Pachymic acid exerts antitumor activities by modulating the Wnt/β-catenin signaling pathway via targeting PTP1B
2024-05-12HaoZhangKunZhuXueFengZhangYiHuiDingBingZhuWenMengQingSongDingFanZhang
Hao Zhang, Kun Zhu, Xue-Feng Zhang, Yi-Hui Ding, Bing Zhu, Wen Meng, Qing-Song Ding, Fan Zhang✉
1Department of Emergency, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou 310006, Zhejiang, China
2Department of Cardiothoracic Surgery, The Third Affiliated Hospital of Qiqihar Medical College, Qiqihar 161000, Heilongjiang, China
3Department of Cardiothoracic Surgery, The Affiliated Heilongjiang Provincial Hospital of Harbin Institute of Technology, Harbin 150036,Heilongjiang, China
ABSTRACT Objective: To determine the inhibitory effects of pachymic acid on lung adenocarcinoma (LUAD) cells and elucidate its underlying mechanism.Methods: CCK-8, wound healing, Transwell, Western blot, tube formation, and immunofluorescence assays were carried out to measure the effects of various concentrations of pachymic acid on LUAD cell proliferation, metastasis, angiogenesis as well as autophagy.Subsequently, molecular docking technology was used to detect the potential targeted binding association between pachymic acid and protein tyrosine phosphatase 1B (PTP1B).Moreover,PTP1B was overexpressed in A549 cells to detect the specific mechanisms of pachymic acid.Results: Pachymic acid suppressed LUAD cell viability, metastasis as well as angiogenesis while inducing cell autophagy.It also targeted PTP1B and lowered PTP1B expression.However, PTP1B overexpression reversed the effects of pachymic acid on metastasis,angiogenesis, and autophagy as well as the expression of Wnt3a and β-catenin in LUAD cells.Conclusions: Pachymic acid inhibits metastasis and angiogenesis,and promotes autophagy in LUAD cells by modulating the Wnt/β-catenin signaling pathway via targeting PTP1B.
KEYWORDS: Pachymic acid; Lung adenocarcinoma; Protein tyrosine phosphatase 1B; Wnt/β-catenin signaling pathway;Metastasis; Angiogenesis; Autophagy
1.Introduction
Lung cancer exhibits high incidence and fatality rates among malignant tumors and brings heavy socio-economic burdens worldwide.According to histology, small cell lung cancer and nonsmall cell lung cancer remain two subtypes of lung cancer and the latter can be further divided into squamous cell carcinoma,adenocarcinoma, and large cell carcinoma[1].Lung adenocarcinoma(LUAD), which accounts for 80%-85% of all lung cancers, is the most prevalent form of adenocarcinoma[2].The malignancy of LUAD is relatively high, and distant metastasis may occur in 70%of patients at diagnosis[3].In spite of the improvements in multimodal treatment patterns, such as immunotherapy and radiotherapy,along with non-invasive surgical excision, the 5-year relative overall survival rate of LUAD patients remains at ~18%[4].Therefore,developing novel drugs for the treatment of LUAD is critical.
The diversified effects of traditional Chinese medicine (TCM)have been displayed in lung cancer.Compared with Western medicine, TCM has many components, fewer adverse reactions,and other characteristics, and has the effect of reducing toxicity and enhancing effect, improving life quality, and prolonging survival time[5,6].As one of the most unique and active components ofPoria cocos, pachymic acid possesses anti-tumor, anti-inflammatory,and antioxidant properties[7,8].A previous study demonstrated that pachymic acid may increase lung cancer cell apoptosisviathe activation of JNK and endoplasmic reticulum stress pathways dependent on reactive oxygen species[9].However, further investigations into the regulatory effects of pachymic acid on LUAD cell metastasis, angiogenesis, and autophagy, and the associated mechanisms are required.
