Recent research progress from biological perspective on the mechanism of formation of osteoarthritis after anterior cruciate ligament injury
2024-05-09ZHOUKaiDUXiupanWANGGuangji
ZHOU Kai, DU Xiu-pan, WANG Guang-ji
Hainan Hospital Affiliated to Hainan Medical University, Department of Sports Medicine, Hainan Provincial People's Hospital, Haikou 570311,China
Keywords:
ABSTRACT The anterior cruciate ligament (ACL) mainly plays a role in stabilizing the knee joint by limiting the forward translation of tibial force and rotational force at the tibial joint, and if this ligament is damaged, it will cause joint pain, limited mobility, knee instability, etc.According to related studies, the incidence of traumatic osteoarthritis (PTOA) after ACL injury is as high as 87%, although many studies have shown that patients with ACL injury are susceptible to PTOA, but the exact mechanism is currently unknown.This may be related to biological,structural, and mechanical factors caused by the ligament injury.Previous studies have shown that elevated inflammatory mediators in the joint cavity following ACL injury can lead to chondrocytes necrosis and degradation of the cartilage matrix.These potential biochemical mediators contribute to PTOA formation, and early intervention can reduce future episodes of PTOA.In recent years, many scholars have devoted themselves to studying the potential important factors and signaling pathways involved in the formation of osteoarthritis after ACL injury, and exploring its molecular mechanism, which has led to great progress in this field.This paper mainly studies and discusses the mechanism of osteoarthritis formation after ACL injury from the biological perspective.
Osteoarthritis is a chronic disease in which damage to cartilage cells and subsequent osteophytes is caused by a variety of factors.The cause of osteoarthritis is currently unknown and occurs mainly following joint injuries (e.g.intra-articular fractures, ligament injuries, meniscal injuries, etc.).[1] The cause is not known.Anterior cruciate ligament injuries are a common cause.Previous studies have shown that damage to this ligament results in biological changes that can lead to cartilage damage and ultimately contribute to the formation of OA.However, according to current research osteoarthritis following ACL injury is due to a variety of factors[2].Most patients with ACL injury or rupture are surgically deintervened.This restores the stability of the knee joint and results in a lower incidence of PTOA.However, after reconstructive surgery it still does not stop the progression of OA[3].This suggests an increased risk of OA after ACL injury and may be closely related to the intra-articular cavity microenvironment.Current research has shown that chondrocytes produce inflammatory and catabolic factors following ACL injury[4].This places their joint cavity in a state of inflammatory environment, which in turn exacerbates the formation of PTOA.One of the most important factors in the pathogenesis of OA has also been found to be an imbalance in the balance of cytokines in the joint cavity.Pro-inflammatory cytokines predominate.Through their action, these factors initiate a vicious cycle that leads to the final effect[5].This paper therefore focuses on a series of changes in the microenvironment of the joint cavity following ACL injury (including changes in cytokines, changes in the protein composition of the synovial fluid and damage and destruction of chondrocytes) and the degradation of the cartilage matrix and damage and necrosis of chondrocytes through relevant molecular mechanisms or mediated signalling pathways, ultimately leading to the formation of PTOA.
1.Mechanism of PTOA formation due to intraarticular cytokine alterations
After an ACL injury, it causes dramatic changes in its intraarticular cytokines.Some studies have shown that intra-articular luminal levels of cytokines such as IL-6, TNF-α, IL-1β, IL-8 and IL-10 are elevated after acute injury in ACL[6, 7].These cytokines are released by activated chondrocytes, synovial cells and synovial fibroblasts.They are also involved in the degradation of the cartilage matrix, causing damage to cartilage and inhibiting matrix synthesis[8].In addi
L-6 to the IL-6 receptor (IL-6R).By forming a complex with the glycoprotein 130 (gp130) homodimer, it activates the JAK/STAT pathway, which in turn leads to the recruitment and activation of STAT1 and STAT3, causing damage to cartilage.IL-6 also activates the MAPK signalling pathway and the PKB/Akt signalling pathway,inducing matrix degradation, while IL-6 trans-signalling is mediated by a complex formed by IL-6 and soluble IL-6R (IL-6/sIL-6R).It inhibits proteoglycan synthesis and promotes proteoglycan loss,causing damage to cartilage.This suggests that IL-6 factors mediate the signalling pathways involved in chondrocyte damage and apoptosis.
