Neoantigen cancer vaccines: a new star on the horizon
2024-05-08XiaolingLiJianYouLipingHongWeijiangLiuPengGuoXishanHao
Xiaoling Li, Jian You, Liping Hong, Weijiang Liu, Peng Guo, Xishan Hao,6
1Cell Biotechnology Laboratory, Tianjin Cancer Hospital Airport Hospital, Tianjin 300308, China; 2National Clinical Research Center for Cancer, Tianjin 300060, China; 3Haihe Laboratory of Synthetic Biology, Tianjin 300090, China; 4Department of Thoracic Oncology, Tianjin Cancer Hospital Airport Hospital, Tianjin 300308, China; 5Department of Thoracic Oncology Surgery, Tianjin Medical University Cancer Institute & Hospital, Tianjin 300060, China; 6Tianjin Medical University Cancer Institute & Hospital, Tianjin 300060, China
ABSTRACT Immunotherapy represents a promising strategy for cancer treatment that utilizes immune cells or drugs to activate the patient’s own immune system and eliminate cancer cells.One of the most exciting advances within this field is the targeting of neoantigens, which are peptides derived from non-synonymous somatic mutations that are found exclusively within cancer cells and absent in normal cells.Although neoantigen-based therapeutic vaccines have not received approval for standard cancer treatment, early clinical trials have yielded encouraging outcomes as standalone monotherapy or when combined with checkpoint inhibitors.Progress made in high-throughput sequencing and bioinformatics have greatly facilitated the precise and efficient identification of neoantigens.Consequently, personalized neoantigen-based vaccines tailored to each patient have been developed that are capable of eliciting a robust and long-lasting immune response which effectively eliminates tumors and prevents recurrences.This review provides a concise overview consolidating the latest clinical advances in neoantigen-based therapeutic vaccines, and also discusses challenges and future perspectives for this innovative approach, particularly emphasizing the potential of neoantigen-based therapeutic vaccines to enhance clinical efficacy against advanced solid tumors.
KEYWORDS Immunotherapy; neoantigen cancer vaccine; solid tumors; high-throughput sequencing; bioinformatics; PDOs; AI; HLA; TCR
I ntroduction
Cancer immunotherapy has revolutionized the paradigm of cancer care in the past decade by encompassing a diverse range of therapeutic approaches aimed at activating and boosting the patient’s own immune system to eradicate cancer cells.These therapeutic approaches include immune checkpoint inhibitors (ICIs), adoptive cell transfer (ACT) therapy, immune cell-secreted cytokine therapy (e.g., IFN-α), oncolytic viruses[Talimogene laherparepvec (T-VEC)], and neoantigen-based therapeutic vaccines, all of which have demonstrated remarkable clinical benefits across various types of cancer1-10.
ICIs are extensively utilized in the clinical management of various cancer types by targeting inhibitory receptors expressed on tumor and immune cells, such as cytotoxic T lymphocyte-associated protein-4 (CTLA-4), programmed cell death protein 1 (PD-1) or its ligand PD-L1, lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin and mucin- domain containing-3 (TIM-3), and T cell immunoglobulin and ITIM domain (TIGIT)11,12.ICIs refer to therapeutic monoclonal antibodies that enhance T cell activation, which enables the immune system to effectively kill tumor cells.Currently, 9 ICIs have been approved in the United States (US), while China has approved 15 ICIs for various types of cancer in clinical practice11,13.In 2011 the first ICI [ipilimumab (Yervoy)]received approval from the US Food and Drug Administration(FDA); ipilimumab is an anti-CTLA-4 monoclonal antibody.Pembrolizumab (Keytruda), the first ICI targeting PD-1,received US FDA approval for melanoma treatment in 2014.Several PD-1/ PD-L1 inhibitors have been since been approved for treating multiple solid tumors.Although this innovative approach has shown promising outcomes in advanced-stage cancer patients; the efficacy remains limited to a range of 10%-40% in solid malignancies, even when used as a part of combination therapy14-16.Ongoing efforts are focused on advancing agents that target additional immune checkpoints as well as co-stimulatory or co-inhibitory receptors involved in regulating T cell function17,18.
ACT therapy is a form of cancer immunotherapy that utilizes modified living immune cells, commonly referred to as“living drugs”8,19-23.Numerous clinical studies have demonstrated remarkable clinical responses in cancer treatment using novel ACT strategies, including tumor-infiltrating lymphocytes(TILs), chimeric antigen receptor-modified T cells (CAR-Ts), T cell receptor-engineered T cells (TCR-Ts), CAR-natural killer cells (CAR-NKs), and CAR-macrophages (CAR-Ms)8,21,24.Among the ACT strategies, CAR-T therapy has made significant breakthroughs in hematologic malignancies, such as CAR T-cell therapy targeting CD19 and B-cell maturation antigen(BCMA)19,25.Two CAR-T therapies were approved by the FDA for the first time in 2017 [tisagenlecleucel (Kymriah®)for acute lymphoblastic leukemia and axicabatagene ciloleucel (Yescarta®) for diffuse large B-cell lymphoma].Currently,six CAR-T cell therapies have been approved by the US FDA and four by the National Medical Products Administration(NMPA) in China.Although CAR-T cells have demonstrated a remarkable success in treating hematologic cancers, application in solid malignancies has remained a challenge, mainly due to limited membrane antigen targets, high heterogenicity,dense extracellular matrix, low infiltration of effector T cells into tumors, and the complex tumor microenvironment19,25-27.
TCR-T cell therapies offer a promising therapeutic alternative by genetically modifying T cells to express a TCR that specifically recognizes antigens presented by the major histocompatibility complex (MHC) on tumor cells8,20.Tumorassociated antigens (TAAs) and tumor-specific antigens(TSAs) have emerged as promising targets for TCR-T cell therapies.TAA refers to antigens that are present on tumor and normal cells, but are overexpressed or mutated in tumors, such as NY-ESO-1, GP100, and MAGE.TAA-targeted TCR-T cell therapies have shown certain effective results in clinical trials20,28.For example, a phase I/II trial of TCR-T cell therapy targeting NY-ESO-1, a TAA present in patients with synovial sarcoma and melanoma, demonstrated objective responses in 50% of patients29,30.Similarly, a phase I/II trial of TCR-T cell therapy targeting MAGE-A4 in patients with HLAA★02:01-positive non-small cell lung cancer showed an objective response rate of 40%31.Additionally, Tebentafusp-tebn is a bi-specific CD3 T cell engaging gp100 peptide and HLA molecules for the treatment of HLA-A★02:01-positive adults with unresectable or metastatic uveal melanoma32.TSAs, also known as neoantigens, are exclusively present in tumor cells and absent in healthy cells33.Currently, there is a growing body of research focused on TSAs.A more comprehensive discussion on neoantigens targeted TCR-T cell therapy (neoTCR-T)will be presented in the upcoming session (Neoantigentargeted adoptive T cell therapy).
Despite the implementation of various advanced immunotherapy strategies in cancer treatment, clinical outcomes for solid tumor patients remain limited due to multiple factors.These advanced immunotherapy strategies include low tumor cell antigenicity, insufficient effector T cell infiltration,and diverse mechanisms of immunosuppression in the tumor microenvironment8,34,35.Therefore, novel strategies, such as neoantigen targeting immunotherapy, have emerged as promising approaches for targeting personalized or shared neoantigens through the use of neoantigen vaccines and ACT8,21,34,36,37.Neoantigens are peptides derived from mutated proteins expressed exclusively by cancer cells, which are recognized as foreign by the immune system and potentially trigger a robust immune response against tumors.The development of diverse neoantigen-targeted therapies has been extensively investigated to enhance and potentiate immune responses against specific neoantigens expressed on the surface of cancer cells, thereby facilitating recognition and subsequent elimination.Recently,there has been significant interest in exploring the potential of neoantigen-based vaccines to induce long-lasting tumor control with curative potential by augmenting immunologic memory.Neoantigen-based vaccines involve immunizing patients with a vaccine that primes and activates T cells or boosts existing weak responsesin vivowithin lymph nodes.These innovative neoantigen vaccines have demonstrated feasibility, safety,and the ability to elicit vaccine-specific immune responses at unprecedented levels compared to previous cancer vaccines.Numerous excellent reviews have highlighted the promising clinical potential of neoantigens1,37-45.We sincerely apologize for any oversights in this review regarding relevant findings and references.Herein we have focused on the most recent advances in personalized neoantigen-based cancer vaccines and the clinical applications, with a particular emphasis on strategies to overcome obstacles that hinder optimal patient outcomes.
Neoantigens
Neoantigens, which are known as TSAs, are a unique class of antigens that arise exclusively from non-synonymous somatic mutations in cancer cells.These alterations occur at the genomic, transcriptomic, and proteomic levels46,47.The resulting mutated peptides can be presented on MHC molecules to generate neoantigens that have been shown to elicit strong and specific immune responses against cancer cells.This characteristic makes TSAs highly appealing targets for cancer immunotherapy aimed at selectively attacking cancer cells while sparing healthy cells.
Tumor neoantigens arise from a variety of somatic mutations and can be categorized into several types based on their origin, including the following: 1) Non-synonymous mutations result in an amino acid change in the protein sequence,which are the most common source of tumor neoantigens and are often found in oncogenes or tumor suppressor genes,such as single nucleotide variants (SNVs) and insertions/deletions (INDELs).2) Gene fusions are a potent neoantigen source and have been reported to drive the development of approximately 16% of all cancers.3) Splicing site mutations can create neoantigens by generating novel peptides or altering the expression of existing peptides.4) Aberrant translation of non-coding RNA, alternative transcription start/stop sites, non-canonical open reading frames (ORFs),mRNA intron retention, and endogenous retro-transposition can generate neoantigens by producing novel peptides that are not present in healthy cells44,48.5) Viral proteins can be regarded as a novel type of neoantigen in virus-induced tumors due to significant dissimilarity from normal cellular proteins and an ability to elicit high T cell responses.Viral proteins are optimal targets for some virus-induced solid tumors, such as Merkel cell carcinoma induced by Merkel cell polyomavirus (MCPyV) infection and nasopharyngeal carcinoma triggered by Epstein-Barr virus (EBV) infection.Additionally, neoantigens arising from structural variants(SVs) may also serve as valuable targets for anti-tumor immunotherapy49.
Given that 99% of tumor-specific mutations occur in non-coding regions of genes and exonic regions represent only 2% of the entire human genome, screening neoantigens solely from exonic region mutations is limited50.Recent studies have revealed coding functions in many regions previously defined as non-coding regions49.The non-canonical variants that are present as valuable targets due to superior immunogenicity compared to SNVs and INDEL-derived neoantigens have been extensively investigated in ongoing clinical trials46,51.Accumulating evidence suggests that other sources of cancer neoantigens, including coding and non-coding sequences,such as gene fusions and alternative splice variants at the transcriptome level, and post-translational modifications (PTMs),proteasome processing, and transporter associated with antigen processing (TAP) at the proteome level, represent promising novel targets for immunotherapy43,50,52.
Neoantigens can be categorized into two groups [shared neoantigens (public neoantigens) and personalized neoantigens]21,45,53.Shared neoantigens exist across multiple cancer patients, often originating from commonly mutated genes, such as TP53 and KRAS.These mutation peptides have commonality among different cancer patients, making them suitable for the preparation of shared neoantigen vaccines,known as off-the-shelf vaccines54.Highly immunogenic shared neoantigens can be screened as broad- spectrum therapeutic cancer vaccines for patients with the same mutation gene.It is worth noting that neoantigens resulting from gene fusions,recurrent mutations in cancer driver genes, non- coding regions, and abnormal PTMs are more likely to be shared among cancer patients.This possibility provides readily available common neoantigens for immunotherapy.Personalized neoantigens are unique mutation peptides predicted based on a patient’s tumor genome mutation profile.The personalized neoantigens exhibit complete variations between patients and tumors, making them a significant source for developing personalized neoantigen-based cancer vaccines.Therefore, there is an urgent need to develop efficient computational algorithms that can rapidly screen these potential neoantigens and verify their suitability for cancer immunotherapy.
Novel immunotherapeutic strategies targeting neoantigens in cancer treatment
With the rapid advancement in precision medicine, biological science, and computer science, novel immunotherapeutic strategies targeting neoantigens have emerged as one of the most promising solutions in recent years for cancer treatment.Novel immunotherapeutic strategies targeting neoantigens include neoantigen-based cancer vaccines,neoTCR-T therapy, neoantigen- targeted chimeric antigen receptor (neoCAR) T-cell therapy, and neoantigen- targeted antibodies.These therapies are currently undergoing extensive clinical investigation due to their potential in inducing an anti-tumor immune response and providing long-term control of recurrences, as well as protection against metastases55-59.Moreover, the development of neoantigen-specific antibodies has demonstrated promising results in selectively
recognizing and binding to specific neoantigens expressed on cancer cells, thereby facilitating their elimination.This approach holds great potential as a therapeutic strategy for cancer treatment.Advanced technologies, such as high-throughput sequencing and artificial intelligence (AI)algorithms, have greatly facilitated the realization of personalized immunotherapies by enabling rapid and cost-effective detection of tumor-specific mutations in individual patients.Moreover, the development of algorithms predicting MHC molecule binding affinity to neoantigens and the ability of TCRs to recognize neoantigens have a crucial role in designing personalized cancer vaccines.However, there is still a need for further improvement in identifying an ideal pipeline for neoantigen identification and the cost and time limitations associated with personalized immunotherapy products.With the continuous development and interdisciplinary integration of fields, such as biotechnology, immunology,bioinformatics, materials science, chemistry, and AI, more ideal neoantigens will be discovered and utilized in diverse strategies for effective cancer treatment.