Protein tyrosine phosphatase 1B (PTP1B) is associated with metabolic signaling and plays a role in the process of cancer and diabetes[10].A previous study demonstrated that PTP1B activates ERK1/2 to accelerate non-small cell lung cancer cell proliferation and metastasis[11].In addition, PTP1B deficiency inhibits adipocyte apoptosis in oval cells by metabolic recombination, thereby promoting autophagy, mitochondrial adaptation, and lipid droplet formation[12].PTP1B deficiency also eliminates endothelial dysfunction and aggravates angiogenesis and wound healingin vivo[13].Zhenget al.have proposed that PTP1B may modulate the Wnt/β-catenin signaling to drive hepatocellular carcinoma (HCC)[14].Interestingly, pachymic acid has been also reported to be involved in chronic kidney diseaseviathe Wnt/β-catenin signaling pathway[15].Considering the well-established oncogenic role of Wnt/β-catenin signaling in LUAD[16,17], it is reasonable to speculate that pachymic acid might inhibit LUAD by mediating PTP1B and the Wnt/β-catenin signaling.Based on the abovementioned aspects, the current work aimed to investigate the effect of pachymic acid on LUAD cell metastasis, angiogenesis, and autophagy, and unravel its underlying mechanism.
2.Materials and methods
2.1.Bioinformatics tools
SwissTargetPrediction database (http://www.swisstargetprediction.ch/) was used to predict the relationship between pachymic acid and PTP1B.
2.2.Cell culture
Human bronchial epithelial cell line (16HBE cells; cat.no MZ-1420, Ningbo Mingzhou Biotechnology Co., LTD) and LUAD cell line (A549 cells; cat.no.CRM-CCL-185, American Type Culture Collection) were hatched in Dulbecco’s Modified Eagle Medium(DMEM, Guangzhou Inst Technology Co., Ltd.) supplemented with 10% fetal bovine serum (FBS, Bioind) at 37 ℃ in an incubator with 5% CO2and 95% O2.Cells were treated with 20, 40, and 80 μM pachymic acid (cat.no.HY-N0371, MedChemExpress) for 24 h at 37 ℃[9,18].
2.3.Cell Counting Kit (CCK)-8 assay
A549 cells were plated in a 96-well plate (2×103cells/well), and treated with 10 μL CCK-8 (Kaiji Biotechnology Co.Ltd.) for 4 h.Absorbance was measured at 450 nm following 24, 48, and 72 h.
2.4.Wound healing assay
A549 cells (3×105cells/well) were dispensed in 6-well plates.Cells were grown in a monolayer, and a scratch was generated by using a 200-µL pipette tip.A total of three fields were randomly selected and imaged under an inverted light microscope (Olympus Corporation), and the cell migration rate was measured following 24 h of incubation at 37 ℃.
2.5.Transwell assay
A total of 1×104A549 cells were plated in the upper chambers of 24-well Transwell plates (pore size, 8 µm; Corning, Inc.) in a serum-deprived medium.The bottom chambers were loaded with a medium containing 10% FBS.Cells were submerged in 4%paraformaldehyde and dyed by crystal violet 24 h later.Stained cells were imaged under an inverted light microscope (Olympus Corporation) and evaluated using Quantity One software (Bio-Rad Laboratories, Inc.).
2.6.Western blot analysis
Total proteins were subjected to isolation and quantification employing RIPA lysis buffer and a Bradford Protein Assay kit (Blue Skies), respectively.A total of 30 μg protein/lane separated by SDS-PAGE on a 12% gel were then shifted to PVDF membranes.Membranes were blocked with 5% bovine serum albumin (BSA) and labeled with primary antibodies for matrix metallopeptidase 9 (MMP9) (1∶1 000; cat.no.ab76003, Abcam),matrix metallopeptidase 2 (MMP2) (1∶1 000; cat.no.ab92536,Abcam), vascular endothelial growth factor (VEGF) (1∶1 000; cat.no.AV202; Beyotime), LC3 (1∶1 000; cat.no.ab192890; Abcam),Beclin-1 (1∶1 000; cat.no.ab207612; Abcam), p62 (1∶1 000; cat.no.ab109012; Abcam), PTP1B (1∶1 000; cat.no.ab244207; Abcam),E-cadherin (1∶1 000; cat.no.ab40772; Abcam),N-cadherin (1∶1 000;cat.no.ab76011; Abcam), vimentin (1∶1 000; cat.no.ab92547;Abcam), Wnt3a (1∶1 000; cat.no.ab219412; Abcam), β-catenin(1∶1 000; cat.no.ab223075; Abcam) and GAPDH (1∶1 000; cat.no.ab9485; Abcam) at 4 ℃ overnight.Following primary incubation,membranes were incubated with goat anti-rabbit secondary antibody(1∶5 000; cat.no.ab6721; Abcam) for 1 h at room temperature.The visualization of proteins was conducted using the ECL kit (Pierce;Thermo Fisher Scientific, Inc.) and protein expression was quantified using Quantity One software (Bio-Rad Laboratories, Inc.).
