]. 沈阳:沈阳农业大学,2023.
MA Yuxin. Analysis of CDPK gene family and identification of resistance function of MdCDPK24 to Colletotrichum gloeosporioides in ‘Hanfu apple[D]. Shenyang:Shenyang Agricultural University,2023.
[86] 赵伟玉. ‘寒富苹果CaMBP基因家族分析及MdCaMBP6抗苹果炭疽叶枯病的功能鉴定[D]. 沈阳:沈阳农业大学,2023.
ZHAO Weiyu. Analysis of CaMBP gene family in ‘Hanfu apple and function identification of MdCaMBP6 against apple Glomerella leaf spot[D]. Shenyang:Shenyang Agricultural University,2023.
[87] 史佳俊. 苹果细胞分裂素合成关键酶基因MdIPT8在抗炭疽病中的功能解析[D]. 沈阳:沈阳农业大学,2022.
SHI Jiajun. Functional analysis of MdIPT8,a key enzyme gene for cytokinin synthesis in apple,in resistance to anthracnose[D]. Shenyang:Shenyang Agricultural University,2022.
[88] GUO T L,BAO R,YANG Z H,FU X M,HU L,WANG N,LIU C H,MA F W. The m6A reader MhYTP2 negatively modulates apple Glomerella leaf spot resistance by binding to and degrading MdRGA2L mRNA[J]. Molecular Plant Pathology,2023,24(10):1287-1299.
[89] 刘源霞,李保华,王彩虹,刘春晓,孔祥华,祝军,戴洪义. 苹果对炭疽菌叶枯病抗性遗传的研究及其分子标记筛选[J]. 园艺学报,2015,42(11):2105-2112.
LIU Yuanxia,LI Baohua,WANG Caihong,LIU Chunxiao,KONG Xianghua,ZHU Jun,DAI Hongyi. Genetic studies and molecular markers screening of apple resistance to Glomerella leaf spot[J]. Acta Horticulturae Sinica,2015,42(11):2105-2112.
[90] 刘源霞,兰进好,柏素花,孙晓红,刘春晓,张玉刚,戴洪义. 苹果抗炭疽菌叶枯病基因SNP和InDel标记的HRM筛选[J]. 园艺学报,2017,44(2):215-222.
LIU Yuanxia,LAN Jinhao,BAI Suhua,SUN Xiaohong,LIU Chunxiao,ZHANG Yugang,DAI Hongyi. Screening of SNP and InDel markers to Glomerella leaf spot resistance gene locus in apple using HRM technology[J]. Acta Horticulturae Sinica,2017,44(2):215-222.
[91] 刘春晓,兰进好,侯鸿敏,张玉刚,戴洪义,刘源霞. 苹果抗炭疽菌叶枯病基因相关的4个分子标记的准确性验证[J]. 园艺学报,2017,44(7):1355-1362.
LIU Chunxiao,LAN Jinhao,HOU Hongmin,ZHANG Yugang,DAI Hongyi,LIU Yuanxia. Accuracy test of four molecular markers of Glomerella leaf spot resistant gene in apple cultivars[J]. Acta Horticulturae Sinica,2017,44(7):1355-1362.
[92] LI W X,PANG S Y,LU Z G,JIN B. Function and mechanism of WRKY transcription factors in abiotic stress responses of plants[J]. Plants,2020,9(11):1515.
[93] 谷彦冰. 苹果两个WRKY转录因子的克隆和表达分析[D]. 北京:中国农业科学院,2016.
GU Yanbing. Cloning and expression analysis of two WRKY transcription factors in apple (Malus domestica Borkh.)[D]. Beijing:Chinese Academy of Agricultural Sciences,2016.
[94] SHAN D Q,CHANYU W,ZHENG X D,HU Z H,ZHU Y P,ZHAO Y,JIANG A W,ZHANG H X,SHI K,BAI Y X,YAN T C,WANG L,SUN Y Z,LI J F,ZHOU Z Y,GUO Y,KONG J. MKK4-MPK3-WRKY17-mediated salicylic acid degradation increases susceptibility to Glomerella leaf spot in apple[J]. Plant Physiology,2021,186(2):1202-1219.
[95] ZHAO X Y,QI C H,JIANG H,ZHONG M S,YOU C X,LI Y Y,HAO Y J. MdWRKY15 improves resistance of apple to Botryosphaeria dothidea via the salicylic acid-mediated pathway by directly binding the MdICS1 promoter[J]. Journal of Integrative Plant Biology,2020,62(4):527-543.
[96] JI Z R,WANG M Y,ZHANG S W,DU Y N,CONG J L,YAN H F,GUO H M,XU B L,ZHOU Z S. GDSL esterase/lipase GELP1 involved in the defense of apple leaves against Colletotrichum gloeosporioides infection[J]. International Journal of Molecular Sciences,2023,24(12):10343.