Neoantigen-targeted adoptive T cell therapy
Neoantigen-targeted adoptive cell therapy (neoACT) is an innovative and personalized approach to cancer treatment that harnesses the power of the immune system to specifically target and eliminate cancer cells.Various strategies have been used, such as the selection of neoantigen-reactive T cells and engineering of TCRs to target neoantigen-specific T cells(neoTCR-T cells).The original T cells can be obtained by harvesting TILs from the tumor or enriching lymphocytes from peripheral blood mononuclear cells (PBMCs).The neoantigen-targeting T cells are then expandedex vivoand infused back into the patient, where the neoantigen-targeting T cells selectively recognize and attack cancer cells expressing targeted neoantigens.
NeoTCR-T cells, also known as TSA-targeting TCR-T cell therapy, involves modifying a patient’s T cells to express T cell receptors (TCRs) that recognize specific neoantigens on cancer cells, which is typically a hotspot mutation containing a shared neoantigen60.Compared to TAAs, which may result in off-target effects and immune-related toxicities,TSA targeting offers higher specificity and reduces the risk of off-target effects, while increasing safety.This method has demonstrated success in treating some types of cancer, such as patients with the KRAS hotspot mutations, G12D/G12V,that are restricted by HLA-C★08:02 and HLA-A★11:0161-63,that H3.3K27M epitope restricted by HLA-A★02:0153, and patients with PIK3CA (H1047L) mutations restricted by HLA-A★03:0164.Refining the search term to “neoantigen TCR-T” on ClinicalTrials.gov yielded a total of six relevant results (NCT05292859, NCT05292859, NCT05194735,NCT04520711, NCT05349890, and NCT03970382).A recent report on Neo-T cell therapy utilizing neoantigen-specific CD8+ T cells derived from PBMCs demonstrated positive clinical benefits in the treatment of advanced solid tumors65.Furthermore, multiple neoTCRs that recognize neoantigen traffic to the tumors of patients have reported long-lasting clinical responses66,67.A recent study demonstrated the feasibility of isolating and cloning multiple TCRs that recognize mutational shared neoantigens by a novel method involving simultaneous knockout of the endogenous TCR and knock-in of neoTCRs.Furthermore, these three gene-edited neoTCR T cells exhibit tumor trafficking capabilities66.However, it should be noted that a limitation of TCR-Ts lies in a dependence on specific HLA alleles for neoantigen presentation and identification of a shared neoantigen target to bind specific HLA alleles.Therefore, a given TCR can only be utilized to treat patients with corresponding HLA alleles64.
Neoantigen-targeted CAR-T cell therapy involves modifying T cells to express chimeric antigen receptors (CARs) that recognize antigens directly through CARs approaches independent of HLA restriction, but only specific surface antigens on cancer cells are targeted, such as unmodified proteins,glycoproteins, glycolipids, and carbohydrates.An example of neoantigen-targeted CAR-T therapy in solid tumors is a neoantigen derived from EGFRvIII mutations, which is caused by the in-frame deletion of exons 2-7 deletions in 30% of newly diagnosed glioblastoma patients, making the neoantigen derived from EGFRvIII mutations an ideal target for CAR-T therapy68.In contrast, TCRs have evolved to recognize epitopes derived from the entirety of the proteome, including cell surface, cytosolic, and intra-nuclear proteins56.Consequently,TCRs may recognize a larger universe of protein-based targets relative to CARs.
Overall, neoantigen-targeted ACT represents a cutting-edge approach to cancer immunotherapy and has shown promising results in early clinical trials for various types of cancers,including melanomas, lung cancer, and colorectal cancer.Neoantigen-targeted ACT offers the potential for improved treatment outcomes and long-term survival for patients with advanced or refractory cancers.By leveraging the unique neoantigens present on cancer cells, this therapy has the potential to provide personalized and effective treatment options for patients with various cancers.Identifying neoantigens can be complex and time-consuming, requiring sophisticated genomic analysis.Additionally, the large-scale production of neoantigen-specific T cells can be technically demanding and expensive.Further research is needed to optimize the efficacy and safety of these therapies, and to determine the optimal use in different cancer types and patient populations.
Neoantigen-based cancer vaccines
Recently, with the advances in high-throughput sequencing and computation technologies, neoantigen-based cancer vaccines have emerged as a primary focus over the past decade,the clinical utility of which will continue to be maximized in the future42,43,69-72.The aim of neoantigen-based cancer vaccines is to stimulate the patient’s own immune system to eliminate cancer cells, which can be personalized to each patient’s specific neoantigen profile and may be administered either pre- or post-operatively or in combination with other standard treatments, which will be discussed in detail below.This approach has shown promising results in clinical trials, with some patients exhibiting long-term tumor regression or even complete remission.
The use of vaccines to treat malignant tumors can be traced back to the injection of a mixture of heat-killed bacteria into tumors in the late 19th century by William Coley (known as the Coley toxin)73, as well as a similar approach by Lloyd Old with Bacillus Calmette-Guérin (BCG) in the 1950s, but with varying degrees of success45,71.In the late 1990s and early 2000s, numerous clinical trials were conducted to develop cancer vaccines targeting TAAs74.These trials often yielded disappointing results, however, due to low efficacy and significant toxicity.The first therapeutic cancer vaccine [Provenge(Sipuleucel-T)] was approved by the US FDA for the treatment of prostate cancer in 201075.Recent technological advancements have enabled the development of more effective cancer vaccines targeting neoantigens, which stimulate immune responses to attack cancer cells46,74.Neoantigen-based cancer vaccines can be classified into two main types (off-the-shelf and personalized)76,77.The off-the-shelf vaccines are designed to target shared neoantigens, which are predicted to exhibit a high frequency of expression and elicit robust anti-tumor immune responses, making off-the-shelf vaccines suitable for a broader range of cancer patients62.Conversely, personalized vaccines are designed based on the unique neoantigens present in an individual patient’s tumor.Due to the high anti-tumor specificity and low central immune tolerance, personalized vaccines induce strong and specific anti-tumor immune responses and immune memory, precisely eliminating cancer cells without harming healthy tissues.
The successful development of a neoantigen cancer vaccine requires several critical steps.The initial step is the identification and characterization of tumor-specific neoantigens, which necessitates the use of advanced genomic sequencing technologies and bioinformatics tools to analyze tumor samples and identify unique gene mutations that give rise to neoantigens for each patient.Subsequently, the design of a vaccine that contains the identified neoantigen targets is undertaken.This process involves selecting the most potent neoantigens and formulating the neoantigens in a way that enhances the immunogenicity and stability.Numerous neoantigen targets that can effectively stimulate autologous specific immune responses and kill tumor cells are selected, followed by preparation of corresponding neoantigen vaccines using advanced biosynthetic technology under strict good manufacturing practice (GMP) conditions for clinical application of cancer immunotherapy (Figure 1).The third step involves evaluation of safety, efficacy, and immunogenicity usingin vitromodels.This process entails utilizing immunologic assays to quantify the immune response of the vaccine and assessing the ability of the vaccine to suppress tumor growth and metastasis.Finally, conducting clinical trials on human subjects is crucial to test vaccine safety and efficacy(Figure 1).Various delivery platforms, such as synthetic peptides, DNA, RNA, and neo antigen-pulsed dendritic cell (DC)vaccines, have been utilized to efficiently deliver neoantigens.This comprehensive process involves several phases of clinical trials to establish the safety, tolerability, and efficacy of the vaccine.Phase 1 and 2 trials evaluate the safety and immunogenicity of the vaccine in a small group of patients,while phase 3 trials evaluate vaccine efficacy in a large patient cohort.
In conclusion, the development of a successful neoantigen cancer vaccine requires a multidisciplinary approach encompassing genomic sequencing, bioinformatics, vaccine design and production, preclinical, and clinical testing of vaccine function, as well as regulatory approval for clinical application.Despite the challenges involved in this process, the creation of a safe and effective neoantigen cancer vaccine holds immense potential for enhancing cancer treatment outcomes.

Figure 1 Schematic for the clinical implementation of neoantigen-based cancer vaccines.The clinical implementation of cancer neoantigen vaccine involves a series of sequential processes, including neoantigen prediction, screening, manufacturing, administration, and evaluation of the safety and clinical efficacy.The workflow begins with the collection of patient samples, followed by the utilization of advanced high-throughput sequencing technologies and bioinformatics tools, as well as computation algorithms to identify potential neoantigens targets (~20-30).Subsequently, in vitro assays further screened and identified candidate neoantigens targets for the development of neoantigen cancer vaccines.These vaccines were then manufactured on demand under Good Manufacturing Practice (GMP) conditions.Longitudinal dynamic clinical assessment was conducted to evaluate immune response and clinical outcomes subsequent to the administration of neoantigen vaccines.
Clinical application of neoantigen vaccine
Neoantigen vaccines represent a promising immunotherapy approach for cancer patients.These vaccines elicit an immune response to recognize and attack the cancer cells specifically,while leaving normal cells unharmed37,58,59.The concept of neoantigen vaccines was initially proposed in the 1990s, but it was not until the emergence of high-throughput sequencing technologies and bioinformatics tools that this field began to gain momentum78.Given the highly variable mutation rates frequently exhibited by cancer cells, neoantigen vaccines hold promise as personalized treatments.Although still in early stages of development, these vaccines are already yielding positive results in clinical trials for a range of cancer types.
Neoantigen vaccines have been developed in various forms,including peptide vaccines, RNA vaccines, DNA vaccines, and neoantigen-pulsed DC vaccines44,79,80.These vaccines elicit an immune response in cancer patients, specifically targeting and eliminating cancer cells.Three initial clinical trials of personalized cancer vaccines have demonstrated the feasibility,safety, and immunotherapeutic efficacy of targeting individual tumor neoantigens in malignant melanoma patients59,77,81.As a result, numerous clinical trials have subsequently evaluated the safety and efficacy of neoantigen-based vaccines across various types of cancer.
The latest updates on clinical trials for neoantigen vaccines targeting various types of cancer follow, all of which are registered on ClinicalTrials.gov specifying “Tumor” as the consistent condition or disease.The four research sessions are dedicated to exploring different types of neoantigen vaccines,including peptide, RNA, DNA, and DC vaccines.We then manually reviewed and selected 102 clinical trials for personalized neoantigen vaccines across various cancer types for further analysis.These trials evaluated the safety and efficacy of a variety of vaccine modalities, including 56 peptide, 13 mRNA, 14 DNA (four of which combine with RNA), and 19 personalized neoantigen-pulsed DC vaccines (Tables 1-4).The US and China are at the forefront of clinical research on cancer neoantigens; however, limitations in search keywords and update frequency may impact data accuracy as of September 2023.
Peptide vaccine
Neoantigen peptide vaccines are comprised of small chains of amino acids that correspond to specific neoantigens identified in a tumor.These peptides are designed to trigger an immune response against the tumor by presenting neoantigens to T cells, which then recognize and attack cancer cells59.
Several clinical trials have investigated personalized peptidebased neoantigen vaccines for the treatment of melanomas and other types of cancer, with the majority of referenced clinical trials on neoantigen vaccines based on peptide vaccines(Tables 1 and 4).The initial clinical study was conducted in 2017 on patients with stage IIIB/C or IVM1a/b melanoma.Following surgery, 6 patients received the NeoVax personalized vaccine after 18 weeks.Of the 6 patients, 4 remained recurrence-free within 25 months after vaccination and 2 achieved a complete response (CR) when anti-PD-1 therapy was initiated following disease recurrence [Tables 1 and 4(NCT01970358)]59.The long-term effects of the vaccine and epitope spreading were detected at a median of nearly 4 years after treatment with NeoVax, during which all 8 patients were alive and 6 had no signs of tumor progression93.In a phase 1b clinical trial, the NEO-PV-01 vaccine was tested in combination with the ICI, pembrolizumab (anti-PD-1 therapy),in 82 patients with advanced melanomas, non-small cell lung cancer, and bladder cancer84.The trial demonstrated the presence ofde novoneoantigen-specific CD4(+) and CD8(+)T cell responses following vaccination, as well as the occurrence of the epitope spreading phenomenon.A separate study subsequently presented promising clinical and immunologic findings derived from a phase Ib clinical trial evaluating the combination of NEO-PV-01 with pemetrexed, carboplatin,and pembrolizumab as first-line therapy in 38 patients diagnosed with advanced NSCLC (Tables 1 and 4)85.
To enhance specific immune responses, neoantigen peptides are often combined with appropriate adjuvants.The TLR3 agonist, poly-ICLC (Hiltonol®), is the most commonly utilized adjuvant for neoantigen peptide vaccines because poly-ICLC robustly promotes T cell expansion through MDA5 stimulation and IFN-I production.In addition to traditional adjuvants, innovative liposome-based cationic adjuvant formulation 09b (CAF®09b), QS-21 (Stimulon®),and GM-CSF have also been utilized in clinical trials86.Furthermore, the administration routes of neoantigen peptide vaccines impact efficacy and safety, and subcutaneous and intradermal injections are frequently used routes of administration.
Neoantigen peptide vaccines present numerous advantages compared to other forms of cancer vaccines due to a capacity to elicit a highly targeted immune response and simplified manufacturing process.Nevertheless, these vaccines face challenges related to neoantigen identification and selection complexity, limited immunogenicity, a short half-life, and potential development of immune tolerance.Although peptide vaccines have achieved substantial advances in clinical development to date, additional research is warranted to address existing limitations.
Messenger RNA (mRNA) vaccines
Neoantigen RNA vaccines are a relatively new type of cancer vaccine that use mRNA to instruct cells to produce tumor-specific neoantigens, which trigger an immune response against the patient’s cancer cells.The rapid development of mRNA vaccines during the COVID-19 pandemic brought mRNA vaccine technology into the mainstream38,91,94,95.RNA vaccines can be delivered through various methods, including viral vectors, micro-vectors (nanocarriers), gene guns,microneedles, orin situelectro- transformation.Technological advances have optimized the stability, backbone structure,delivery methods, and cost- effectiveness of mRNA-based vaccines.As a result, multiple clinical trials investigating mRNA vaccine therapy for various cancers are currently enrolling patients.
