Mechanical force-mediated interactions between cancer cells and fibroblasts and their role in the progression of hepatocellular carcinoma
2024-05-17ZhengPengYanlingDingHongyuZhangXiaMengYiyongHuangPengfeiZhangZepengLiXiaolingZhou
Zheng Peng, Yanling Ding, Hongyu Zhang, Xia Meng, Yiyong Huang, Pengfei Zhang, Zepeng Li,Xiaoling Zhou
1Department of Clinical Laboratory, Liuzhou Traditional Chinese Medical Hospital, Liuzhou 545001, Guangxi, China.
2Department of Clinical Laboratory, Liuzhou Maternity and Child Healthcare Hospital, Liuzhou 545001, Guangxi, China.
3Department of Pulmonary and Critical Care Medicine, Liuzhou Traditional Chinese Medical Hospital, Liuzhou 545001, Guangxi,China.
4Department of Gastroenterology, Liuzhou Traditional Chinese Medical Hospital, Liuzhou 545001, Guangxi, China.
Abstract Mechanical forces play a key role in the initiation and progression of cancer.Intercellular interactions between fibroblasts and cancer cells contribute a large portion of the mechanical forces in tumor tissue.Hence, further investigation of the mechanical force-mediated intercellular interactions between cancer cells and fibroblasts is urgently needed, given the slow progress in the management of various solid cancers.In our previous study, we observed obvious mechanical force-mediated interactions between hepatocellular carcinoma (HCC) cells and fibroblasts through integrins and ECM proteins by using our coculture model and discovered that these interactions play important roles in 3D structure formation and tumor growth, suggesting their potential application in HCC treatment.In this review, we summarize the recent research progress in this field in hopes of providing insight into the development of potential anticancer strategies, with a special focus on HCC.
Keywords: Mechanical force, intercellular interactions, fibroblast, hepatocellular carcinoma
INTRODUCTION
A solid tumor comprises not only cancer cells or cancer stem cells but also a heterogeneous group of host stromal cells, an intricate network of extracellular matrix (ECM), and a series of secreted factors, which are known as the tumor microenvironment (TME)[1].Instead of being considered a silent and passive“bystander”, the TME is now believed to be a key promoter of cancer progression through active interactions with cancer cells[2].
From the perspective of physics, on the one hand, uncontrolled growth of solid tumors in confined spaces causes a dramatic increase in compressive pressure on the surrounding TME[3,4]; on the other hand, cancer cells are continuously exposed to mechanical forces in the TME, including compressional and tensional forces[5].Moreover, many solid tumors, including hepatocellular carcinoma (HCC), share several characteristics, such as the loss of mechanical homeostasis, changes in tissue-level mechanical forces, and the stiffening of pathological tissue[6-8].Initially, these physical characteristics were perceived as the natural consequences of tumor development until scientists discovered that these mechanical properties could also contribute to many key biological events in tumor progression, such as cancer cell proliferation/survival[9,10]and epithelial-mesenchymal transition (EMT)[11,12].Since then, mechanical forces have attracted increasing attention in cancer-related studies[13-18].For example, it has been reported that mechanical forces can direct cancer stem cell behavior during development and metastasis[18].
Mechanical forces stimulate cancer cells through mechanoreceptors, while cancer cells respond by exerting reciprocal forces generated by actin and myosin networks, e.g., myosin-generated contraction; this process is termed mechanoreciprocity[19].Mechanoreciprocity can maintain tensional homeostasis in tumor tissue,and its loss promotes disease progression, including invasion and metastasis[20].For instance, scientists initially discovered that cancer cells possess unique mechanical properties compared to their normal counterparts[21].The TME also has several mechanical characteristics, which could result in disturbances in cellular cytoskeletal tension and mechanosignaling transduction[22,23]and ultimately contribute to malignant transformation, tumorigenesis, invasion, and metastasis[22,24,25].