2.7.Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was extracted from A549 cells using TRIzol®reagent (Generay Biotech, Co., Ltd.) and the reverse transcription(RT) of isolated total RNA was performed using a PrimeScript One Step RT-PCR kit (Takara Bio, Inc.).qPCR was performed using SYBR Green PCR kit (Beijing Dingguo Changsheng Biotechnology Co., Ltd.).The following thermocycling conditions were used for the qPCR: initial denaturation at 95 ℃ for 2 min;40 cycles of 94 ℃ for 15 s, 58 ℃ for 30 s, and 70 ℃ for 30 s;extension at 72 ℃ for 5 min.Based on the 2-ΔΔCqmethod, the processing ofPTP1Bexpression was performed, withGAPDHas an internal control.The primer sequences forPTP1B(PTPN1)are as follows: forward 5’-GGCCATTTACCAGTTGACCA-3’and reverse 5’-ATGACGACACCCCTGCTTTT-3’; forGAPDHforward 5’-CATGAGAAGTATGACAACAGCCT-3’ and reverse 5’-AGTCCTTCCACGATACCAAAGT-3’.
2.8.Tube formation assay
A549 cells were treated with pachymic acid and then cell supernatants were collected.A 96-well plate was coated with Matrigel, and human umbilical vein endothelial cells (HUVECs,BeNa Culture Collection) were cultured in 100 µL complete medium(4×104cells/well).Following 24 h, tubes were imaged using a light microscope (Olympus Corporation).
2.9.Immunofluorescence (IF)
A549 cells were stained using 4% paraformaldehyde, penetrated using 0.1% Triton X-10, and blocked using 5% non-fat dry milk.Subsequently, cells were incubated with a primary antibody specific to LC3 (1∶200; cat.no.ab192890; Abcam), followed by the incubation with FITC-conjugated IgG secondary antibody (1∶500;cat.no.ab150077; Abcam).Cell nuclei were stained using DAPI and visualized using an LSM 880 confocal microscope (Zeiss GmbH).
2.10.Molecular docking
To convert the acquired pachymic acid structure in the ChemDraw software into a mol2 format file, it was imported into OpenBabel software (version 2.2.1) for hydrogenation.Using the RCSB PDB webpage (https://www.rcsb.org/), the structure of PTP1B (PDB ID: 1C83) was obtained.To maintain the PTP1B protein structure,excess water molecules were removed and any irrelevant small ligands originally carried were expurgated in PyMOL software(version 2.2.0).After running, the specific docking energy value was displayedviaAutoDock (v4.2).Protein-Ligand Interaction Profiler(PLIP; https://plip-tool.biotec.tu-dresden.de/plip-web) was used for analysis.
2.11.Cell transfection
According to the manufacturer’s protocol, PTP1B overexpression plasmid (oe-PTP1B) and the corresponding empty vector (oe-NC)purchased from GeneChem, Inc.were transfected into cells using Lipofectamine®2000 at a final concentration of 50 nM at 37 ℃.Transfection efficiency was determined following 48 h.