In 2017 the first clinical study evaluating neoantigen mRNA vaccines in patients with advanced melanoma demonstrated that all patients developed T cell responses against multiple vaccine targets81.Post-vaccination resected metastases from two patients showed vaccine-induced T cell infiltration and neoepitope-specific killing of autologous tumor cells.Two of five individuals with metastatic disease exhibited objective responses related to the vaccine, while a third patient achieved a CR to vaccination in combination with PD-1 blockade therapy (NCT02035956)81.
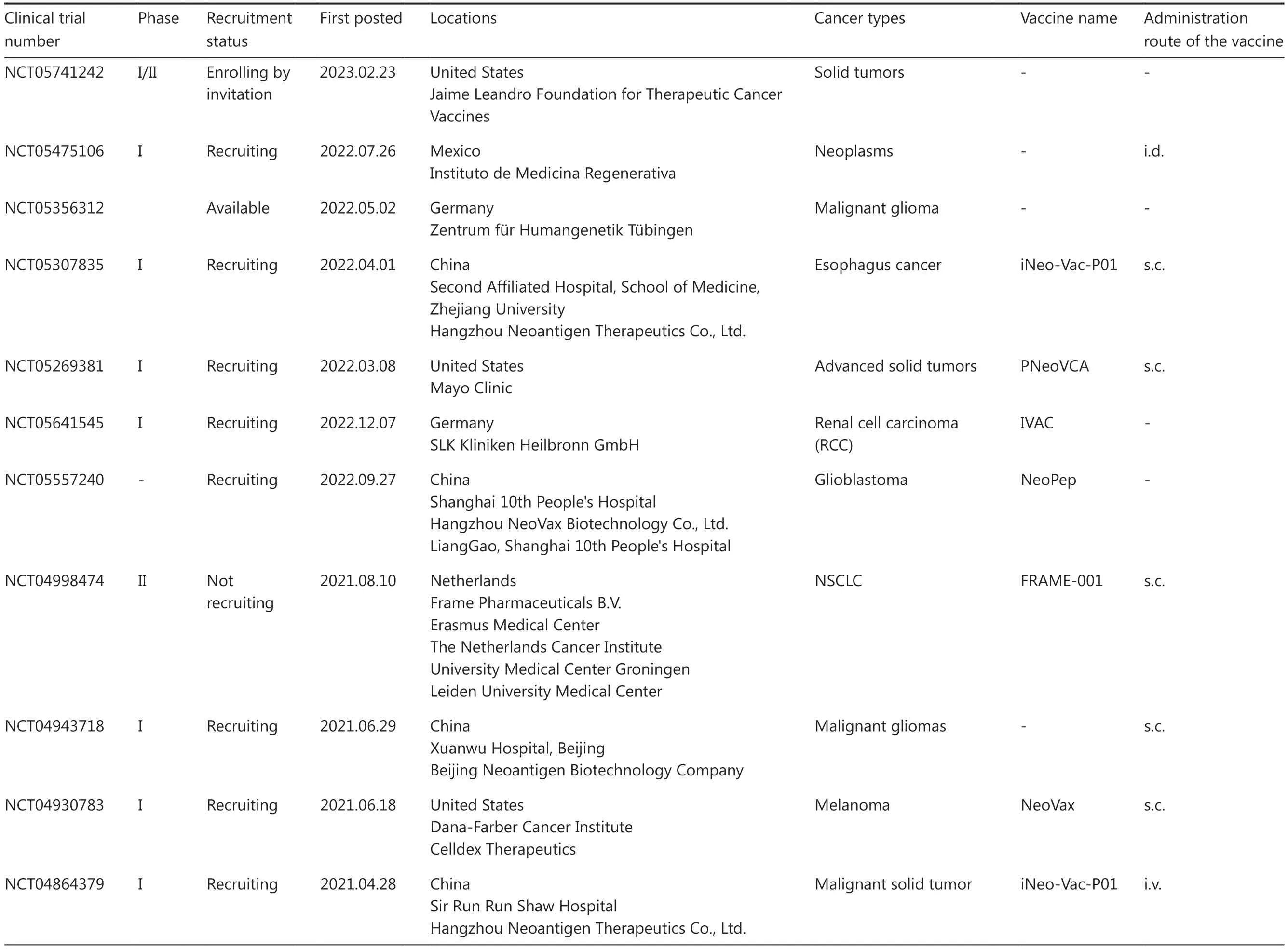
Table 1 An overview of clinical trials for personalized neoantigen-based peptide vaccines
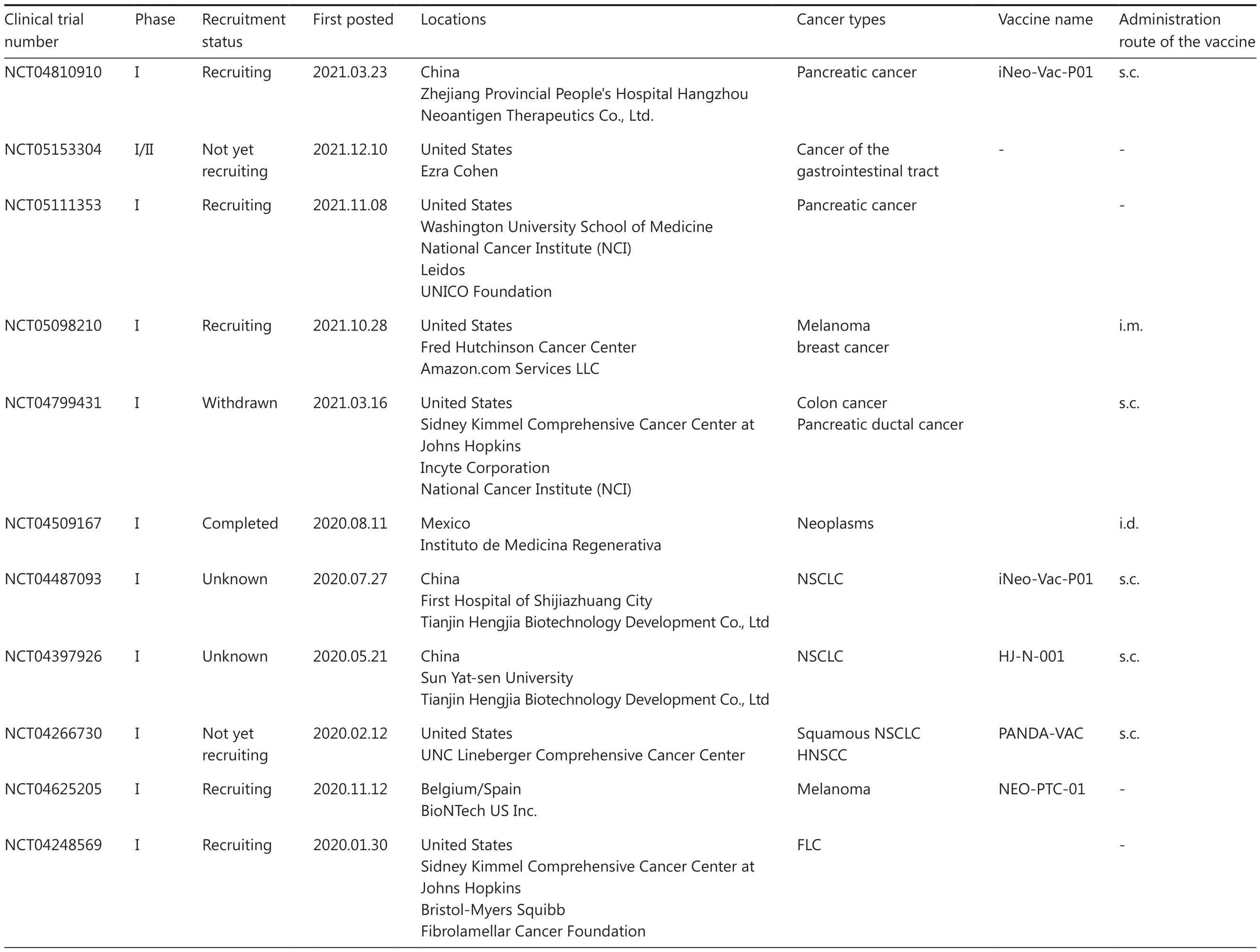
Table 1 Continued
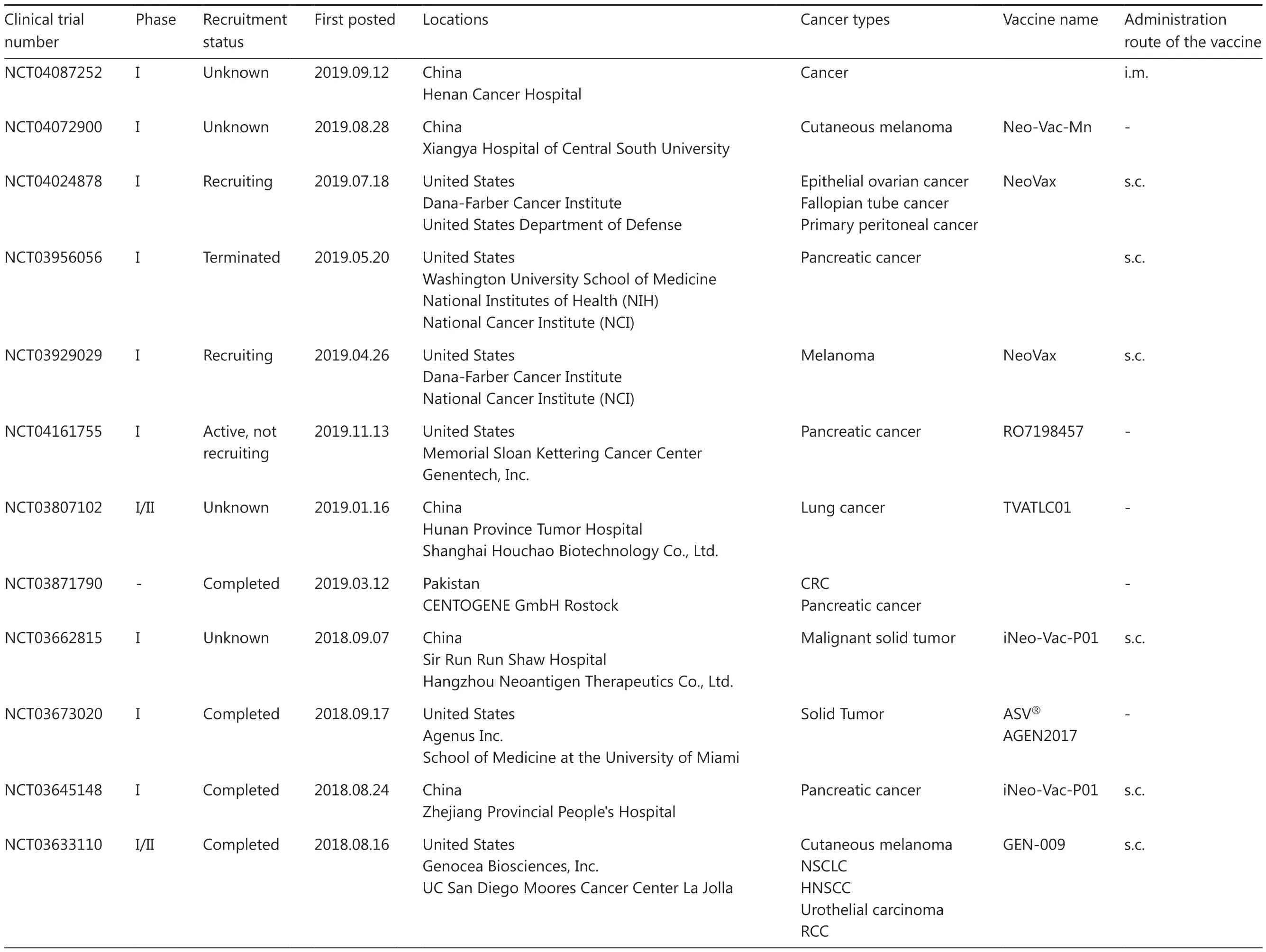
Table 1 Continued
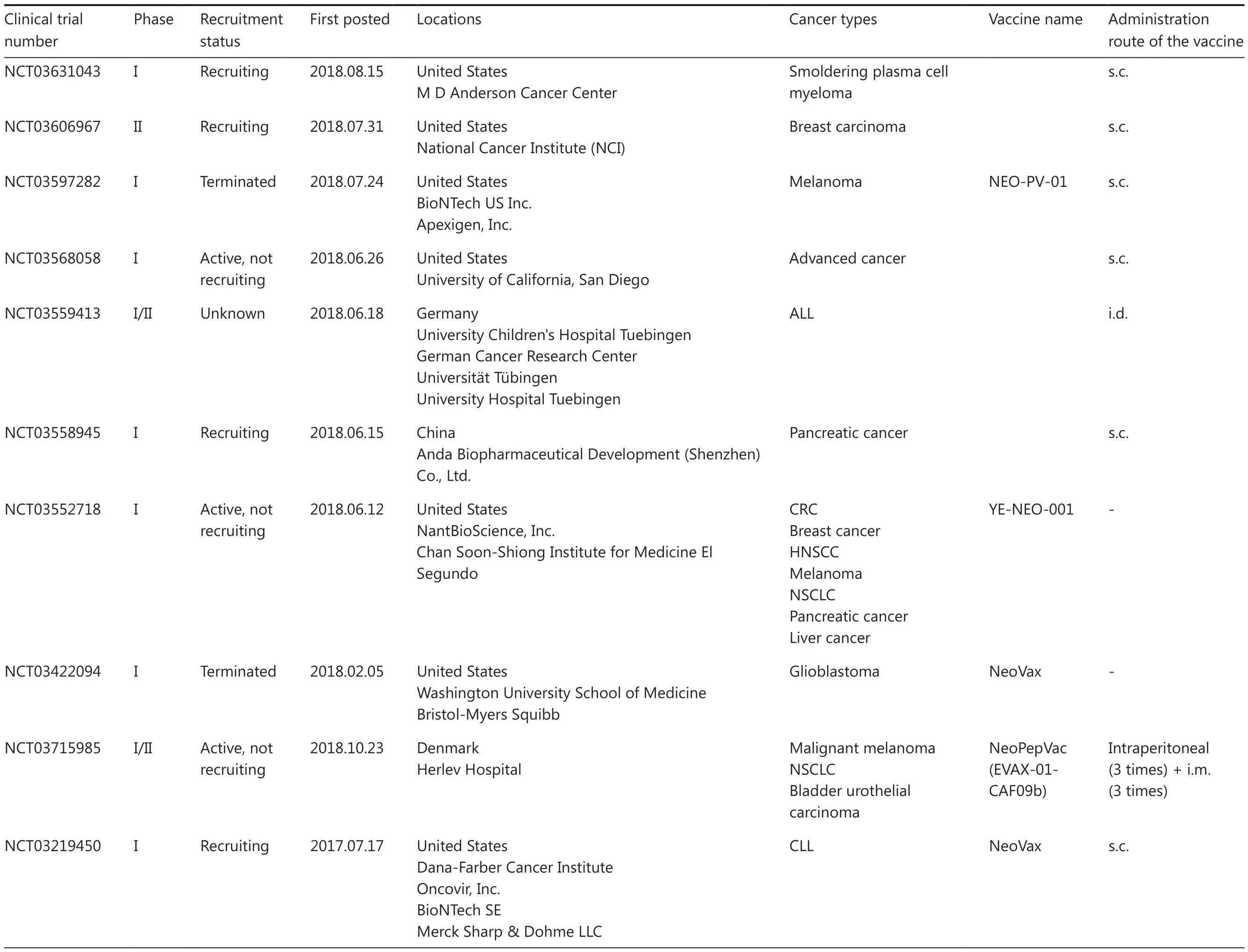
Table 1 Continued
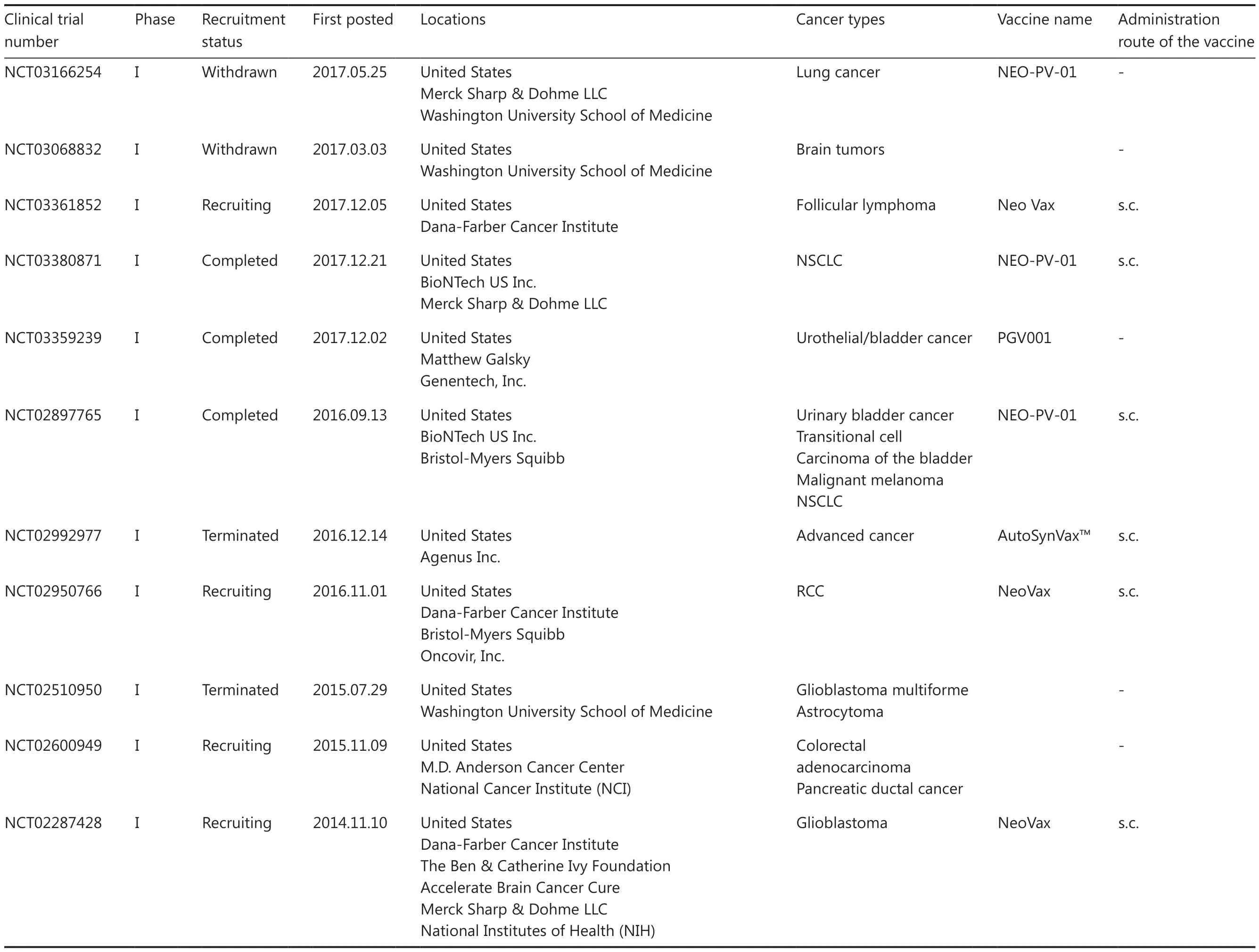
Table 1 Continued

Table 1 Continued
Currently, mRNA vaccines are the forefront of personalized neoantigen vaccine development.The lipid nanoparticle (LNP) mRNA cancer vaccine, mRNA-4157/V940, which encodes 34 neoantigens in a single synthetic mRNA molecule,is being assessed as an adjuvant therapy for high-risk cutaneous melanoma patients who have undergone complete resection when combined with pembrolizumab (NCT03313778 and NCT03897881)96.A study presented at the 2019 ASCO Annual Meeting assessed the efficacy of this treatment as a monotherapy and in combination with pembrolizumab in 33 patients with unresectable solid tumors.In the adjuvant monotherapy group, which comprised 13 patients receiving mRNA-4157, all but 1 patient remained disease-free during the study period, with a median follow-up of 8 months.Among the 20 patients who received combination therapy, 1 achieved a CR prior to vaccination, 2 had partial stable disease, 5 had confirmed disease progression, 2 had unconfirmed disease progression, and 1 had non-evaluable response96.These data supported the advancement of mRNA-4157 to phase 2.As reported at the 2023 American Association for Cancer Research (AACR) Annual Meeting, the combination of mRNA-4157/V940 vaccine and pembrolizumab exhibited a significant 44% reduction in the risk of disease recurrence based on data derived from the phase IIb KEYNOTE-942 trial involving 157 patients with operable high-risk