Among all stromal cells in the TME, cancer-associated fibroblasts (CAFs) are the most abundant cell type[26].Under normal physiological conditions, fibroblasts are quiescent spindle-shaped cells distributed in the connective tissue of different organs[27].Nevertheless, they display an activated phenotype in cancerous tissue[28].Initially, scientists believed that CAFs are activated by secreted factors from cancer cells, as experiments have shown that conditioned medium from cancer cells has similar effects[29].However, this notion is being questioned by reports showing that physical interactions between stromal cells and cancer cells are also indispensable when fixed tumor cells and conditioned media are used separately[30].Moreover,maintenance of the myofibroblast phenotype reportedly depends on the mechanical tension within the tissue[31].
In addition to their indispensable role in ECM deposition, degradation, and remodeling, which ultimately changes the mechanical properties of the TME, CAFs have recently been found to also interact with tumor cells by exerting mechanical forces on tumor cells through physical contact[25,32].Moreover, CAFs can also form adhesive structures with cancer cells, such as focal adhesions and adhesion junctions.Taken together,these findings indicate that CAFs are key regulators of the biological behaviors of tumor cells, such as proliferation, migration, and invasion[33-35].Hence, understanding the mechanical aspects of these interactions may provide insights into the development of novel therapeutic strategies targeting the tumor microenvironment.
In this review, we focus on the recent progress in mechanical force-mediated interactions between cancer cells and fibroblasts with the aim of providing some insights into their biological basis, the three main generation mechanisms, the conversion of mechanical forces into biochemical signaling pathways, and their main effects on the biological behaviors of tumor cells.In the end, we elaborate on recent related advances in HCC research and introduce our findings during the experiments[36].We hope that this review will provide a better understanding of related issues and aid in the development of anticancer strategies[37].
THE BIOLOGICAL BASIS OF MECHANICAL FORCES GENERATED BY CAFS AND TUMOR CELLS
In normal tissue, mechanical homeostasis is maintained by many mechanisms, such as growth arrest and contact inhibition[38].However, during malignancy, cancer cells lose contact inhibition and undergo uncontrolled growth, which disrupts the delicate equilibrium between cell division and cell death(apoptosis), and generates mechanical stress on the surrounding cells[39].Moreover, by interacting with stromal cells, cancer cells actively remodel the ECM, resulting in increased deposition and crosslinking of fibrous proteins, which eventually hardens the cancerous tissue[40].As a result, cancerous tissue has a greater elastic modulus than its normal counterpart, as shown in Table 1[41-43].In this process, fibroblasts are activated into CAFs, which secrete various cytokines and matrix metalloproteinases (MMPs).
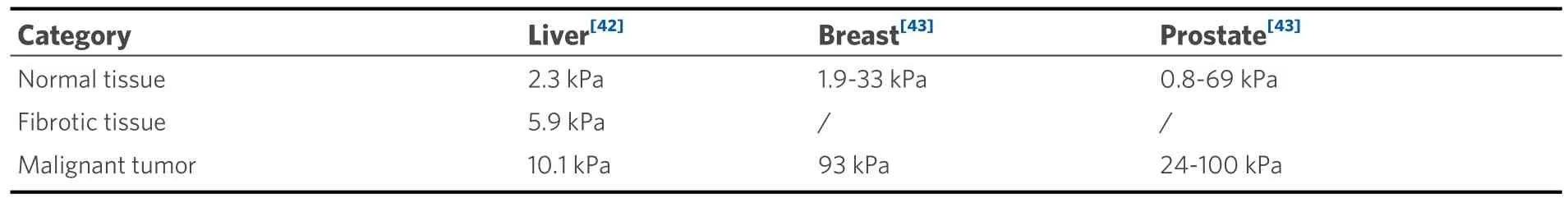
Table 1.The elastic modulus of normal and cancerous tissues
Moreover, from the cellular mechanics perspective, due to their key function in connective tissues,compared with other cell types, fibroblasts possess a more organized cytoskeleton that results in distinct mechanical properties, such as stiffer cell bodies and greater contractile forces [Table 2[44-49]].In addition,fibroblasts exhibit greater mobility than other cells[50,51].Due to their reorganized cytoskeleton and reduced cell-cell adhesion, cancer cells often have a lower elastic modulus than their normal counterparts[Table 2][52].