1.2 IL-1β factor
StudiesStudies have shown that IL-1β is one of the major inflammatory and catabolic cytokines in OA pathophysiology.It has a significant catabolic effect on cartilage by increasing the expression and activity of key enzymes in matrix degradation[11].IL-1β can directly induce activation of matrix degrading enzymes(MMP), leading to cartilage damage[12].In general, IL-1β factor, by binding to the receptor (IL-1RI), activates the signalling pathways NF-κB and MAPK pathways, leading to enhanced catabolism by increasing the expression of proteolytic enzymes.Namely,proteoglycan degradation and collagen destruction[13, 14].Through the same signalling pathway, IL-1β inhibits the synthesis of transcription factors (e.g.SOX-9) thereby inhibiting the synthesis of type II collagen as well as aggregated glycans[15].Notably, IL-1β can promote the expression of COX-2, PGE-2 and NO to inhibit proteoglycan formation in the joint cavity.Also, IL-1β upregulates the expression of MMP3 and MMP9 by activating the JNK pathway[16].This activates the PI2K and Akt pathways to upregulate the concentrations of NO, PGE3, MMP and ADAMT[17], thereby contributing to the degradation of the cartilage matrix.In addition,IL-1β can also inhibit type II collagen synthesis by inducing the NF-κB signalling pathway[18].The above mechanisms of these studies show that IL-1β factor causes great damage to the cellular cartilage.
1.3 TNF-alpha factor
Related studies have clarified[19] that TNF-α binding to the TNF receptor (TNFR) causes conformational changes that promote the recruitment of a DD-containing kinase by intracytoplasmicassociated factors.RIP1 in turn recruits TNF receptor-associated factor-2 (TRAF2) and TRAF5 as well as cytostatic inhibitors of apoptosis protein-1 (cIAP1) and cIAP2.These factors act synergistically with RIP1 and participate in a downstream phosphorylation cascade to activate the NF-κB, JNK and p38 pathways, thereby indirectly upregulating matrix degrading enzymes that contribute to the degradation of articular cartilage.Such as collagen type 10 (Col10), MMP13, ADAMT5 and ADAMT9[20].These can lead to the degradation of cartilage structures and thus the development of osteoarthritis.Meanwhile, Li et al[21] reported that the TNF-α pathway can upregulate IL-1β factors acting together (see Figure 1).Interestingly, TNF-α directly induces the production of MMP and prostaglandins and inhibits the synthesis of proteoglycan and type II collagen[22].Thus, TNF-α plays a key role in cartilage matrix degradation and bone resorption in OA.

Fig 1 IL-1β and TNF-α factors lead to degradation of ECM through relative signal pathway
1.4 IL-8 factor
The IL-8 factor consists of four exons and three introns.IL-8 is produced by various cell types in inflammation.the 5’ flanking region of the IL-8 gene contains multiple nuclear factor binding sites.NF-κB binding to AP-1 or C/EBP synergistically activates the IL-8 gene in response to IL-1 and T NF-α, thereby contributing to cartilage degradation[23].In addition, IL-8 signaling promotes activation of the major effector phosphatidylinositol-3 kinase or phospholipase C, which in turn promotes activation of the Akt and MAPK signaling cascades[24].MAPK signaling can lead to cartilage damage.In contrast, the PI3K/AKT signalling pathway promotes cell proliferation and inhibits chondrocyte apoptosis.It has also been shown that IL-8 enhances chondrogenesis in vivo through the CXCR2-mediated PI3k/Akt signaling pathway[25].Therefore, the effect of IL-8 on chondrocytes after ACL injury is twofold.Most of the current research on IL-8 factors has focused on tumour effects.No studies have yet clearly demonstrated the relationship between IL-8 factors and PTOA formation, and it is believed that future studies will make a breakthrough in this area.