melanoma.Furthermore, most adverse effects were reported to be mild97.A larger phase III trial for mRNA-4157/V940 is currently underway.Based on these findings, the combined use of mRNA-4157/V940 with Merck’s anti-PD-1 therapy Keytruda has been granted breakthrough therapy designation by the US FDA for adjuvant treatment of high-risk melanoma patients following complete resection.
Notably, an ongoing phase I/II clinical trial is currently investigating mRNA-4650, a neoantigen vaccine designed with the same mRNA skeleton to target both shared and personalized neoantigens in patients diagnosed with metastatic gastrointestinal cancer (NCT03480152)94.Another phase 1 clinical trial is currently assessing the efficacy of mRNA-5671, a lipid nanoparticle-based mRNA cancer vaccine that specifically targets four frequent KRAS mutations (G12D, G13D, G12C, and G12V)91.This innovative mRNA vaccine is being administered as monotherapy or in combination with pembrolizumab to patients diagnosed with KRAS-mutated NSCLC, colorectal cancer, or pancreatic cancer91.Moreover, a recent report highlighted the significant T cell activity induced by the personalized RNA neoantigen vaccine, autogene cevumeran, whencombined with atezolizumab and mFOLFIRINOX, potentially leading to a delayed recurrence of pancreatic ductal adenocarcinoma (PDAC)98.
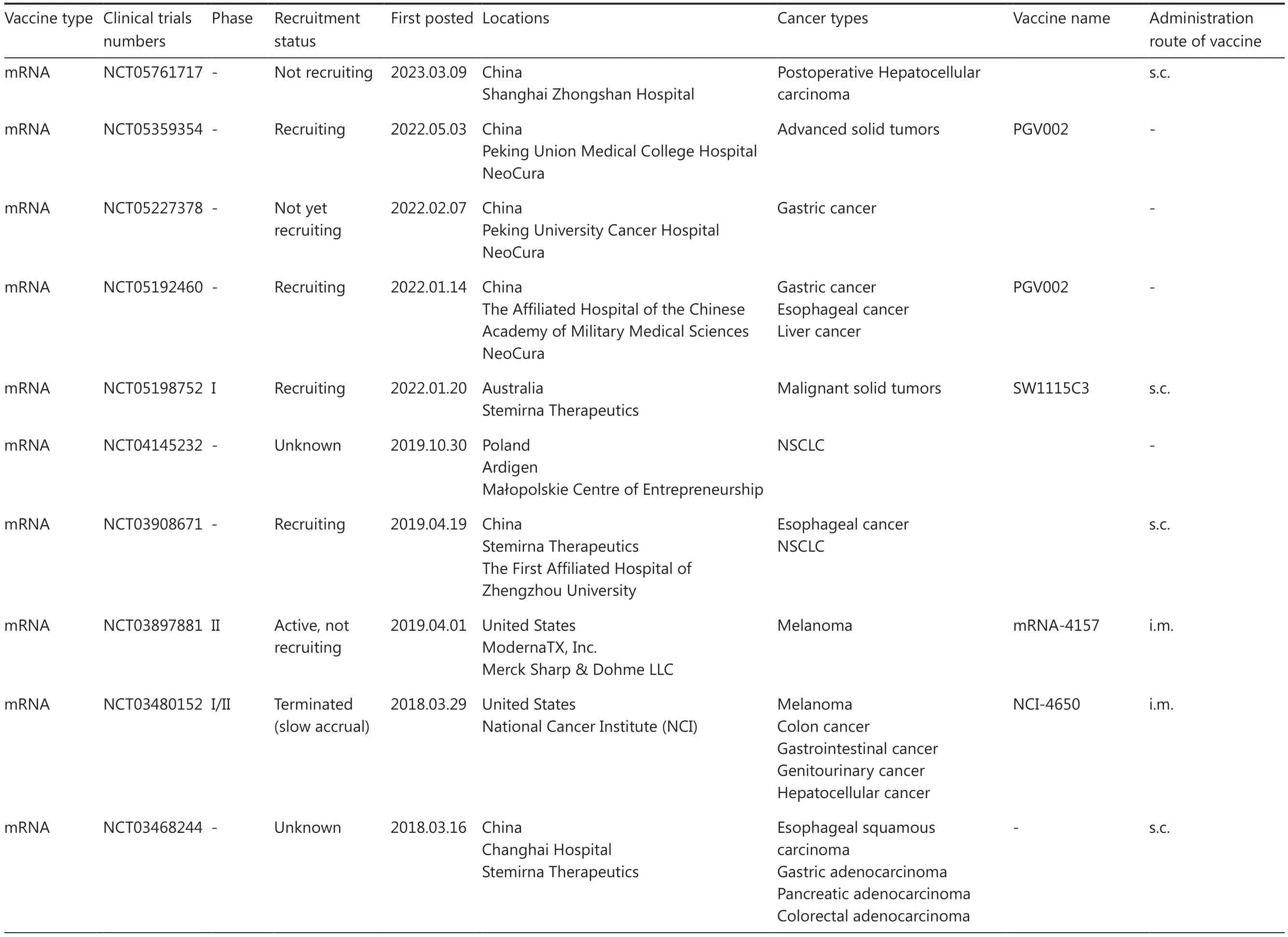
Table 2 An overview of clinical trials for personalized neoantigen-based mRNA and DNA vaccines
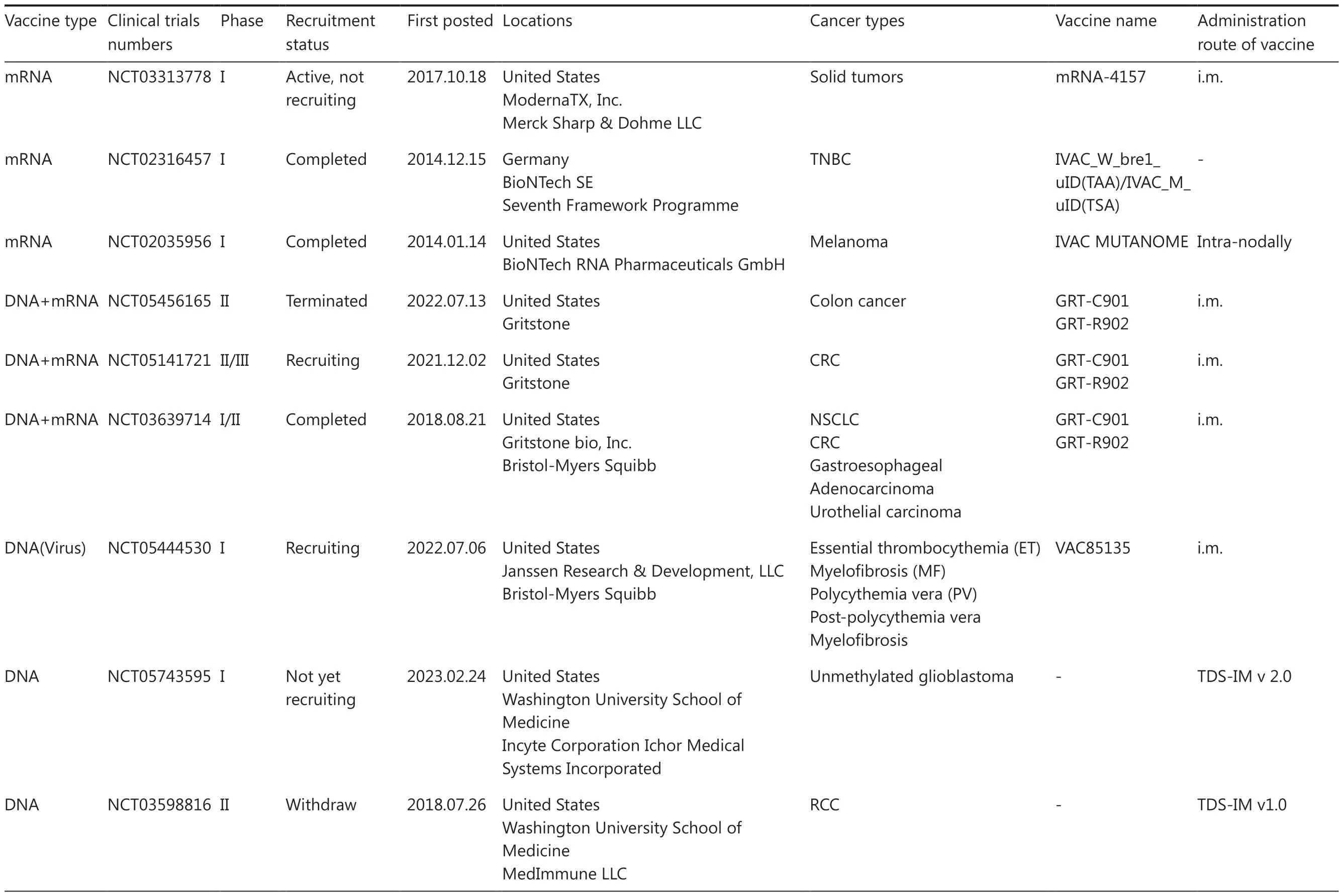
Table 2 Continued

Table 2 Continued
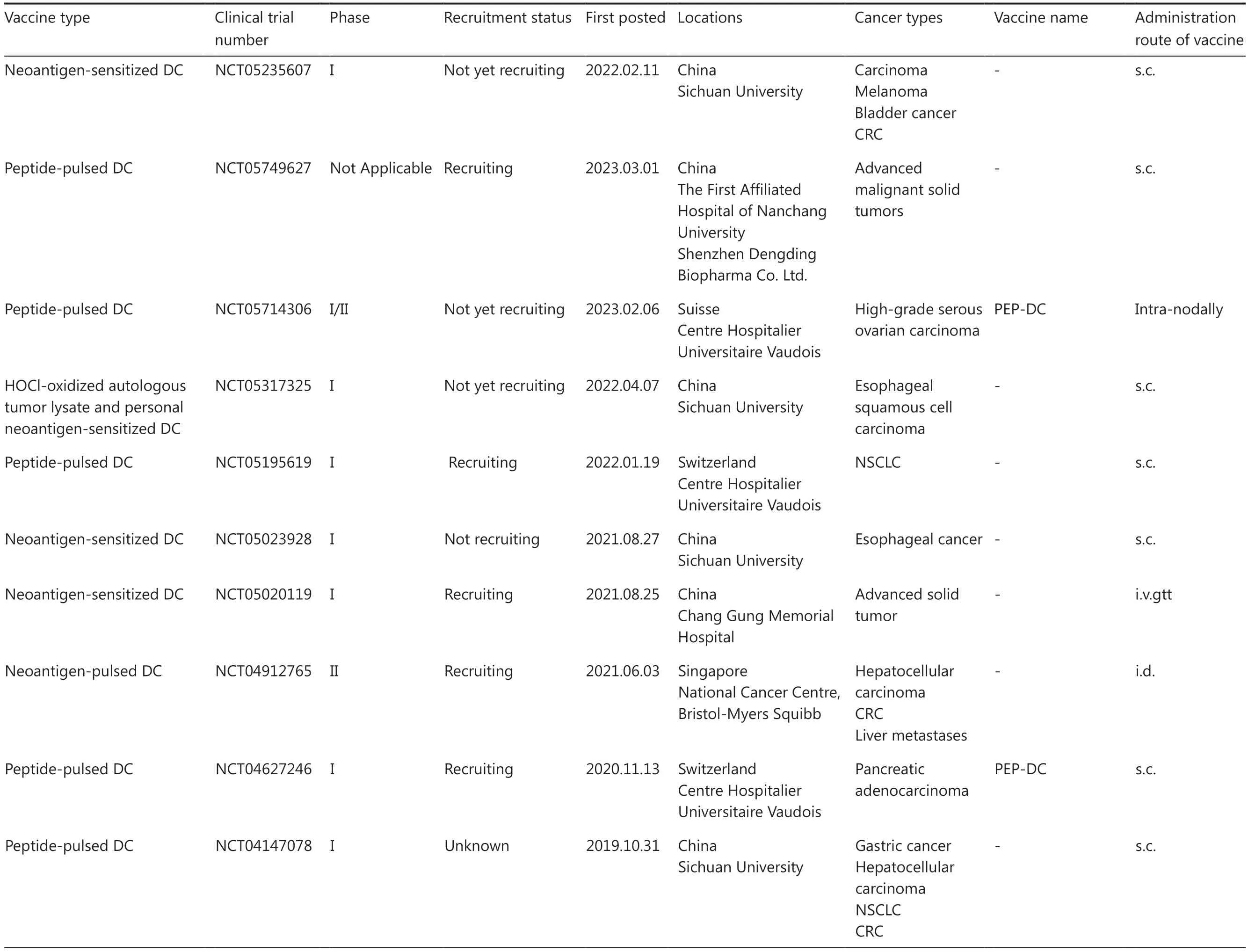
Table 3 An overview of clinical trials for personalized neoantigen-based DC vaccines
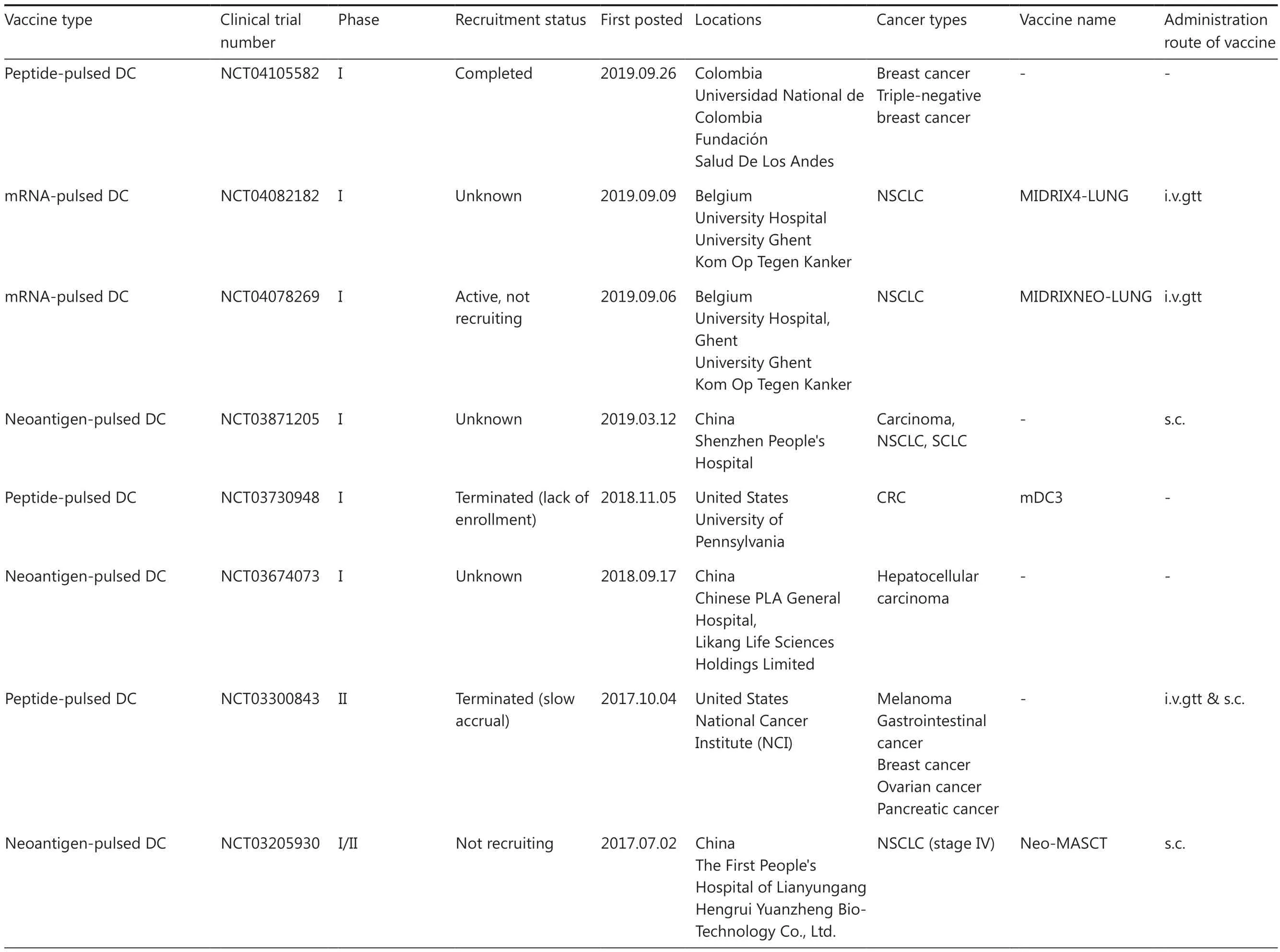
Table 3 Continued
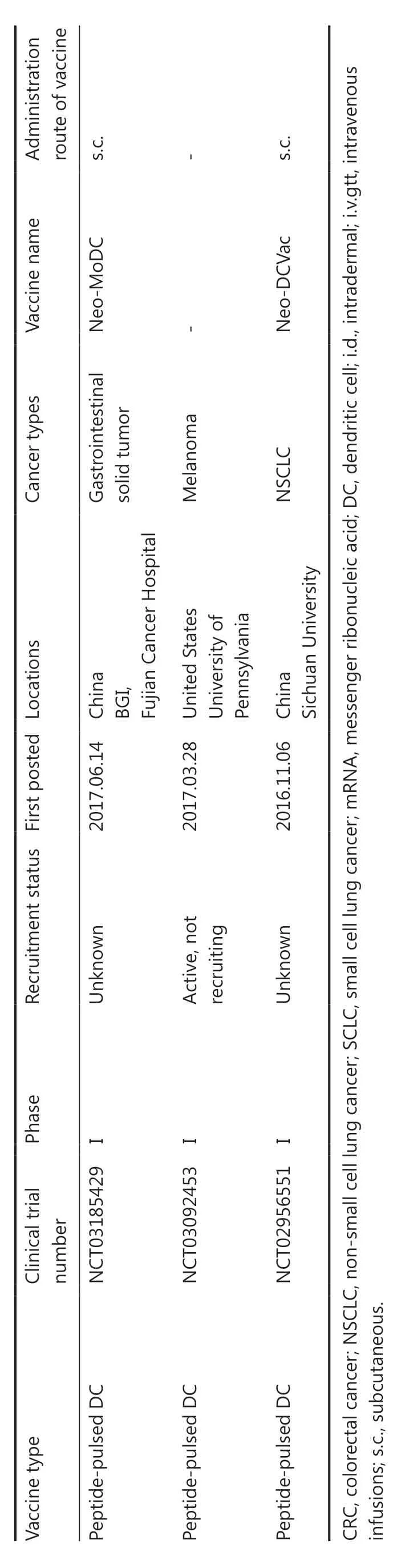
Table 3 Continued
Taken together, neoantigen RNA vaccines hold great potential for cancer immunotherapy due to the ease and rapidity of mRNA production, lower risk of inducing insertional mutagenesis or autoimmunity, absence of HLA restriction,and capability to simultaneously target multiple neoantigens.Efficientin vivodelivery can be achieved through various carriers, such as LNPs.Furthermore, neoantigen RNA vaccines have the capacity to elicit a more robust and enduring immune response by activating both CD8+ and CD4+ T cells94.Although further research is necessary to optimize stability, design, delivery route, dosage, and clinical efficacy, RNA vaccines may provide a valuable alternative to current cancer treatments.