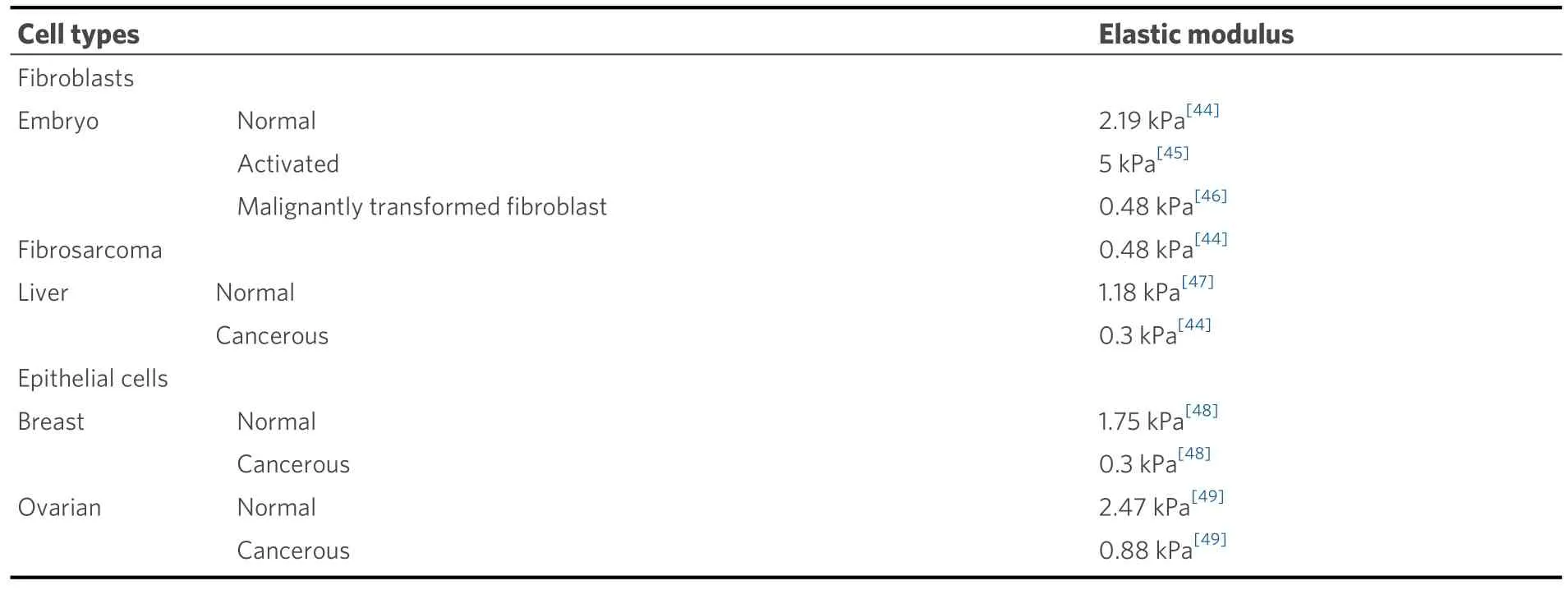
Table 2.The elastic modulus of a few common cell types
Third, scientists recently discovered that CAFs can form mechanically active heterophilic adhesions with cancer cells, as observed in patient-derived material[53], and can exert pulling forces on cancer cells while CAFs contract or migrate.These findings indicate a new mechanical force generation and transduction mechanism in which CAFs can lead cancer cells to invade by traction and dragging, giving rise to a new invasion and metastasis strategy named the “cooperative invasion/metastasis strategy”[53].