2.12.Statistical analysis
Data are presented as mean ± standard deviation (SD).Each experiment was repeated at least three times.One-way ANOVA followed by Tukey’spost hoctest was used to demonstrate the differences among multiple groups.SPSS software (version 19.0;IBM Corp.) was used for statistical analysis.P<0.05 was the threshold of significance.
3.Results
3.1.Pachymic acid inhibits the viability, migration, invasion,and epithelial-mesenchymal transition (EMT) of LUAD cells
Different doses of pachymic acid (20, 40, and 80 μM) were used to treat human bronchial epithelial cell line 16HBE and LUAD cell line A549, and cell viability was determined using CCK-8 method.Pachymic acid at all doses exerted no significant effect on 16HBE cell viability.However, A549 cell viability was markedly decreased by pachymic acid in a dose-dependent manner (P<0.01) (Figure 1A).Results of wound healing and Transwell assays demonstrated that cell migration and invasion were markedly reduced following treatment with pachymic acid when compared with the control group (P<0.01) (Figure 1B-C).Moreover, the expression levels of metastasis-associated proteins MMP9 and MMP2 were markedly reduced by pachymic acid, compared with the control group(P<0.01) (Figure 1D).In addition, the expression levels of EMTassociated proteins were investigated.The expression level ofE-cadherin was markedly increased, while those ofN-cadherin and vimentin were markedly reduced by pachymic acid in a concentration-dependent manner (P<0.01) (Figure 1E).

Figure 1.PA inhibits the viability, migration, invasion, and epithelial-mesenchymal transition of LUAD cells.(A) Different concentrations of PA (20, 40, and 80 μM) were used to induce A549 cells, and cell viability was detected using a Cell Counting Kit-8 assay.(B) Cell migration and (C) invasion were measured using wound healing and Transwell assays, respectively.(D-E) Western blot analysis was used to detect the expression levels of invasion- and migrationassociated proteins as well as EMT-associated proteins.**P<0.01, ***P<0.001 vs. the control.PA: pachymic acid; LUAD: lung adenocarcinoma; EMT: epithelialmesenchymal transition.
3.2.Pachymic acid inhibits the angiogenesis of LUAD cells
The tube-forming ability of HUVECs was markedly decreased following the treatment of pachymic acid (Figure 2A).Moreover,Western blot analysis demonstrated that angiogenesis-associated VEGF expression was notably downregulated by pachymic acid in a concentration-dependent manner (P<0.01) (Figure 2B).
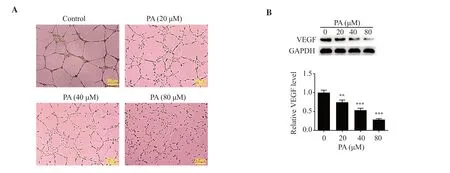
Figure 2.PA inhibits the angiogenesis of LUAD cells.(A) A tube formation assay was used to determine the effect of PA on the tube-forming ability of HUVECs.(B) Western blot analysis was used to detect the expression levels of VEGF.**P<0.01, ***P<0.001 vs. the control.VEGF: vascular endothelial growth factor; HUVECs: human umbilical vein endothelial cells.
3.3.Pachymic acid induces the autophagy of LUAD cells
LC3 expression was investigated using an IF assay to evaluate the effect of pachymic acid on LUAD cell autophagy.LC3 expression was markedly enhanced by pachymic acid in a concentrationdependent manner (Figure 3A).The expression levels of proteins associated with autophagy were examined by Western blot analysis.LC3Ⅱ/Ⅰand Beclin-1 expression were pronouncedly raised,whereas p62 expression declined following treatment with pachymic acid in a concentration-dependent manner (P<0.01) (Figure 3B).
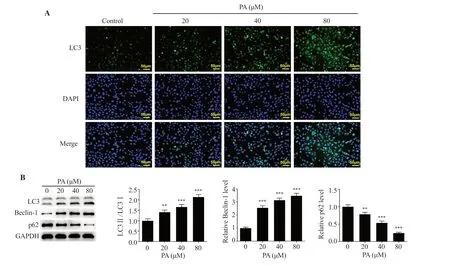
Figure 3.PA induces the autophagy of LUAD cells.(A) An immunofluorescence assay was used to determine the expression of GFP-LC3.(B) Western blot analysis was used to detect the expression levels of autophagy-associated proteins.**P<0.01, ***P<0.001 vs. the control.GFP: green fluorescent protein.