DNA vaccines
Neoantigen DNA vaccines involve the introduction of synthesized DNA fragments encoding specific neoantigens, which are subsequently translated into proteins within cells and presented to the immune system, thereby eliciting an immune response that results in the eradication of cancer cells expressing targeted neoantigens.
One advantage of DNA vaccines is the capacity to deliver multiple neoepitopes simultaneously using the vectors designed to carry genes encoding tumor neoantigens and built-in adjuvants.There are two main types of neoantigen DNA vaccines (plasmid- and viral vector-based).Plasmidbased vaccines involve the direct injection of DNA encoding for the neoantigens into target tissues.Viral vector-based vaccines, in contrast, use a genetically modified virus to deliver the DNA encoding the neoantigens into cells.The virus acts as a delivery vehicle, allowing the DNA to enter cells more efficiently and produce the respective neoantigen.
Delivery techniques have a pivotal role in the efficacy of plasmid vector DNA vaccines.Electroporation (EP), a technique that uses electrical pulses to create transient pores in cell membranes, has been used to enhance the uptake and expression of DNA vaccines in DC.This approach has been used in a phase I trial of a DNA-based neoantigen vaccine for glioblastoma.Viral vector-based DNA vaccines have made significant strides in clinical trials and surpassed plasmids, which are constrained by delivery systems.For example, VAC85135, which was developed utilizing Nouscom’sexclusive viral vector platform, represents a pioneering vaccine candidate entering clinical trials.This innovative platform integrates multiple neoantigen-based viral vector vaccines with other immunomodulatory agents(NCT05444530).Another vaccine, GRT-C901/GRT-R902,is a personalized neoantigen vaccine that uses adenoviral(GRT-C901) and self-amplifying mRNA (GRT-R902) vectors(NCT03639714)92.Currently, a phase 2/3 study is underway to combine GRT-C901/GRT-R902 with immune checkpoint blockade for patients with metastatic colorectal cancer(NCT05141721; Table 2).The primary administration route for these vaccines is intramuscular injection.