Finally, unlike cytokines secreted by cells, ECM proteins secreted by CAFs remain attached to their surface and can form a junction structure called the “fibronexus”, which is a specialized focal adhesion complex on the myofibroblast surface, as shown in Figure 1.Since many tumor cells, including liver cancer cells, tend to upregulate integrin expression, they easily adhere to ECM proteins attached to fibroblasts.In this way, these two cell types are connected by ECM proteins, and tensional force is generated and passed to cells connected when fibroblasts contract or migrate[36].Considering that crosstalk between CAFs and cancer cells, in large part, is carried out through the ECM, the involvement of ECM proteins and integrins in their interactions is highly important.
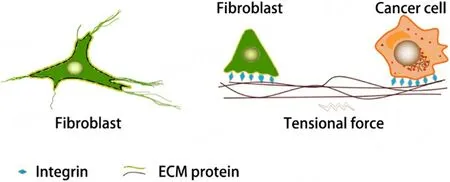
Figure 1.ECM proteins as mediators of mechanical force-mediated interactions between cancer cells and fibroblasts.The left panel shows the tight connection between ECM proteins and fibroblasts, while the right panel shows the mechanical interactions between fibroblasts and cancer cells mediated by ECM proteins.Tensional force is generated as fibroblasts migrate or contract.
THREE TYPES OF MECHANICAL FORCE-MEDIATED INTERCELLULAR INTERACTIONS BETWEEN CAFS AND TUMOR CELLS
Mechanical force-mediated intercellular interactions refer to the communication between cells that occurs through the application of physical forces[54].Mechanical forces can be generated through cell-cell or cellextracellular matrix interactions and can be transmitted through direct contact or through mechanical signaling pathways[55].Understanding the mechanisms by which cells sense and respond to mechanical forces can provide insights into the regulation of these processes and potentially lead to the development of therapeutic approaches for various diseases[56].
During normal physiological processes, cells are exposed to a variety of mechanical stimuli, including compression and tensile force[57].Specifically, for CAFs and tumor cells, we summarize three types of mechanical force-mediated intercellular interactions between CAFs and tumor cells, as shown below.
Direct extrusion through ECM remodeling mediated by CAFs (stiffening deposition topology reorganization)
During tumor expansion, a direct extrusion force will be naturally generated due to the limited growth space, as shown in Figure 2[10,58,59].However, the mechanical stress generated by tumor cells during expansion depends not only on their growth rate but also on the compliance of the surrounding ECM, in which CAFs play a crucial role[34].CAFs can change the components of the ECM by specifically upregulating secretion or degradation to affect its rigidity, as suggested by the finding that tumor cells can migrate along fibroblast protrusions[60].Moreover, the remodeling process generates physical forces that can influence tumor cell behavior[24].For example, the increased stiffness of the ECM due to CAF-mediated remodeling can promote tumor cell invasion[61]and migration[62].
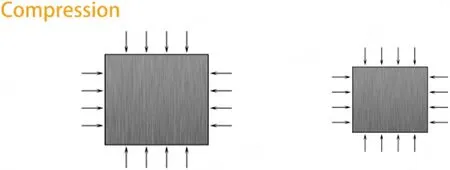
Figure 2.Diagram depicting the main effects of the compression force applied to tissues/cells.Compression stress is the kind of force applied perpendicularly to the cells, resulting in compaction, which is generated in conjunction with the uncontrolled proliferation of tumor cells[58] or the formation of CAF capsules[59].