3.4.Pachymic acid inhibits the expression of PTP1B in LUAD cells
In the present study, SwissTargetPrediction database predicted that PTP1B may serve as a potential target of pachymic acid.Pachymic acid significantly lowered PTP1B expression in LUAD cells(P<0.001) (Figure 4A).Moreover, results of the molecular docking assay demonstrated that pachymic acid may exhibit a targeted binding with PTP1B (Figure 4B).
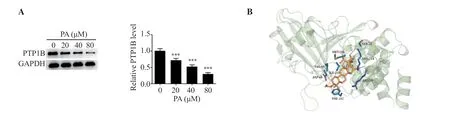
Figure 4.PA inhibits the expression of PTP1B in LUAD cells.(A) Western blot analysis was used to detect the expression levels of PTP1B in LUAD cells.(B) Molecular docking was carried out to determine the targeted binding association of PA with PTP1B.Yellow color represents charge center, blue represents protein and orange represents ligand.***P<0.001 vs. the control.PTP1B: protein tyrosine phosphatase 1B.
3.5.Pachymic acid regulates cell biological behaviors and the Wnt/β-catenin signaling in LUAD via targeting PTP1B
Following PTP1B overexpression, PTP1B expression was determined using RT-qPCR and Western blot analysis (Figure 5A).Results of the present study demonstrated that 80 μM pachymic acid exerted the most notable effects on LUAD cells; thus, this concentration was chosen for the follow-up assays.Cells were categorized into the control, pachymic acid, pachymic acid + oe-NC, and pachymic acid + oe-PTP1B groups.In the wound healing and Transwell assays, compared with the pachymic acid + oe-NC group, cell migration and invasion were significantly increased in the pachymic acid + oe-PTP1B group (Figure 5B and C).Results of the Western blot analysis also demonstrated that MMP9 and MMP2 expression levels were markedly increased in the pachymic acid +oe-PTP1B group (Figure 5D).In addition, PTP1B overexpression abrogated the effects of pachymic acid onE-cadherin,N-cadherin,and vimentin expressions (Figure 5E).The pachymic acidinduced inhibitory effect on tube formation in HUVECs as well as downregulated VEGF expression were also reversed by PTP1B overexpression (Figure 6A-B).Results of the IF assay indicated that LC3 expression levels were markedly decreased in the pachymic acid + oe-PTP1B group compared with the pachymic acid + oe-NC group, and pachymic acid-induced changes in the expression levels of autophagy-associated proteins were reversed following PTP1B overexpression (Figure 6C-D).Moreover, Wnt3a and β-catenin expressions were significantly downregulated in the pachymic acid group, while they were fortified in the pachymic acid + oe-PTP1B group (P<0.001) (Figure 6E).
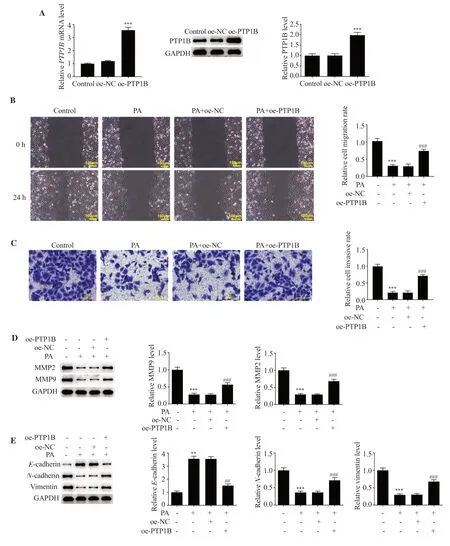
Figure 5.PA regulates cell migration, invasion as well as EMT in LUAD via targeting PTP1B.(A) PTP1B overexpression plasmid was constructed, and transfection efficiency was determined using reverse transcription-quantitative PCR and Western blot analysis.***P<0.001 vs. the oe-NC group.(B) Cell migration and (C) invasion were measured using wound healing and Transwell assays, respectively.(D-E) Western blot analysis was used to detect the expression levels of invasion- and migration-associated proteins as well as EMT-associated proteins.**P<0.01, ***P<0.001 vs.the control; ##P<0.01, ###P<0.001 vs.the PA + oe-NC group.Oe-NC: overexpression-negative control; oe-PTP1B: PTP1B overexpression.