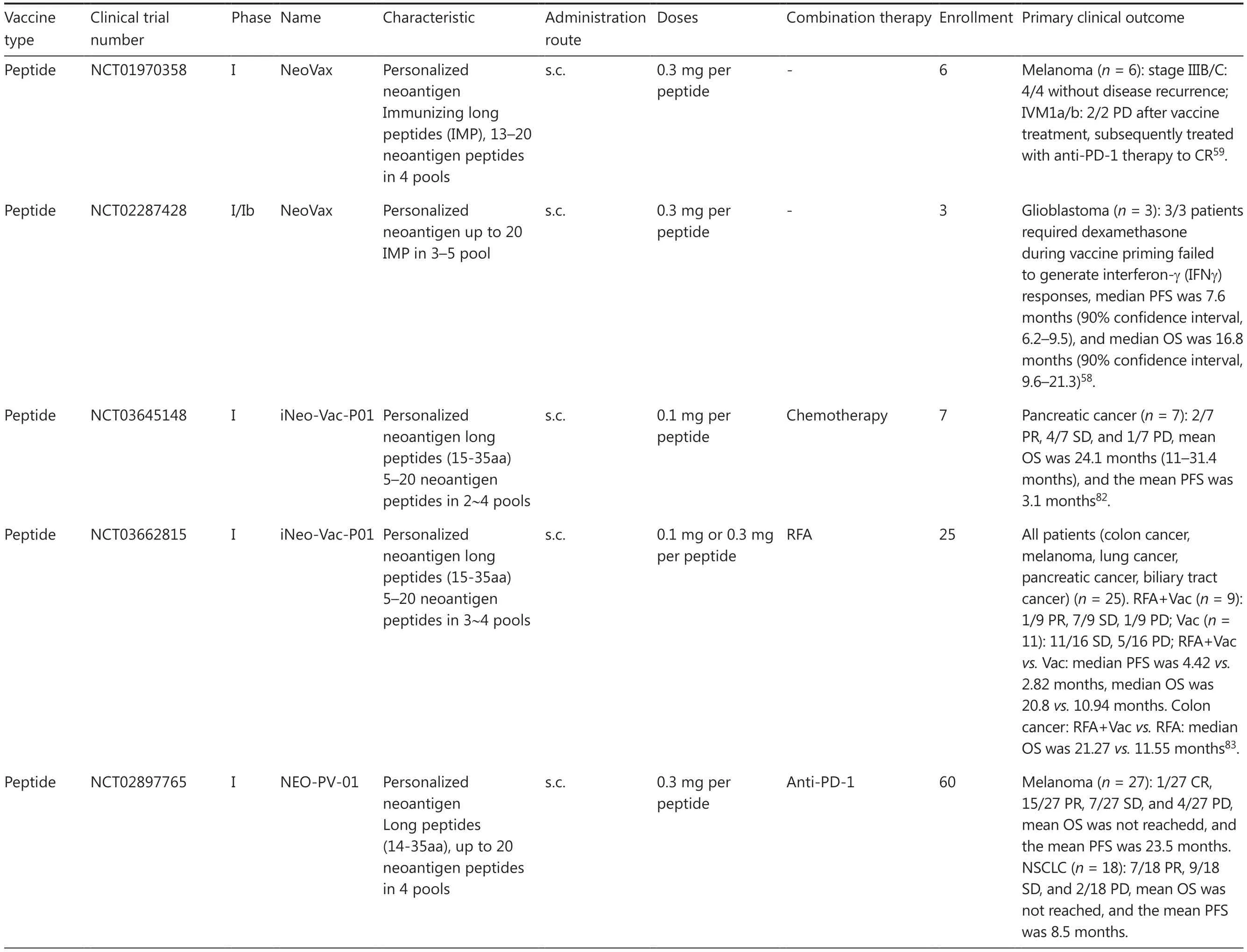
Table 4 Advances in clinical trials for personalized neoantigen-based cancer vaccines

Table 4 Continued
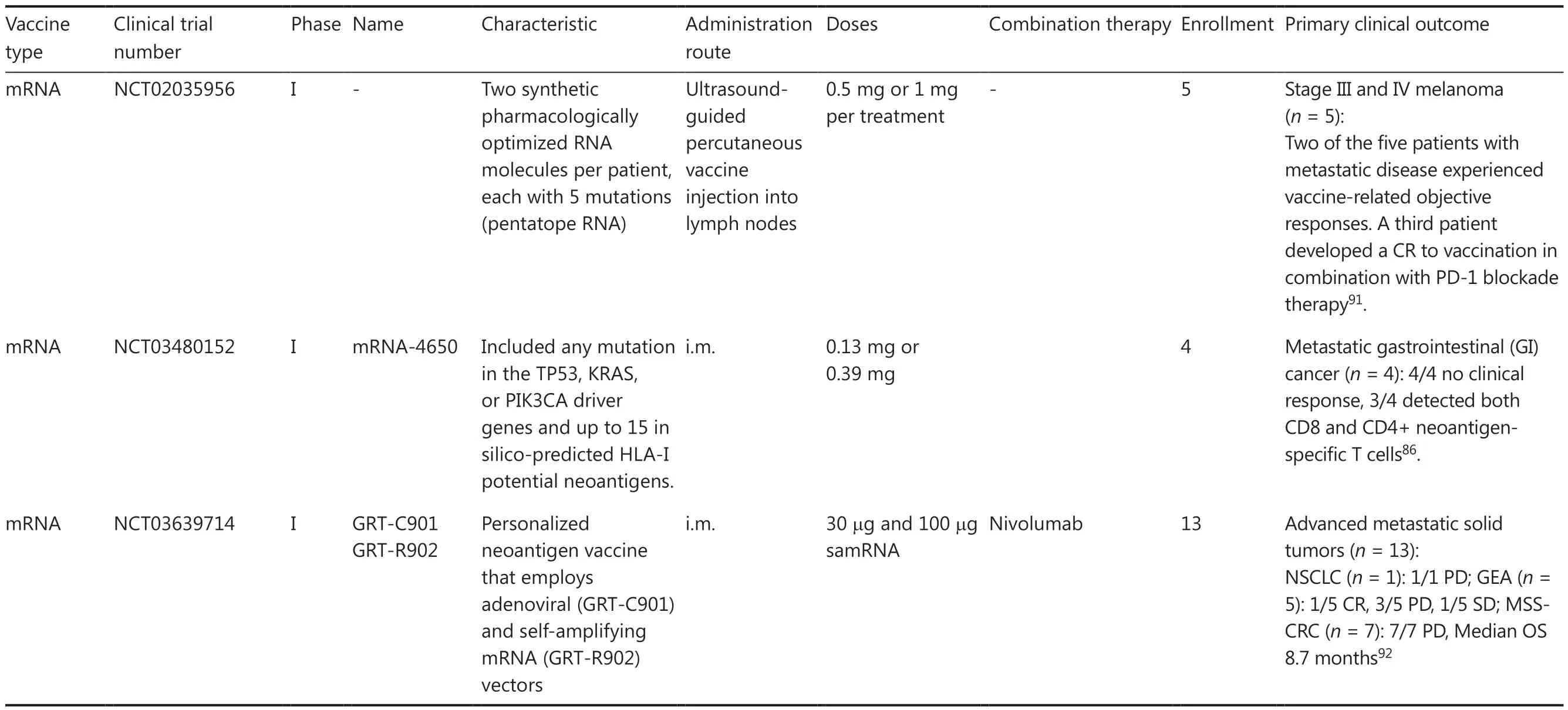
Table 4 Continued
While neoantigen DNA vaccines have demonstrated potential in preclinical studies, the clinical efficacy has been limited due to the weak and short-lived immune response generated by these vaccines.Furthermore, there are concerns regarding the safety of viral vector-based vaccines because viral vectorbased vaccines may elicit unwanted immune reactions or may be integrated into the host genome.Therefore, further research is necessary to optimize efficacy and safety for use in clinical settings.
Neoantigen-pulsed DC vaccines
Neoantigen-based DC vaccines have emerged as a promising approach for cancer immunotherapy due to the high immunogenicity, specificity, safety, and potential for long-lasting immunity.Neoantigen-based DC vaccines have demonstrated exciting efficacy in the treatment of some malignant tumors.DCs are specialized antigen-presenting cells that have the ability to initiate and modulate immune response,which facilitates the transfer of antigens from tumors-tolymph nodes, while ensuring the activation and expansion of tumor-specific T cells within the tumor microenvironment(TME)99,100.Neoantigen-based DC vaccines involve taking a patient’s DCs and subsequently loading the DCs with neoantigen, which can be in the form of peptides, mRNA, or DNA.The neoantigen-loaded DC vaccines are then administered back into the patient’s body, where the neoantigen-loaded DC vaccines stimulate a specific T cell response against the tumor.
The primary routes of administration for DC vaccines are intravenous infusion and subcutaneous injection, while alternative methods, such as intra-tumoral, -nodal, and lymphatic injections, are currently being explored.The use of DC vaccines loaded with various types of antigens, including TAA,TSA, and whole tumor cell lysates, has been extensively investigated.In terms of TSA, peptide-loaded DC vaccines are currently more commonly utilized in clinical trials compared to mRNA-loaded DC vaccines.Peptide-pulsed DC vaccines have demonstrated an augmented spectrum and diversity of neoantigen-specific T cells in melanomas and advanced lung cancer89,101,102(Table 3).
Several clinical trials have investigated the use of neoantigen-based DC vaccines in various types of cancer.The first single-arm study demonstrated that neoantigen short peptide-loaded DC vaccines induce a T cell-specific immune response resulting in antigen spreading in melanoma patients77.In a phase I trial with Neo-DCVac, a peptide-pulsed DC vaccine, has demonstrated remarkable therapeutic efficacy in 12 lung cancer patients with an objective effectiveness rate was 25%, the disease control rate was 75%, the median progression-free survival was 5.5 months,and the median overall survival was 7.9 months89.Another phase I trial described complete regression in a metastatic gastric cancer patient mediated by Neo-MoDC vaccine when used in conjunction with ICI therapy, suggesting promising results for the treatment of patients with metastatic gastric cancer90.
DC-based neoantigen vaccines represent a promising approach to cancer immunotherapy that can leverage the power of DC to prime a specific T cell response against the tumor.However, the use of neoantigen-pulsed DC vaccines is currently limited by the complexity and cost of preparing the DC cells, as well as the difficulty in optimizing the vaccine regimen for individual patients.Further research is needed to optimize the efficacy of these vaccines in the clinic setting.
Overall, numerous clinical trials of neoantigen vaccines are currently underway for a variety of different cancer types, such as NEO-PV-0184,85, NeoVax93, EVX-0186,103, Neo-MoDC90,Neo-DCVac89, iNeo-Vac-P0183,104, mRNA-4157/V94097, and VAC8513541(Table 4).These clinical trials have demonstrated the potential of neoantigen vaccines as a novel approach to cancer immunotherapy, including melanoma, lung cancer,glioblastoma, gastrointestinal tumors, pancreatic adenocarcinoma, and ovarian cancer.In the majority of clinical trials,personalized neoantigen cancer vaccines have been combined with ICIs, such as ipilimumab, pembrolizumab, and nivolumab.Ongoing clinical trials will provide further insight into the safety and efficacy of these vaccines and combination therapy to enhance clinical outcome.
Challenges and opportunities of neoantigen-based cancer vaccine therapy
Despite the promising advances in neoantigen-based cancer vaccines, there remain several challenges that need to be addressed.One major obstacle lies in the identification and characterization of neoantigens because distinguishing between immunogenic and non-immunogenic mutations can be a complex task.The expression of neoantigens varies among tumors, and even within different regions of the same tumor,posing another challenge when optimizing vaccine design and delivery to target patient-specific neoantigens.This complexity is further compounded by the unique nature of each patient’s neoantigens, which necessitates personalized vaccine design.Additionally, it is crucial to ensure the safety and efficacy of these vaccines while avoiding unwanted immune responses.Determining optimal dosing and timing for administration also poses a challenge that can only be addressed through clinical trials.Furthermore, logistical issues, such as cost, manufacturing, and distribution, need to be resolved for personalized neoantigen-based vaccines.
Recent advances in this field have addressed some of the previous challenges and have provided promising opportunities for personalized cancer vaccine therapy in the clinic setting.Further research and development are needed to optimize the design and delivery of these vaccines and to improve the efficacy.Collaboration between researchers, clinicians, and industry partners can help overcome these challenges.Another important development is the use of neoantigen-based vaccines in combination with other therapies, such as ICIs, to further boost the immune response and improve treatment outcomes.Advances in technology will also continue to enhance our ability to identify and characterize neoantigens,unlocking the full potential of these vaccines for patients with solid tumor cancers.
Identification of immunogenic neoantigen
Identification of immunogenic neoantigens is the crucial initial step in the development of effective cancer immunotherapies.Advances in next-generation sequencing technology and computational bioinformatics have facilitated the rapid and cost-effective identification of genomic alterations and putative neoantigens105.To date, most studies have primarily utilized whole-exome sequencing (WES) and RNA-seq analysis of tumor samples as the main source for identifying somatic mutations and neoantigens, but with limited success.Currently, a plethora of novel multi-omics approaches, such as single-cell sequencing and spatial transcriptomics, have emerged.The novel multi-omics approaches are accompanied by advanced bioinformatic algorithms and pipelines that are utilized to investigate tumor heterogeneity, immune repertoire, and somatic mutations across diverse cancer types.This promising approach integrates diverse types of data to enhance precision in identifying potential targets that may have been overlooked by other methodologies, entailing the integration of genomic, transcriptomic, and proteomic information for identifying neoantigen candidates linked with specific tumor mutations.