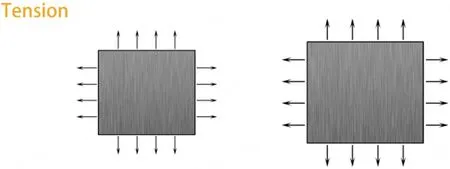
Figure 3.Diagram depicting the main effects of tensional force applied on tissues/cells.Tensional stress is the kind of force applied perpendicular to cells, resulting in expansion, which is usually generated after the formation of adhesion junctions with other cells.In addition to the direct adhesion junctions formed by cadherin, there are indirect adhesion interactions mediated by ECM proteins.
Indirect intercellular interactions mediated through the ECM and mechanoreceptors
Molecular mechanoreceptors include several families of transmembrane proteins, such as mechanosensitive ion channels and integrins.Mechanosensitive ion channels are ion channels that open in response to mechanical stimuli such as compression or tensional force, as shown in Figures 2 and 3[13].As another important mechanoreceptor, integrins play a crucial role in mechanosensing and transducing mechanical stimuli into biochemical signals that can be interpreted and responded to by the cell[63].Moreover, integrins can regulate mechanosensitive ion channels and form macromolecular complexes that help localize the channel to the plasma membrane and regulate downstream signaling proteins such as tyrosine kinases and GTPases[64].Common ECM ligands of integrins include fibronectin, laminin, and collagen[65].For example,integrin expression is reported to be much greater in many types of cancer cells than in their normal counterparts.It has been reported that mechanical forces and increased rigidity of the ECM can also upregulate integrin expression[13].Furthermore, integrin α5β1, in association with fibronectin, has been found to facilitate the differentiation of liver cancer cells and subsequently promote tumor formation and angiogenesis[36].
Heterotypic intercellular interactions (HIIs) mediated by shear forces and N-cadherin
Shear forces or stresses play a critical role in the mechanical aspects of heterotypic intercellular interactions.Shear forces are parallel forces that act in opposite directions, causing one layer of a material to slide or deform with respect to an adjacent layer, which can influence the nature of cell adhesion, migration, and communication[66].
Cadherins are a family of transmembrane proteins that are responsible for mediating cell-cell adhesion and are crucial for the formation and maintenance of adhesion junctions[67].E-cadherin primarily mediates homophilic interactions within the same cell type, whereas N-cadherin, on the other hand, plays a significant role in facilitating heterotypic interactions.The cadherin interactions between adjacent cells create strong cell-cell adhesion, which helps to maintain the structural integrity of tissues and allows for the coordination of cell movement and the transmission of mechanical forces[68].CAFs and other cells can also form heterotypic adhesion junctions with cancer cells via cadherin interactions and lead to collective migration or invasion; CAFs and other cells act as “leaders”, as their invasion is followed by cancer cell invasion[69].
MECHANOTRANSDUCTION: FROM MECHANICAL FORCES TO BIOCHEMICAL SIGNALS
Mechanotransduction refers to the biological process whereby cells convert mechanical stimuli or forces into biochemical signals through various mechanisms, including intracellular mechanosensory mechanisms[70].In addition to the activation of mechanosensitive ion channels, other membrane proteins and intracellular mechanosensory proteins can also play a role in converting mechanical forces into biochemical signals, such as adhesion receptors (integrin, cadherin), cytoskeletal proteins (actin, myosin),and signaling molecules (protein kinases, phosphatases)[71].Moreover, changes in force distributions across adhesion receptors result in both local and global restructuring of the tensionally integrated cytoskeleton network and cause subsequent biochemical signaling events[72].Mechanical forces trigger multiple signaling pathways, several of which converge and result in the activation of the GTPase RhoA and downstream consequences of this pathway[73].
Overall, mechanotransduction is a complex process that involves the coordination of the above components that interact with each other to modulate the cellular response to mechanical stimuli[70].Understanding the above mechanism is essential in various fields, including touch sensation, tissue engineering, and regenerative medicine, as it provides insights into how mechanical forces influence cell behavior, tissue function, and cancer development.