In summary, ACL injury leads to a dramatic rise in inflammatory factors in the joint cavity and contributes to morphological changes in the joint through relevant signalling pathways (e.g.PKB/Akt,MAPK, NF-κB and other signalling pathways) or related molecular mechanisms.For example, cartilage degeneration, bone flab formation and other inflammatory changes.Could PTOA formation be better avoided by regulating the balance between inflammatory factors or inhibiting related signalling pathways after injury to this ligament.In recent years, many studies have identified miRNAs involved in the process of disruption of cartilage homeostasis caused by inflammatory factors such as IL-1β and TNF-α[26].This suggests that the development of osteoarthritis can be inhibited by regulating the relevant miRNAs.Also included are studies of various signalling pathways, such as Ge Q et al[27] who de-induced PKDI protein expression via IL-1β, which in turn phosphorylated p38,leading to apoptosis.The results suggest that PKDI may contribute to apoptosis through the p38 MAPK signalling pathway.However,there are no reports of this protein acting to promote osteoarthritis formation after ACL injury.In addition, there are many genemediated signalling pathways that have not been investigated or are currently being investigated.For example, the role of the NFκB pathway mediated by the Gremlin-1 gene after ACL injury.Therefore, with a better understanding of the mechanisms that lead to PTOA formation after ACL injury, they can be investigated in depth to uncover the relevant genes or factor-mediated mechanisms.This may provide new therapeutic strategies to prevent the development of PTOA after ACL injury.
2.Changes in protein composition of synovial fluid in relation to PTOA
According to studies, anterior cruciate ligament injury leads to an increase in synovial fluid-related protein components such as matrix metalloproteinases (MMP), osteopontin and cystein 3[28, 29].These protein components are mainly produced by synovial cells,chondrocytes and other intra-articular tissues that are activated and prompted by inflammatory mediators.They can contribute to cause cartilage matrix degradation as well as collagen destruction.The role of the protein components and the mechanisms involved in contributing to the development of osteoarthritis are discussed below.
2.1 Matrix metalloproteinases
The characteristic sign of OA is progressive cartilage destruction,culminating in the complete loss of chondrocytes.The key enzymes responsible for the degenerative changes in cartilage are matrix metalloproteinases, specifically MMP-13, which is currently considered to be the main protease involved in the degradation of ECM[30].Previous studies have suggested that the mechanism of action exerted by MMP is mainly due to its ability to cleave glycosaminoglycans (GAGs) of aggregated sugars at specific peptide bonds and to release the associated fragments.The proteolytic cleavage process is destructive and results in the loss of GAG from the matrix[31].At the same time, MMP has a catabolic effect on the ends of the aggregated sugars.This may accelerate the loss of matrix aggregated glycans from articular cartilage.In addition,MMP has collagenase activity.This can directly degrade collagen in the extracellular matrix (ECM)[32].Thus, elevated MMP levels lead to degradation of the joint ECM, including GAG, proteoglycans and collagen, which triggers further activation of MMP, creating a positive feedback loop[33].Notably, the loss of proteoglycan and collagen in articular cartilage is a significant alteration.Once collagen is lost, cartilage cannot be repaired[34].It is thus clear that MMP can lead to irreversible changes in cartilage.
2.2 Periosteal proteins
Periostin (POSTN) belongs to a group of secreted matricellular proteins that have been shown to be expressed in a variety of connective tissues, such as bone and periosteum and tendon[35].According to current studies the mechanism of action of POSTN protein in cartilage matrix degradation and osteoarthritis is not yet clear.Some related studies indicate that periosteal proteins can induce degradation of cartilage matrix by NF-κB signalling pathway.ADAMTS5 protein has extracellular matrix degrading enzyme activity and can contribute to the development of osteoarthritis through degradation.The relationship between ADAMTSs and OA is currently receiving increasing attention from the medical community[36].Also, studies have used exogenous periosteal proteins to treat chondrocytes and found that POSTN increases the expression of MMP13, which in turn promotes cartilage degradation.However,it did not alter periostin mRNA expression[34].Therefore, increased periosteal proteins after ACL injury can lead to a risk of cartilage degradation.