The single-cell transcriptome sequencing (scRNA-seq)method, which was first developed by Tang et al.in 2009,has been widely utilized in the fields of cancer biology and immunology106-108.The scRNA-seq method enables us to precisely unravel intricate tumor heterogeneity and the complex microenvironment at a single-cell resolution60,109,110.Currently, a novel solution for single-cell immune profiling enables high-throughput sequencing of both immune cell gene expression and immune repertoire (IR) at the single-cell level, making single-cell immune profiling an invaluable tool for identifying cancer neoantigens and anti-tumor TCRs60.TCR sequencing allows for the identification of TCRs that recognize specific neoantigens, and thus play an important role in generating an immune response against cancer cells.Advanced high-throughput sequencing techniques, including high-throughput single-cell DNA sequencing (scDNA-seq),single cell whole genome sequencing [scWGS (SMOOTHseq)]111, and a spatial model of allelic imbalance for identifying somatic mutations in single cell DNA-seq, have been developed112.The utilization of these innovative technologies enhances the efficiency of neoantigen identification and improves clinical outcomes in cancer immunotherapy.
Spatial transcriptomics was awarded the title of “Method of the Year 2020” by Nature Method113,114.To date, over 100 studies have employed this technique in cancer biology and immunology115-120.Various commercial platforms, such as Visium (10X Genomics), Stereo-seq (BGI), and MERFISH(SeqFISH), have been developed for high-throughput spatial transcriptomics118,121.These methods enable researchers to gain novel insight into tumor tissue architecture, heterogeneity, microenvironment, and infiltrating cell populations122-124.However, the limited use of spatial transcriptomics in uncovering cancer neoantigens may be attributed to constraints,such as sequencing depth, high cost, and availability of bioinformatics tools116,125,126.
With the aid of more advanced high-throughput sequencing techniques, we can obtain a more comprehensive molecular profile of tumors and immune systems, which enables identification of TCR sequences that are associated with an immune response against a specific neoantigen.A growing number of studies have highlighted the relationship between neoantigens and T cell recognition of cancer cells127.The distribution and localization of T cells with distinct TCR sequences offer valuable insights into the mechanisms and functional role of T cells in effective cancer immunotherapy,particularly their ability to recognize neoantigens presented by the MHC complex116,125, and have a critical role in the recognition of neoantigens presented by the MHC.Spatial transcriptomics sequencing can reveal the subtypes and TCR profiles of T cells at a spatial resolution, enabling quick identification of neoantigen-reactive TCRs through computational predictions for putative neoantigen-MHC complexes.
Despite the potential benefits of multi-omics approaches,significant challenges remain to be addressed, including the development of robust and accurate algorithms for integrating different data types, as well as establishing standardized protocols for sample collection and processing.Overall, these methodologies hold great promise in improving the accuracy of identifying immunogenic neoantigens and will be critical for advancing the field.
Computational prediction of immunogenic neoantigens
Neoantigen prediction is a crucial initial step in neoantigen-based immunotherapy.Advances in sequencing technologies, computational algorithms, and machine learning techniques have led to improved accuracy and efficiency in predicting neoantigens with high immunogenicity, which are used for personalized vaccines for individual patients.However, due to the diversity and stochastic of T cell immune responses and tumor heterogeneity,the majority of reported neoantigen prediction pipelines have exhibited a limited accuracy rate1,43,70,105,128,129.
The process of neoantigen prediction typically involves the identification of somatic mutations, HLA typing, peptide processing, peptide-MHC binding affinity and presentation,as well as TCR recognition of the neoantigen-MHC complex.Subsequently, candidate neoantigens are scored and ranked based on the higher likelihood of immunogenicity.Recently,the Tumor Neoantigen Selection Alliance (TESLA) has identified a set of key parameters for tumor epitope immunogenicity to enhance the accuracy of neoantigen prediction.These parameters include binding affinity and stability, gene expression level, peptide hydrophobicity, and foreignness130.Over time, all tools and software packages required for neoantigen prediction and selection have evolved with technologic advancements.In this discussion, we will explore the latest developments in this field along with the most commonly used tools.
First, tumor-specific mutations are identified by analyzing genomic and transcriptomic data from tumor and normal samples (typically peripheral blood mononuclear cells)obtained from patients through WES and RNA sequencing.A variety of software programs has been utilized to analyze the sequencing data, such as Alfred and Qualimap2 for WESseq data analysis, minimap2 for RNA-seq analysis, STAR and HISAT for SVs analysis, and MuTect2, CaVEMan, Strelka,VarDict, and MuSE for SNVs and indels analysis127,131-133.Additionally, fusion genes were analyzed using STAR-Fusion,EricScript, and Breakfast70,134.
Next, MHC binding prediction tools evaluate the binding affinity of the predicted mutant peptides to specific MHC molecules.HLA molecules present neoantigens to T cells,including MCH class I or II molecules, hence accurate HLA typing is vital to predict neoantigens binding to the HLA alleles.A wide range of tools have been developed and utilized for various purposes70,128.Specific HLA typing methods are utilized to determine an individual’s precise HLA alleles, such as HLA-VBSeq, seq2HLA, HLAminer, HLAscan, HLA-HD,HLAforest, and HISAT-genotype.To be presented to T cells effectively, neoantigens must bind efficiently with MHC molecules.However, predicting MHC binding specificity with precision is challenging due to the diverse array of MHC alleles and the distinct binding preferences.The development of algorithms that successfully anticipate MHC binding is pivotal in enhancing neoantigen prediction accuracy.Additionally, various tools, such as NetMHCpan4.1, MHCflurry, MHCnuggets,EDGE, MHCRoBERTa, MixMHCpred2.0.1, NetMHCcons1.1,DeepSeqPanII, TransPHLA, pVACseq, EpiScan Predictor,TLimmuno2, NetChop20S, NetChopCterm, ProteaSMM, and NetChop-3.0 Cterm, are utilized to determine neoantigen binding affinity with specific patient MCH class I or II molecules (8-15 AA for MHC-I and 13-25 AA for MHC-II)1,135-144.
Accurately predicting the immunogenicity of neoantigens remains a challenge because not all neoantigens induce an immune response.The intricate nature of the cancer genome and the diverse TCR repertoire pose significant challenges in identifying neoantigens through TCR sequencing.Because each T cell possesses unique TCR sequences, not all TCRs recognize a specific neoantigen.However, this obstacle can be overcome by utilizing prediction algorithms that identify TCR sequences for a given neoantigen.Immune repertoire profiling is performed by deep sequencing T-cell receptor genes of TILs and peripheral blood lymphocytes (PBLs) to identify TCR sequences that bind to the predicted neoantigens145,146.The binding between TCR and tumor neoantigen has been investigated using several public datasets, including TBAdb147, VDJdb148-150, McPAS-TCR151, IEDB152, and the Cancer Epitope Database and Analysis Resource (CEDAR)153.Furthermore, various deep learning models have been explored for predicting TCR-peptide binding, such as pMTnet154, NetTCR155, DeepTCR156, ImRex157, AttnTAP145, ATMTCR158, DLpTCR159, TITAN160, dbPepNeo2.0161, epiTCR140,and TCR-Pred162.Additionally, assessing TCR clonality and spatial localization allows for molecular tracking of antitumor T cell trajectories, clonal dynamics, and phenotypic changes during treatment.Ongoing research and clinical trials are exploring the potential application of this approach in cancer treatment55,126.
Several software pipelines have been developed to streamline the process for neoantigen prediction and to rank the most promising neoantigens based on the predicted binding affinities, functional effects, and other contextual information.These pipelines integrate various tools and algorithms to automate prediction and analysis with limited accuracy, such as pVAC-Seq and NetMHCpan.However, challenges related to identifying immunogenic neoantigens and accurately predicting the interactions with the immune system remain areas of active research.
In vitro validation of immunogenic neoantigens
Despite being a promising and innovative approach for attacking and eliminating tumor cells, the efficacy of neoantigen-based cancer immunotherapy relies heavily on the quality of selected neoantigens.Computational prediction pipelines are currently used to identify and prioritize potential neoantigens that frequently generate false-positive neoantigens which only elicit limited anti-tumor immune responses in the clinic setting.Assessing the precision of prediction algorithms in identifying genuine neoantigens recognized by the patient’s own T cells remains a challenge given that only a small proportion of predicted neoepitopes are typically recognized by T cells and elicit specific T cell responsesin vivo163.Therefore,it is crucial to accurately identify true-positive neoantigens for effective clinical cancer treatment.
Multiplein vitroverification methods are utilized to identify immunogenic neoantigens that truly stimulate autologous specific immune responses and eliminate tumor cells.The immune response of neoantigen-reactive T cells is traditionally detected using various techniques, including enzyme-linked immunosorbent spot (ELISpot), flow cytometry, multi- color labeled MHC tetramers, and T cell sequencing164-168.Flow cytometric measurement detects T cell activation markers(CD4, CD8, CD137, CD107a, and INFγ), while ELISpot assay detects the secretion of INFγ after stimulation with candidate neoantigen vaccines derived from patient PBMCs or TILs169,170.Multicolor-labeled MHC tetramers can also be screened for epitope short peptide and TCR affinity studies to evaluate the reactivity of various potential epitopes of T cells following candidate neoantigen stimulation171,172.Additionally, single-cell RNA and TCR sequencing can be utilized to analyze neoantigen-stimulated T cells, facilitating the identification of activated T cells responding to neoantigens166,167.Other methods include chimeric receptors, known as signaling and antigen-presenting bifunctional receptors (SABRs), for validating MHC-TCR complex interactions, as well as T-Scan for detecting the physiologic activity of T cell killing has also been explored in this application142.
The further validation of neoantigens often relies onin vitrotumor models.Nevertheless, conventional approaches using primary cells, cell lines, or animal models possess inherent limitations when studying cancer-immune interactions that are directly applicable to human biology and clinical translation.For example, 2D cell line cultures lack the spatial architecture found in real tumors, while patient-derived xenograft (PDX) models often better emulation of biological characteristics but struggle with incorporating a patient’s unique TME and immune system into mice.Consequently,these methods fail to accurately replicate the intricate tumorimmune microenvironment and the significant heterogeneity of solid tumors among patients173.Henceforth, there is a need for a novel approach capable of identifying immunogenic neoantigens that can effectively induce an anti-tumor immune response and selectively eliminate tumor cells in cancer treatment.
Patient-derived organoids (PDOs) have recently emerged as a powerful tool for cancer research and personalized medicine174,175.PDOs are three-dimensional cell culture models that faithfully recapitulate the architecture, characteristics, and functionality of original tumors176.In contrast to cell line cultures and PDX models, PDO models provide a wider range of cell types and maintain spatial structures that closely resemble the corresponding tumor tissuesin vivo.Moreover, the origin of PDOs can be more diverse because PDOs can be derived from surgically resected tissues, needle biopsy samples, circulating tumor cells, and malignant effusions177.The pioneering establishment of colon cancer PDOs was reported in 2011, followed by subsequent studies of PDO models for liver178, pancreatic179, breast180, and lung cancers181,182.
The ability of PDO to recapitulate patient-specific tumor characteristics and mimic the TME has made PDOs a valuable and reliable tool for validating and screening neoantigen cancer vaccines183,184.There are two distinct methods for constructing organoids within the immune microenvironment185,186.The first approach involves establishing a co-culture system at the air-liquid interface in a Transwell chamber to promote the proliferation of both organoids and endogenous immune cells187-189.This culture system maintains the heterogeneity of the original TCR due to the presence of endogenous immune cells; however, the culture system is limited to maintaining the immune microenvironment for only 2 months189.The second approach involves a submerged culture, in which exogenous immune cells are introduced into tumor organoids for co-culture.It should be noted that typically, submerged Matrigel PDO can only support the growth of cancer cells; therefore, exogenous immune cells isolated from the PBMCs or TILs must be introduced to create a co-culture system that mimics the internal microenvironment of the tumor patient190,191.This submerged culture method facilitates efficient interaction between cancer and immune cells, allowing for long-term cultivation of tumor organoids that can induce an immune microenvironment at any given time.A PDO model with immunocompetent microenvironments was established to conduct T cell cytotoxicity assays for neoantigen vaccines with the aim of improving the precision in identifying neoantigen vaccines that elicit an immune response (Figure 2).This framework provides a robust platform for screening and validating neoantigen candidates, ultimately leading to the development of personalized vaccines with high immunogenicity and potent tumor-killing capabilities for clinical applications (Figure 2)184,192.
Optimization of neoantigen vaccine platform
To enhance the accessibility and scalability of clinical applications, ongoing efforts are being made to optimize the manufacturing and delivery procedures of neoantigen-based vaccines.The production process of neoantigen vaccines involves synthesizing neoantigen vaccines in various formats,such as peptides, DNA/RNA, or neoantigen-loaded DC vaccines.However, this production process is intricate and expensive.Producing autologous DC cell-based neoantigen-loaded vaccines incurs exorbitantly high costs and poses greater challenges in terms of quality control and cold chain logistics compared to conventional preparations.Furthermore, currently it is not feasible to make these treatments universally applicable due to the cost, which remains difficult to reduce,which poses a significant financial burden for cancer patients without medical insurance.One strategy that shows promise is utilizing synthetic biology methods to increase the efficiency of vaccine production.It should be noted that with advancing technology and streamlining of the manufacturing process,there is an expectation for a decrease in the cost of these vaccines over time.
To elicit an effective immune response, a major challenge lies in ensuring the precise delivery of the cancer vaccine to its intended target site.Various administration methods have been explored, including intravenous (IV), intramuscular(IM), subcutaneous (SC), or intracutaneous (IC) injections,and directly into lymph nodes.These approaches aim to effectively introduce the neoantigen into the antigen-presenting cells and generate a robust T cell response against cancer.Each approach possesses distinct advantages and disadvantages depending on factors, such as vaccine type, disease stage, and patient immune status79,193-196.