THE MAIN EFFECTS OF MECHANICAL FORCE-MEDIATED INTERCELLULAR INTERACTIONS BETWEEN CAFS AND TUMOR CELLS
As discussed above, mechanical stimuli can be transmitted and converted into biochemical signals through mechanical signaling pathways, which results in a series of biological events and effects, such as proliferation, survival, invasion, migration, and metastasis, as shown in Figure 4[22,74-79].The major effects are summarized below:

Figure 4.Mechanotransduction of cancer cells in the context of the TME and intercellular interactions and its main effects.Diagram showing the conversion of mechanical forces into biochemical signals within the context of mechanical force-mediated intercellular interactions in solid tumors.Intercellular interactions can be initiated by mechanical forces and can also result in mechanical forces such as compression and tension[83].Intercellular interactions can be initiated by the proliferation of cancer cells or the migration of connected fibroblasts, and mechanical force-mediated intercellular interactions can also promote the migration, invasion, proliferation,and survival of cancer cells[84].Mechanical forces can reinforce focal adhesions, stimulate the Rho-ROCK-MLC pathway, and increase cytoskeletal tension[85].Thus, these reactions will form a self-enforcing (positive) feedback loop.Crosstalk between the FAK-Src-ERK pathway and Rho-ROCK-MLC pathway leads to the activation of ERK and the formation of a FAK-Rho-ERK signaling loop, resulting in increased proliferation and survival[86].Src is a nonreceptor tyrosine kinase that can activate FAK, which in turn activates RAS and Rho signaling[87].Rho GTPases, including Rho, are involved in regulating the actin cytoskeleton and cell motility.Rho can activate c-Jun Nterminal kinase (JNK), which is a protein kinase that plays a crucial role in regulating migration, while c-JUN is a transcription factor that is a downstream target of the JNK signaling pathway.Moreover, activation of the JNK signaling pathway can promote metastasis[88].
Proliferation
Mechanical forces play a key role in spindle orientation, which is an important event during cell division[80].Notably, mechanical signaling can also regulate cell proliferation by coordinating focal adhesion assembly and reinforcing Rho GTPase signaling[81].Additionally, mechanical tension causes an increase in cytoskeletal tension, resulting in upregulated extracellular-signal-regulated kinase (ERK) activation[22].Similarly, indirect mechanical stimuli from ECM proteins increase integrin-related ERK signaling and focal adhesion clustering, which work together with other kinases, such as Src and FAK, to promote tumor cell proliferation and survival.The Hippo signaling pathway is another key regulator of tissue growth, organ size, and cell proliferation[82].Mechanical signals can impact the Hippo pathway through several mechanisms.For example, when cells experience high mechanical stress or are subjected to increased extracellular matrix stiffness, the Hippo pathway can be activated, resulting in the phosphorylation and inhibition of YAP/TAZ and, as a result, the activation of genes related to cell proliferation and survival[83].
Invasion
Cancer cells detach from a primary tumor, penetrate the surrounding tissue, and invade at the interface,which is a prerequisite for metastasis[84].As a long-neglected aspect of mechanical force, it is gaining increasing attention[61].During invasion, mechanical forces can modulate cancer cell motility in the TME with a complex architecture, while in intravasation and extravasation, mechanical forces could modulate the motility of cancer cells in the vasculature[62].Moreover, as discussed above, fibroblasts can work with cancer cells and adopt an invasion and metastasis method named the “cooperative invasion/metastasis strategy”, in which fibroblasts mechanically pull on the surrounding ECM and open up paths for cancer cells[53].
Moreover, mechanical forces could also promote the invasion of cancer cells in other ways.As previously reported, mechanical compression could facilitate a shift towards an invasive phenotype, in which a subset of cancer cells will extend filopodia at their leading edge[85].This finding was confirmed by another report that indicated malignant cells actively respond to the mechanical force-mediated stimuli in their TME[86].Further study revealed that invasion requires dramatic cytoskeleton remodeling, which can also be regulated by mechanical forces provided by the surrounding TME[24].Another study revealed that changes in ECM components influence key steps in cancer metastasis, such as adhesion, contractility, and motility[61].