2.3 Caspase-3 and various proteins
Caspases are important proteins in the development of apoptosis,and caspase-3 is the main terminal shear enzyme in apoptosis.Studies have shown that its main mechanism is to activate caspases under the induction of various signals, and the protein is activated after binding to cofactors, which can play the role of hydrolyzed protein.while continuing to activate downstream effector caspases, the effector caspase is activated, it can hydrolyze the relevant substances in the cell on a large scale,.thereby degrading the intracellular protein,and eventually apoptosis of the cell[37].In addition, proteins that change in the joint cavity after ACL injury include ApoA1, α-2-macroglobulin, and more than 30 proteins that bind to bead proteins,among others[38].However, the relationship between these proteins and OA has been less studied.Therefore more studies are needed in the future to refine them in order to be able to better assess the risk of PTOA occurrence.
In summary, ACL injury promotes elevation of relevant proteins in the synovial fluid within the joint cavity.This leads to the destruction of the cartilage matrix and chondrocytes, which in turn affects the progression of OA.The most important of these proteins on articular cartilage is MMP, which is currently the subject of a number of studies on MMP inhibitors.As MMP inhibitors play a crucial role in remodelling the extracellular matrix after ligamentous injury.For example, Malemud CJ[39] found in animal experiments that MMP was inhibited by PI3Kδ,γ inhibitors, which reduced MMP gene expression and reduced cartilage degradation by protein hydrolases.There are various classifications of this class of inhibitors.However,all produce side effects such as pain and joint stiffness in patients,called musculoskeletal syndrome (MSS)[40].MMP-13 inhibitors,on the other hand, avoid such adverse effects.There is currently considerable interest in selective MMP-13 inhibitors.We believe that specific MMP-13 inhibitors will eventually yield breakthrough treatments that will alleviate progression for OA in the near future.
3.Chondrocyte damage and degradation
Cartilage Cartilage damage and degradation are central to the formation of the etiology of osteoarthritis (OA).Chondrocytes are cartilage tissue cells that secrete extracellular matrix (ECM) such as collagen and proteoglycans.OA is caused by excessive degradation of the extracellular matrix (ECM) and chondrocyte differentiation and degradation, the structural components of the matrix (mainly aggregated glycans and collagen) being mediated by proteases and cytokines in the synovial fluid.Due to the dramatic changes in the intra-articular environment following ACL injury, the secretion of collagenases, cytokines and genes related to chondrocyte terminal differentiation (e.g.COL10A1, MMP-13, MMP-9) is increased,affecting chondrocyte growth and leading to chondrocyte necrosis.It has been suggested that increased levels of inflammatory, catabolic factors in the joint cavity following ACL injury are associated with chondrocyte apoptosis and may lead to cartilage degradation and the development of osteoarthritis[41].Specifically, the degradation of cartilage resulting from ACL injury is closely associated with changes in cytokines as well as proteins in the synovial fluid (see Figure 2).Many studies have been conducted to demonstrate that imbalances in cytokines and increases in proteins contribute to the degradation of articular cartilage morphology through a variety of signalling pathways[42].Thus, changes in the microenvironment of the articular cavity following ACL injury lead to apoptosis of chondrocytes and degradation of the cartilage matrix.ACL injury contributes to the development of PTOA through the action of relevant molecular mechanisms, and the process of PTOA formation is progressively worse.Current clinical treatments are mainly through non-steroidal anti-inflammatory drugs and painkillers.However, these methods can only reduce the pain and delay the OA process in patients with osteoarthritis, but cannot alter the course of OA caused by irreversible degeneration of articular cartilage.There are no drugs available today that can potentially intervene against PTOA.It is theoretically possible to intervene at an early stage to prevent the progression of the disease.