Furthermore, the optimal timing and frequency of vaccination have not been extensively investigated to date.Some studies suggested that repeated or combination dosing might be necessary to maintain an effective immune response,but this strategy could increase the risk of adverse effects.Additionally, patient-related factors, including immune status,co-morbidities, and previous treatments, can all influence the efficacy of neoantigen vaccines.For example, patients with immunosuppression or a high tumor burden may have a weaker response to the vaccine.The selection of an appropriate

Figure 2 Illustration for the in vitro validation platform of the efficacy of neoantigen cancer vaccines.The framework provides an in vitro platform for the screening and validating neoantigen candidates, including TCR-guided strategies, T cell activation validation, and organoid killing assays, with the aim of improving the precision in identifying neoantigen vaccines that elicit a robust immune response and effectively eliminate cancer cells.Consequently, positive outcomes facilitate the development of personalized vaccines with enhanced immunogenicity and potent tumor-killing capabilities for clinical applications, thereby enabling the evaluation of neoantigen cancer vaccines suitable for implementation in a clinical setting.
delivery method for neoantigen vaccines will depend on multiple factors and may vary among patients.Ongoing clinical trials are currently exploring diverse vaccine platforms and delivery methods to determine which vaccine platforms and delivery methods offer the optimal balance between safety,efficacy, and patient convenience.
Combination therapy
Considering the intricate mechanisms of the immune system and the high heterogenicity and adaptability of solid tumors during the treatment, combination therapies that target different phases of the cancer-immune cycle simultaneously may prove more effective.Cancer cells have evolved innate defense systems to evade immune recognition at each stage of the cancer immune cycle5,197.Most single-agent monotherapies only target one or two stages of the anti-cancer immune pathway,resulting in limited effectiveness for patients with advanced malignancies.In contrast, combination therapies concurrently target multiple phases of the cancer immune cycle, including antigen release and presentation, immune cell priming and activation, immune cell migration and invasion to tumors,as well as cancer cell recognition and killing.Dead cancer cells generate additionalde novoneoantigens that augment and strengthen the immune response in subsequent cycles.Therefore, combining neoantigen vaccines with other therapies has been shown to enhance the anti-tumor immune response and improve clinical outcomes in preclinical studies and clinical trials, thereby initiating or amplifying the selfsustaining cancer-immune cycle71,76,198-200.
Recently, a variety of immunotherapeutic strategies have been developed and integrated with neoantigen-based vaccines to create a platform for simultaneous administration of multiple drugs or therapeutic agents.These agents synergistically activate different stages of the cancer-immune cycle, reverse immunosuppression, and foster an immunesupporting TME.By combining therapeutic agents with different mechanisms of action, these strategies induce robust,effective, durable, and tumor-specific immunity.Numerous clinical studies have shown that combining neoantigen-based cancer immunotherapy with traditional cancer treatments,such as ICIs, radiotherapy, and chemotherapy, can result in more substantial and long-lasting therapeutic outcomes201,202.Combination strategies involve diverse approaches to induce cell death, including chemotherapy, radiotherapy, targeted therapy, and oncolytic viruses, which all enhance the release ofde novoneoantigens71,83,203,204.Furthermore, modifying the TME and intra-tumoral cytokine milieu, such as IL-2,IFN, and transforming growth factor (TGF)-β, which may promote differentiation of immature T cells into effector T cells and infiltration into tumors.Overcoming immunosuppression by using ICIs (anti-CTLA4/PD-1/PD-L1 antibodies)and indoleamine-pyrrole 2,3-dioxygenase (IDO) inhibitors is also crucial in activating different T cell populations.Lastly, augmenting both the quantity and quality of tumorspecific T cells through cancer vaccines and ACT is crucial for enhancing the clinical efficacy of immunotherapyviaimplementation of effective recombination strategies22,94,205,206.To achieve improved clinical outcomes in solid tumors,further exploration into recombination strategies involving neoTCR-T, neoCAR-T, and CAR-M cell therapy for solid tumors is necessary8.
Combination therapies have shown promising results in preclinical and clinical studies, with improved response rates and prolonged progression-free survival.Nevertheless, the efficacy of these treatments may vary depending on factors,such as cancer type and stage, patient immune status, and treatment regimen.Further research is needed to determine the optimal combination strategies of neoantigen vaccines with other therapies.
Regulatory framework of neoantigen cancer vaccines
Recent advances in cancer vaccines have expedited the development and regulatory approval of neoantigen-based cancer vaccines.Due to the individualized nature of neoantigens among patients, neoantigen cancer vaccines are tailored specifically for each person, which ensures a personalized treatment approach.To ensure the safety, quality, and efficacy of vaccines, neoantigen cancer vaccines encounter various regulatory and policy challenges domestically and internationally.Government regulatory departments have a crucial role in overseeing, monitoring, and enforcing stringent standards for vaccine manufacturing, while also providing essential support through funding basic and translational research, as well as offering guidance.
Stringent regulations governing neoantigen cancer vaccines are implemented to minimize risks and protect patients72.The regulatory process for clinical trials and vaccine approval is overseen by the FDA in US.Prior to authorization for human use, neoantigen cancer vaccines must undergo rigorous preclinical and clinical trials to demonstrate both safety and efficacy.The Center for Biologics Evaluation and Research(CBER), a division of the US FDA, actively develops specific guidelines and regulations pertaining to these vaccines.The European Medicines Agency (EMA) is responsible for regulating neoantigen cancer vaccines in Europe.The Committee for Medicinal Products for Human Use (CHMP) evaluates data submitted by companies, including manufacturing quality, non-clinical data, and clinical trial results.Based on this assessment, CHMP provides a recommendation for approval,which is then finalized by the European Commission.The Pharmaceuticals and Medical Devices Agency (PMDA) is the regulatory authority for pharmaceuticals in Japan.The PMDA ensures compliance with Japanese standards through comprehensive scientific evaluations that include facility inspections,as well as assessments of quality management systems207.
Based on the current legal and regulatory framework in China, combined with actual cases, the clinical trial of neoantigen cancer vaccine adopts a dual-track regulatory mode,also known as “conditional approval.” This approach involves pharmaceutical companies initiating clinical trials, while researchers initiate a separate clinical trial regulatory mode208.The dual track regulation approach grants conditional approval based on initially promising safety and efficacy data,while further clinical data is collected after the therapy is marketed.Conditional approval has been utilized by the US FDA and EMA for new therapies addressing severe or lifethreatening conditions, such as cancer and Ebola virus infection.To address this issue more effectively, the NMPA recently released guidelines for developing and evaluating therapeutic cancer vaccines.These guidelines include “Guiding Principles for Clinical Trials of Tumor Therapeutic Vaccines (Draft)”(9 October 2022) and “Guidelines for Quality Management of Cell Therapy Products (Trial Implementation)” (31 October 2022), which are based on existing regulatory documents for clinical trial supervision.These guidelines not only regulate and guide pharmaceutical research and development, production, and registration of immune cell therapy products,but also pay special attention to personalized or autologous vaccines.The guidelines provide explicit and comprehensive instructions regarding production processes and quality control measures for cancer vaccines.Moreover, the guidelines ensure compliance with Good Clinical Practice (GCP)standards that mandate safety and efficacy assessments, while facilitating effective connection between investigator-initiated clinical trial data for new drug research applications.
While specific regulatory frameworks have been established for neoantigen cancer vaccines in all of these countries and regions, it should be noted that these frameworks are subject to continuous updates and refinements as scientific knowledge progresses.The development of personalized vaccines based on an individual’s unique cancer neoantigens poses various challenges, such as the absence of standardized guidelines,variations in evaluation criteria across different regulatory authorities, difficulties in regulating the dynamic nature of neoantigen cancer vaccines effectively, and ensuring robust post-market surveillance along with long-term safety monitoring.Overcoming these obstacles requires collaborative efforts between regulatory authorities, industry stakeholders, and scientific communities to establish comprehensive globally harmonized regulations that guarantee adherence to necessary standards regarding safety, quality, and efficacy of cancer vaccines.
Collaboration
The development of neoantigen-based cancer vaccines is technology-intensive and talent-oriented.From the perspective of the entire workflow and industrial chain, manufacturing neoantigen vaccines, such as peptides, mRNA/DNA vaccines,and neoantigen-loaded DC therapies, are highly complex.The emergence of this vaccine therapy has led to extensive collaboration among research institutions, hospitals, and biotech and pharmaceutical companies involving professionals from diverse fields, including researchers and physicians in frontline clinical hospitals, gene sequencing firms, AI big data companies, and CRO companies.Such collaborations facilitate crucial exchange of knowledge, technology, and resources that are vital for advancing the discovery and production of effective vaccines.
Although many clinical trials of neoantigen vaccines are still in the early phase, this field is rapidly advancing globally.Research institutions within universities and other academic organizations possess the valuable scientific expertise and resources to conduct fundamental research, identify neoantigens, develop techniques and technology to deliver vaccines,and study the correlates of vaccine-mediated protection.Hospitals play a vital role in conducting clinical trials of neoantigen vaccines as well as providing patient samples for vaccine development and evaluation.Biotech and pharmaceutical companies have a crucial role in propelling the clinical application of neoantigen vaccines, often having specific expertise in areas, such as high-throughput sequencing, recombinant DNA technology, protein engineering, biosynthetic technology, and vaccine delivery platforms.Many companies cooperate with each other to explore the clinical effect of neoantigen cancer vaccine in cancer treatment, and some potential products have received a US FDA fast track designation and clinical trial approval, such as mRNA-4157/940 (Moderna and Merck,2022), BNT111(BioNTech), NEO-PV-01(Neon Therapeutics),GRANITE (Gritstone Oncology), AutoSynVax™ [ASV™(Agenus Inc.)], and GEN-009 (Genocea Biosciences) (Table 4).Several emerging biotech companies are actively developing neoantigen-based cell immunotherapy, including peptide,DC, and mRNA vaccines in China.To date, many products have received implied approval for the clinical trial by the NMPA, such as Neo-T (Beijing Genomics Institute Jinuoyin),LK101 injection (Likang Life Sciences), XH101 injection(NeoCura BioTech), iNeo-Vaccine (Shanghai GeneChem), and ZSNEO-DC1.1 (Zhongsheng Kangyuan Biotechnology).The aforementioned advances have been achieved through a strong collaborative partnership with hospitals and researchers.
By leveraging collaboration and resource-sharing among diverse organizations, it is possible to accelerate the development and clinical implementation of neoantigen vaccines.This collaborative approach also aids in identifying ideal vaccine targets, thus generating a range of candidate vaccines customized for each cancer patient.Moreover, a collaborative approach ensures adherence to regulatory standards,while paving the way for both personalized neoantigen vaccines targeting specific tumors and shared neoantigen vaccines that benefit public health.
Future perspectives
As additional scientific and clinical evidence continues to emerge demonstrating the feasibility, safety, and promising clinical efficacy of neoantigen-based cancer immunotherapy in patients with advanced solid tumors.By leveraging the immune system recognition of tumor-specific neoantigens,these vaccines hold promise in improving patient clinical outcomes by addressing tumor heterogeneity and immune evasion challenges.However, it is evident that several obstacles still need to be addressed before widespread adoption in clinical practice.
Cancer cells have developed intrinsic mechanisms to evade immune responses at every stage of the cancer immune cycle,posing a formidable challenge for vaccine treatment that must be addressed comprehensively5,197.These challenges encompass identifying the essential properties of immunogenic neoantigens that elicit potent tumor-specific immunity, including coding and non-coding regions, efficiently and accurately detecting tumor-specific neoantigens, optimizing production and delivery of personalized cancer vaccines, devising strategies to overcome immune resistance and tumor heterogeneity, ensuring durability of immune memory and adaptability through epitope spreading, and minimizing potential toxicity and off-target effects.
Given the intricate mechanisms of immune evasion in cancer, combination therapies that simultaneously target distinct stages of the cancer immune cycle may exhibit enhanced efficacy.The induction of cancer cell death through chemotherapy, radiotherapy, targeted therapy, oncolytic virus therapy, and other cellular immunotherapies will stimulate the generation and release of additionalde novoneoantigens,thereby further augmenting the anti-tumor immune response and promoting durable immune memory.It is important to note that the development of personalized neoantigen-based cancer vaccines entails individualized costs and timelines.Moreover, liquid biopsy-based high-throughput sequencing holds promise as a viable alternative for assessing a patient’s complete somatic tumor mutations because obtaining multiple tissue samples from different regions is often impractical in clinical settings.Overall, while neoantigen vaccines hold potential in solid tumor treatment, further research is imperative to address challenges and optimize the clinical effectiveness.Insight derived from clinical trials has paved the way for personalized neoantigen cancer immunotherapy, particularly benefiting patients who are unresponsive to standard-of-care immunotherapies and striving towards achieving a cure for cancer.
Acknowledgments
The authors express their sincere gratitude to all members of the Cell Biotechnology Laboratory and the staff at Tianjin Cancer Hospital Airport Hospital.
Grant support
This study was supported by grants from the National Clinical Research Center Cancer Fund, and the Haihe Laboratory of Synthetic Biology (22HHSWSS00004).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceptualized and wrote the manuscript: Xiaoling Li.
Revised a portion of the manuscript and provided valuable insight: Jian You.
Prepared tables and figures, as well as contributed to the conceptualization and writing of specific sections in the manuscript: Liping Hong, Weijiag Liu, Peng Guo.
Reviewed and refined the manuscript, laying a solid foundation for the review: Xishan Hao.
All authors actively participated in editing the manuscript and have read and approved its final version.
杂志排行
Cancer Biology & Medicine的其它文章
- Hodgkin’s lymphoma: 2023 update on treatment
- Deciphering gastric inflammation-induced tumorigenesis through multi-omics data and AI methods
- Blockade of CD300A enhances the ability of human NK cells to lyse hematologic malignancies
- Influence of sex on outcomes of liver transplantation for hepatocellular carcinoma: a multicenter cohort study in China