Migration/metastasis
Metastasis accounts for more than 90% of cancer-related deaths and usually occurs together with migration after invasion[87].In addition to genetic factors, the mechanical force-mediated physical interactions of cancer cells with the TME are another key determinant of the metastatic process[62], since remarkable alterations in the mechanics of malignant cells and their surrounding TME are found during metastasis[88].Metastasis is a complex process involving the activation of various cellular signaling pathways, including the JNK and c-JUN signaling pathways.
RECENT RELATED ADVANCES IN HCC
Specifically, for liver cancer, scientists have shown that mechanical force-mediated intercellular interactions also occur between fibroblas61ts and liver cancer cells[32,53].In our related research project[36], to observe the direct mechanical interactions between liver cancer cells and fibroblasts, first, we labeled the two cell types with different fluorescent proteins by using lentivirus for live-cell imaging (liver cancer cells with tdTomato,red; fibroblasts with clover, green); second, we adopted the direct coculture method to study their physical intercellular interactions by live-cell imaging.Then, we observed that these two types of cells formed a 3D microstructure with a height of about 80 µm on day 10 [Figure 5A].However, the height fluctuated around 35 μm for monocultured HepG2-tdT cells and 20 μm for MEF-clover cells, respectively, on day 10[Figure 5B and C].Importantly, in immunofluorescence (IF) staining, when we labeled HepG2 cells with species-specific integrin-α5antibodies (which recognize only human antigens) to examine the microstructures after fixation and permeabilization, we observed obvious signs of mechanical forces, as indicated by the severely deformed tumor cell contours (as shown by the white arrows in Figure 5D).Combined with the presence of fibronectin and the distribution of fibroblasts (as shown by the typical nuclei in Figure 5D), these results clearly prove the occurrence of mechanical force-mediated intercellular interactions between these two cell types during coculture.Hence, we propose that after binding with fibronectin from fibroblasts, cancer cells are dragged by mechanical forces generated as fibroblasts migrate or contract (as demonstrated in Figure 1).In our further experiments, the administration of (±)-blebbistatin(a potent and selective inhibitor of myosin II ATPase) obviously abolished the formation of 3D microstructures (data not shown).All these results showed the indispensable role of intercellular mechanical forces in intercellular interactions between fibroblasts and liver cancer cells.
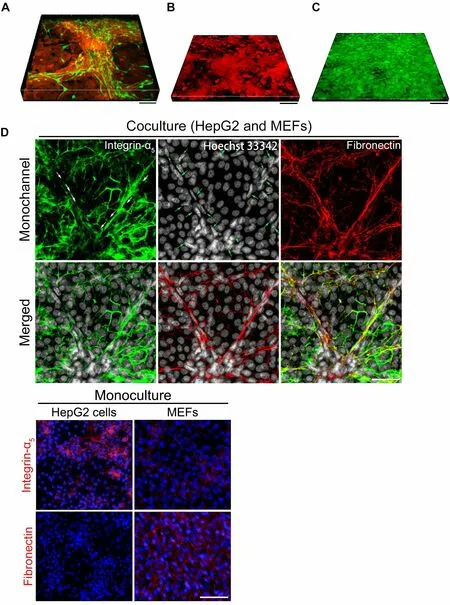
Figure 5.Evidence for the existence and effects of mechanical forces between fibroblasts and liver cancer cells in coculture.(A)Representative fluorescent live-cell image showing the 3D microstructure generated by the coculture of liver cancer cells and fibroblasts on day 10.HepG2-tdT cells (red) and MEF-clover cells (green) were plated together in a confocal dish at a ratio of 1 to 1.Scale bars, 100 μm.(B) Representative fluorescent live-cell image showing the 3D microstructure generated by monocultured HepG2-tdT cells on day 10.Scale bar, 100 μm.(C) Representative fluorescent live-cell image showing the 3D microstructure generated by monocultured MEF-clover cells on day 10.Scale bar, 100 μm.(D) Representative IF staining images of integrin-α5 (human antigens only), fibronectin, and nuclei in the coculture of HepG2 cells and MEFs.HepG2 cells and MEFs were plated together in a 6-well plate at a ratio of 1 to 1.Sterile coverslips were first placed in each well before plating.IF staining images of monocultured cells are provided below.Scale bars, 50 μm.Figures are modified with permission from Ref.[36] © (2022) the Ivyspring International Publisher.