Figure 6.PA regulates cell angiogenesis, autophagy, and the Wnt/β-catenin signaling in LUAD via targeting PTP1B.(A) Tube formation assays were used to determine the effect of PA on the tube-forming ability of HUVECs.(B) Western blot analysis was used to detect the expression levels of VEGF.(C) The expression of GFP-LC3 was determined by immunofluorescence.(D-E) Western blot analysis was used to detect the expression levels of autophagy-associated as well as Wnt/β-catenin signaling-associated proteins.***P<0.001 vs. the control; ###P<0.001 vs.the PA + oe-NC group.
4.Discussion
LUAD is a common disease that may not respond to conventional chemotherapeutic drugs[19].Notably, the use of chemotherapeutic drugs may lead to adverse reactions in patients, and in severe cases, these may aggravate the disease condition[20].In addition,the diagnostic rate is higher in both intermediate and late stages of disease; however, conventional treatments, such as chemotherapy,radiotherapy, and combined therapy may not be effective in increasing survival rates[21].Therefore, developing novel effective anti-LUAD drugs with fewer adverse effects is required.
Recent research has focused on the use of TCM monomers and associated active ingredients for the treatment of tumors,due to its potential multi-pathway, multi-target, and multi-effect characteristics[22].Pachymic acid demonstrates great potential as an anticancer drug and has been used for the treatment of nasopharyngeal carcinoma, gastric cancer, and liver cancer.Wanget al.clarified that pachymic acid markedly decreased gastric cancer cell invasion and metastasis[23].A previous study demonstrated that pachymic acid inhibited non-small cell lung cancer A549 cell growth and the metabolism of arachidonic acid in cells[18].The present study revealed that pachymic acid inhibited the viability,migration, and invasion of A549 cells in a concentration-dependent manner.Moreover, pachymic acid markedly inhibited LUAD cell angiogenesis and induced autophagy.In a previous study, pachymic acid-modified carbon nanoparticles reduced the angiogenesis of tumor cellsviainhibiting MMP3 expression[24].
Autophagy is a process in which eukaryotic cells use lysosomes to degrade their cytoplasmic proteins and damaged organelles under the regulation of autophagy-related genes[25].It can either play a dynamically inhibiting or promoting role in the occurrence and development of cancer and is involved in the regulation of tumor formation, proliferation, metastasis, and response to anticancer therapy.On the one hand, autophagy can inhibit the development of cancer by inducing lethal autophagy.Exosome LOC85009 inhibits cell proliferation and docetaxel resistance in LUAD by regulating ATG5-induced autophagy[26].Furthermore, autophagy mediates the progression of cancer, and the induction of protective autophagy can play a role in promoting cancer.The previous study showed that Apelin/APJ signaling activates autophagy to promote human LUAD cell migration[27].Results of a previous study further demonstrate that pachymic acid-induced autophagy is associated with the IGF-1 signaling pathway in senescent cells[28].Moreover, pachymic acid exerted dose-dependent toxic effects on lung fibroblasts, and significantly potentiated autophagy[28].In our experiment, pachymic acid significantly induced autophagy of LUAD cells and inhibited the development of cancer cells.