[97] LI Y X,CUI Y L,LIU B Y,XU R X,SHI Y J,LV L L,WANG H T,SHANG Y M,LIANG W,MA F W,LI C Y. γ-aminobutyric acid plays a key role in alleviating Glomerella leaf spot in apples[J]. Molecular Plant Pathology,2023,24(6):588-601.
[98] LIPPOK B,BIRKENBIHL R P,RIVORY G,BR?MMER J,SCHMELZER E,LOGEMANN E,SOMSSICH I E. Expression of AtWRKY33 encoding a pathogen- or PAMP-responsive WRKY transcription factor is regulated by a composite DNA motif containing W-box elements[J]. Molecular Plant-Microbe Interactions,2007,20(4):420-429.
[99] ZHAO X Y,QI C H,JIANG H,ZHONG M S,YOU C X,LI Y Y,HAO Y J. MdHIR4 transcription and translation levels associated with disease in apple are regulated by MdWRKY31[J]. Plant Molecular Biology,2019,101(1/2):149-162.
[100] HOU Y J,YU X Y,CHEN W P,ZHUANG W B,WANG S H,SUN C,CAO L F,ZHOU T T,QU S C. MdWRKY75e enhances resistance to Alternaria alternata in Malus domestica[J]. Horticulture Research,2021,8(1):225.
[101] ZHANG X W,XU R R,LIU Y K,YOU C X,AN J P. MdVQ10 promotes wound-triggered leaf senescence in association with MdWRKY75 and undergoes antagonistic modulation of MdCML15 and MdJAZs in apple[J]. The Plant Journal,2023,115(6):1599-1618.
[102] ZHANG S S,WU Y Q,HUANG X,WU W L,LYU L F,LI W L. Research progress about microRNAs involved in plant secondary metabolism[J]. International Journal of Biological Macromolecules,2022,216:820-829.
[103] SALVADOR-GUIRAO R,BALDRICH P,WEIGEL D,RUBIO-SOMOZA I,SAN SEGUNDO B. The microRNA miR773 is involved in the Arabidopsis immune response to fungal pathogens[J]. Molecular Plant-Microbe Interactions,2018,31(2):249-259.
[104] ZHANG Y,ZHANG Q L,HAO L,WANG S N,WANG S Y,ZHANG W N,XU C R,YU Y F,LI T Z. A novel miRNA negatively regulates resistance to Glomerella leaf spot by suppressing expression of an NBS gene in apple[J]. Horticulture Research,2019,6:93.
[105] ZHANG Q L,XU C R,WEI H Y,FAN W Q,LI T Z. Two pathogenesis-related proteins interact with leucine-rich repeat proteins to promote Alternaria leaf spot resistance in apple[J]. Horticulture Research,2021,8:219.
[106] ZHANG Q L,WANG Y H,WEI H Y,FAN W Q,XU C R,LI T Z. CCR-NB-LRR proteins MdRNL2 and MdRNL6 interact physically to confer broad-spectrum fungal resistance in apple (Malus × domestica)[J]. The Plant Journal,2021,108(5):1522-1538.
[107] 张亚楠,陈新慧,张杰. 苹果炭疽病抗性miRNA的筛选[J]. 中国农学通报,2021,37(7):106-111.
ZHANG Yanan,CHEN Xinhui,ZHANG Jie. Screening of Malus miRNA:Mediated resistance to Colletotrichum gloeosporioides[J]. Chinese Agricultural Science Bulletin,2021,37(7):106-111.
[108] SHEN X X,PING Y K,BAO C N,LIU C,TAHIR M M,LI X W,SONG Y,XU W R,MA F W,GUAN Q M. Mdm-miR160-MdARF17-MdWRKY33 module mediates freezing tolerance in apple[J]. The Plant Journal,2023,114(2):262-278.
[109] YU X Y,HOU Y J,CAO L F,ZHOU T T,WANG S H,HU K X,CHEN J R,QU S C. MicroRNA candidate miRcand137 in apple is induced by Botryosphaeria dothidea for impairing host defense[J]. Plant Physiology,2022,189(3):1814-1832.
[110] LIU K,YANG A,YAN J D,LIANG Z L,YUAN G P,CONG P H,ZHANG L Y,HAN X L,ZHANG C X. MdAIL5 overexpression promotes apple adventitious shoot regeneration by regulating hormone signaling and activating the expression of shoot development-related genes[J]. Horticulture Research,2023,10(11):uhad198.
收稿日期:2024-03-13 接受日期:2024-04-07
基金项目:中央级公益性科研院所基本科研业务费专项(1610182023012);国家现代农业产业技术体系(CARS-27);中国农业科学院科技创新工程专项(CAAS-ASTIP-2021-RIP-05)
作者简介:冀志蕊,女,在读博士研究生,研究方向为果树病害流行与综合防控。Tel:0429-3598236,E-mail:xinyu_jzr@163.com
*通信作者 Author for correspondence. E-mail:xubl@gsau.edu.cn;Tel:0429-3598268,E-mail:zhouzongshan@caas.cn