Apart from direct interactions, fibroblasts can also exert their influence by actively being involved in ECM remodeling[89], which changes the stiffness or viscosity of the ECM; subsequently, these changes in substrate properties influence the behavior of HCC cells[90].Moreover, researchers have shown that, compared to normal hepatocytes, liver cancer cells have distinct lifetimes of binding to the substrate, which might explain their differences in cell spreading and motility within diseased tissue[91].
OPPORTUNITIES AND PROSPECTS
Understanding that mechanical force is an important determinant and the first and key step in understanding the pathogenesis of cancer naturally leads us to conclude that the loss of the ability to sense,respond to, and adapt appropriately to mechanical forces will eventually lead to malignancy[6].
To investigate mechanical force-mediated intercellular interactions, the development of appropriate models that allow for the measurement of mechanical forces and functional analysis with high resolution is an important prerequisite[92].To date, many different models have been developed to achieve this purpose, and they are broadly classified into two categories: two-dimensional models and three-dimensional models[93,94].On the one hand, two-dimensional systems could recapitulate both the architecture and the phenotypical behavior of different cell types with reasonable fidelity to some extent.On the other hand, threedimensional model systems can be used to study force and its effects on cell behavior in the context of an organized tissue structurein vitro.Primary or immortalized cells with synthetic hydrogels are used in these systems to mimicin vivoconditions, while protein and polysaccharide concentrations in these gels are manipulated to modify their mechanical properties to approximate the proportional elastic modulus needed[95].With the improvement of model systems, sooner or later, we will solve the mysteries of mechanical force-mediated intercellular interactions.
CONCLUSION
This review not only summarizes the mechanism underlying the generation of mechanical forces between CAFs and tumor cells but also covers the three main types of mechanical force-mediated intercellular interactions, the conversion of mechanical stimuli into intracellular biochemical signals, and the main effects of these interactions.Considering our latest research findings, we elaborate on the integrin- and ECM-mediated mechanical interactions between liver cancer cells and fibroblasts.Therefore, this review provides valuable insight into the mechanical force-mediated interactions between cancer cells and fibroblasts, especially in HCC.
DECLARATIONS
Authors’ contributions
Conceptualized the idea: Peng Z, Ding Y Provided resources: Zhou X, Li Z Helped with the resources: Ding Y, Meng X, Zhang P, Huang Y Wrote the manuscript: Zhang H, Peng Z
Availability of data and materials
All the data utilized in this study are publicly available.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (No.82160882), the Guangxi Natural Science Foundation (No.2022JJA141302), and the Scientific Research Fund of Guangxi University of Chinese Medicine (No.2023MS044 and 2023LZ002).
Conflicts of interest
All the authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
杂志排行
Journal of Cancer Metastasis and Treatment的其它文章
- Feature interview with Dr.William C.CHO -“Clarivate 2023 Highly Cited Researcher”
- Prognostic value of clonal evolution identified by sequential FISH in untreated chronic lymphocytic leukaemia
- Fast-tracking drug development with biomarkers and companion diagnostics
- Leveraging metformin to combat hepatocellular carcinoma: its therapeutic promise against hepatitis viral infections
- Regulation and function of the RSK family in colorectal cancer