Moreover, molecular docking technology was used in the present study to demonstrate that the activity of pachymic acid may be associated with PTP1B.PTP1B expression was markedly reduced in pachymic acid-treated LUAD cells.Inhibiting the expression of PTP1B as an intracellular checkpoint in tumor cells may alleviate the restriction of the inhibition of T cells and enhance the efficacy of adoptively transferred chimeric antigen receptor T cells against cancer, thus suppressing tumor growth[29].Ophiopogonin-B inhibits the malignant process of hepatocellular carcinomaviatargeting PTP1B and modulates the PI3K/AKT and AMPK signalings[30].In addition, PTP1B may be used as a regulatory factor of angiogenic endothelial cell signal transduction in heart hypertrophy and heart failure, and participate in cell growth metabolism and angiogenesis[31].PTP1B inhibition in podocytes led to improved renal insulin signaling and enhanced autophagy, as well as reduced inflammation and fibrosis[32].Results of a previous study demonstrated that a high-fat diet inhibited myocardial autophagy,as manifested by LC3-Ⅱ transformation and Beclin-1 reduction,an increase in p62 expression, and a decrease in AMPK and raptor phosphorylation, which were all reversed by PTP1B deletion[33].The present study demonstrated that PTP1B overexpression in LUAD cells significantly reversed the effects of pachymic acid on the migration, invasion, EMT, angiogenesis, and autophagy of LUAD cells.In conclusion, pachymic acid might inhibit the aggressive behaviors of LUAD cells by targeting PTP1B.
Previous research demonstrated that pachymic acid may play a role in chronic kidney disease in ratsviathe Wnt/β-catenin signaling pathway[15].In addition, PTP1B deletion may activate Wnt/β-catenin signaling; thus driving HCC advancement[14].Notably, the Wnt/β-catenin signaling pathway is implicated in LUAD initiation and progression[34,35].The activation of Wnt/β-catenin signaling primarily leads to the malignant development of LUAD[16,36].Moreover, Wnt3a is considered as a prognostic marker of LUAD[37].The activation of the Wnt3a and Wnt/β-catenin signaling is accountable for the tumor-promoting role of PITX2 in LUAD[38].GPC5 may competitively bind to Wnt3a and block the Wnt/β-catenin signaling to hamper EMT in LUAD[39].The present study also demonstrated that the Wnt/β-catenin signaling-associated Wnt3a and β-catenin were depleted in pachymic acid-treated LUAD cells, which were abolished by PTP1B overexpression.Therefore,pachymic acid may also act as a regulator of the Wnt/β-catenin signaling in LUADviatargeting PTP1B.
In conclusion, pachymic acid suppressed cell migration, invasion,EMT, and angiogenesis, induced autophagy and regulated the Wnt/β-catenin signaling pathway in LUADviatargeting PTP1B,which may provide a novel theoretical basis for the therapeutic use of pachymic acid in clinical practice.However, there are some limitations in the current study.Firstly, the role of pachymic acid is limited to the cell experiments and further animal experiments need to be conducted to confirm the protective role of pachymic acid in LUAD.Besides, our research only preliminarily revealed the impacts of pachymic acid on the expression of Wnt3a and β-catenin in the Wnt/β-catenin signaling in LUAD cells and whether pachymic acid functions in LUADviamediating the Wnt/β-catenin signaling needs further exploration by the addition of Wnt agonists or antagonists.
Conflict of interest statement
The authors have no conflict of interest to declare.
Funding
The study was supported by the Zhejiang Province Traditional Chinese Medicine Health Science and Technology Program(2023ZL570).
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’contributions
FZ designed the experiment.HZ and KZ operated the experiment and wrote the article.XFZ, YHD, BZ, WM, and QSD processed the experimental data and ensured the authenticity and accuracy of the experimental data.All the authors agreed to the publication of the article.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Ellagic acid inhibits gastric cancer cells by modulating oxidative stress and inducing apoptosis
- Methanolic extract of Ephedra alata inhibits breast cancer cells in vitro and in vivo
- Isoimperatorin alleviates acetic acid-induced colitis in rats
- Ethyl acetate fraction of Sargassum pallidum extract attenuates particulate matterinduced oxidative stress and inflammation in keratinocytes and zebrafish