4.Discussion
The The incidence of PTOA is higher in patients with a previous history of ACL injury.The incidence of PTOA is higher in patients with intra-articular damage.Patients with more severe ACL injuries and treated surgically can re-stabilise the knee joint to reduce rotational instability, inhibit anterior tibial translation and restore function[43].Postoperative training for rehabilitation can greatly improve structural, mechanical and neuromuscular deficits.Biologically, the postoperative patient is still in an inflammatory environment, which has an impact on exacerbating PTOA.In addition, for patients with minor ACL injuries that can be treated conservatively, there are multiple factors that contribute to the formation of PTOA after this ligament injury[44], and it is complex,thus bringing limitations to the current treatment.Pre- and postoperative patients can benefit from improving the state of the microenvironment in the joint cavity.Future research directions could be directed towards biological studies.The effects of drug therapy are also currently being studied extensively in animal studies.As our understanding of the biological mechanisms underlying ACL damage increases.And selective inhibition of inflammatory chemokines (e.g.IL-1 and TNF-α) in animal studies has shown potential to prevent degeneration of injured joints[45].However, at present, prevention of PTOA progression by drugs is only based on the animal experimental aspect.Prevention in the human context is not yet clear.Furthermore, the development of PTOA is a chronic as well as a progressive disease and PTOA should be detected at an early stage to monitor the progression and severity of OA and to assess the effectiveness of relevant treatments.Finally, a better understanding of the changes in the intra-articular environment should be essential for intervention after ligamentous injury.
5.Problems and prospects
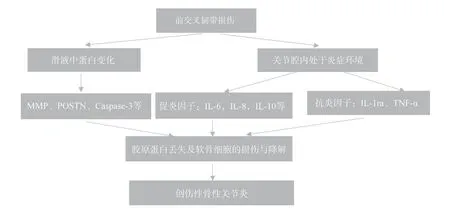
Fig 2 Injury to the anterior cruciate ligament leads to a disturbance in the cytokine balance in the joint cavity and an in-crease in proteases, resulting in loss of collagen and damage to cartilage cells
The development of osteoarthritis in ACL injuries is caused by a variety of factors.The biological effects are mainly due to changes in the substances in the joint cavity following ligament injury,resulting in an imbalance between pro- and anti-inflammatory factors, increased proteolytic enzymes and damage to chondrocytes.This leads to the development of osteoarthritis through potentially important factors and signalling pathways.There are currently very few molecular and protein-based drugs in development,making prevention of osteoarthritis a challenge.In contrast, PTOA is a chronic, damaging disease of the joint structure.In advanced stages, the changes in the knee joint are irreversible.It can only be improved by surgery.Therefore, early prevention and detection of OA is necessary to guide treatment and prevent irreparable damage to the knee.And in the future, research should be directed towards targeting factors or proteolytic enzymes to develop new drugs to reduce the incidence of osteoarthritis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (2021MSXM10) and the Hainan Medical College Research Incubation Fund Project (HYPY2020014).
Author’s contribution
Kai Zhou: The main tasks are literature searching, trawling,recording and writing.Guangji Wang: Overall control of the article and proofreading of the article content.Xiupan Du: A member of the subject team, providing literature in their field and relevant field revisions.
Conflict of interest
The content of this article does not involve a relevant conflict of interest and the study did not involve direct or indirect financial and profit sponsorship by any manufacturer or other economic organization.
杂志排行
Journal of Hainan Medical College的其它文章
- Advances of lncRNA MAFG-AS1 in cancer
- Research progress on dynamic monitoring of ctDNA and drug resistance related concomitant mutations in non-small cell lung cancer
- Prognostic characterization of copper death-related immune checkpoint genes and analysis of immunologic and pharmacologic therapy in bladder cancer
- Meta-analysis of the efficacy and safety of Bushen Huoxue decoction in the treatment of Osteoporosis
- To explore the mechanism of Fuyang Jiebiao granules against viral pneumonia based on network pharmacology and pharmacodynamics
- Sequential experimental observation on the curative effect of Yingbupu decoction of Zhuang medicine on stage I and II acute kidney injury
