Lean body mass index is a marker of advanced tumor features in patients with hepatocellular carcinoma
2024-05-08AndrewScottdeLemosJingZhaoMilinPatelBanksKookenKaranMathurHieuMinhNguyenAreejMazharMaggieMcCarterHeatherBurneyCarlaKettlerNagaChalasaniSamerGawrieh
Andrew Scott deLemos, Jing Zhao, Milin Patel, Banks Kooken, Karan Mathur, Hieu Minh Nguyen, Areej Mazhar, Maggie McCarter, Heather Burney, Carla Kettler, Naga Chalasani, Samer Gawrieh
Abstract BACKGROUND Obesity is an independent risk factor for the development of hepatocellular carcinoma (HCC) and may influence its outcomes. However, after diagnosis of HCC, like other malignancies, the obesity paradox may exist where higher body mass index (BMI) may in fact confer a survival benefit. This is frequently observed in patients with advanced HCC and cirrhosis, who often present late with advanced tumor features and cancer related weight loss.AIM To explore the relationship between BMI and survival in patients with cirrhosis and HCC.METHODS This is a retrospective cohort study of over 2500 patients diagnosed with HCC between 2009-2019 at two United States academic medical centers. Patient and tumor characteristics were extracted manually from medical records of each institutions' cancer registries. Patients were stratified according to BMI classes: < 25 kg/m2 (lean), 25-29.9 kg/m2 (overweight), and > 30 kg/m2 (obese). Patient and tumor characteristics were compared according to BMI classification. We performed an overall survival analysis using Kaplan Meier by the three BMI classes and after adjusting for Milan criteria. A multivariable Cox regression model was then used to assess known risk factors for survival in patients with cirrhosis and HCC.RESULTS A total of 2548 patients with HCC were included in the analysis of which 11.2% (n = 286) were classified as noncirrhotic. The three main BMI categories: Lean (n = 754), overweight (n = 861), and obese (n = 933) represented 29.6%, 33.8%, and 36.6% of the total population overall. Within each BMI class, the non-cirrhotic patients accounted for 15% (n = 100), 12% (n = 94), and 11% (n = 92), respectively. Underweight patients with a BMI < 18.5 kg/m2 (n = 52) were included in the lean cohort. Of the obese cohort, 42% (n = 396) had a BMI ≥ 35 kg/m2. Out of 2262 patients with cirrhosis and HCC, 654 (29%) were lean, 767 (34%) were overweight, and 841 (37%) were obese. The three BMI classes did not differ by age, MELD, or Child-Pugh class. Chronic hepatitis C was the dominant etiology in lean compared to the overweight and obese patients (71%, 62%, 49%, P < 0.001). Lean patients had significantly larger tumors compared to the other two BMI classes (5.1 vs 4.2 vs 4.2 cm, P < 0.001), were more likely outside Milan (56% vs 48% vs 47%, P < 0.001), and less likely to undergo transplantation (9% vs 18% vs 18%, P < 0.001). While both tumor size (P < 0.0001) and elevated alpha fetoprotein (P < 0.0001) were associated with worse survival by regression analysis, lean BMI was not (P = 0.36).CONCLUSION Lean patients with cirrhosis and HCC present with larger tumors and are more often outside Milan criteria, reflecting cancer related cachexia from delayed diagnosis. Access to care for hepatitis C virus therapy and liver transplantation confer a survival benefit, but not overweight or obese BMI classifications.
Key Words: Hepatocellular carcinoma; Cirrhosis; Obesity; Body mass index class; Sarcopenia; Chronic hepatitis C
INTRODUCTION
The epidemiology of cirrhosis and hepatocellular carcinoma (HCC) is evolving as the burden of disease shifts toward a future predominated by alcoholic liver disease (ALD) and nonalcoholic fatty liver disease (NAFLD). A recent study from Canada projects that 92% of incident cases of cirrhosis will be due to either NAFLD or ALD in 2040[1]. The incidence of NAFLD-related HCC in the United States is predicted to increase by 137% to 12240 cases by 2030[2]. These alarming estimates underscore the present mandate to identify patients at risk for cirrhosis and HCC, presently the third leading cause of cancer death worldwide[3].
While the risk of HCC development varies depending on the underlying etiology of liver disease, ample data now supports a higher risk among chronic liver disease (CLD) patients with superimposed metabolic syndrome[4]. In a retrospective cohort of NAFLD patients, the presence of diabetes, hypertension, and dyslipidemia was shown to confer the highest risk for progression to HCC relative to patients with obesity alone[5]. A report from the International Agency for Research on Cancer, however, clearly establishes a higher body mass index (BMI) as a risk factor for HCC with a relative risk of 1.8 compared to a normal reference BMI[6]. A recent meta-analysis of 22 prospective studies encompassing over 6 million patients followed for liver cancer occurrence found that a higher BMI was associated with an increased risk of HCC, with hazard ratios (HR) that increased from 1.36 to 1.77 to 3.08 in overweight, obese class I, and obese class II/III patients respectively[7].
Although obesity is a recognized risk factor for incident HCC, whether a high BMI translates into poorer survival following the diagnosis of HCC remains unclear. In fact, a survival analysis of a nationwide cancer registry of 10578 patients with HCC from South Korea found that overweight men with a BMI of 25-29.9 kg/m2had a better prognosis than normal weight men[8]. This “obesity paradox”, or a survival benefit in overweight or mildly obese patients with cancer may in fact be apparent in patients with HCC such as has been shown in other types of cancer[9,10]. The “obesity paradox” may also be applicable in the context of cirrhosis. The presence of obesity was found by multivariate analysis to be associated with a lower risk of inpatient mortality in 32000 cirrhotic patients from the Nationwide Inpatient Sample[11]. Additionally, a BMI ≥ 30 kg/m2was recently identified as a variable associated with improved survival in cirrhotic patients undergoing surgery[12].
This study aims to investigate the relationship between BMI at diagnosis of HCC, tumor characteristics and patient survival. We contrasted patient and tumor characteristics, as well as overall survival across 3 BMIs: BMI < 25 kg/m2(lean), BMI 25-29.9 kg/m2(overweight), and BMI ≥ 30 kg/m2(obese) in over 2500 patients diagnosed with HCC over the last decade. To our knowledge, this is the first United States-based study comprised of individually collected patient data to address the “obesity paradox” in patients with HCC.
MATERlALS AND METHODS
Study design
This retrospective study included patient data from 2 academic medical centers (Atrium Health in Charlotte, North Carolina and Indiana University School of Medicine in Indianapolis, Indiana). HCC cases diagnosed from January of 2009 through June of 2019 were identified from each institutions’ cancer registries. A detailed explanation of the cohort composition was described previously[13]. A confirmation of the HCC diagnosis based upon histological and/or radiographic evidence consistent with American Association for Study of Liver Disease guidelines was made by direct review of the individual electronic health record (EHR)[14]. Following verification of the HCC diagnosis, patient and tumor characteristics were then manually extracted from the EHR into a shared REDCap database. Tumor variables collected included alpha fetoprotein (AFP), largest tumor diameter, tumor-node-metastasis stage, and whether the HCC was within Milan criteria[15,16]. The method of HCC diagnosis was ascertained whenever possible and categorized as by routine screening, symptom work-up, and/or incidentally. All HCC treatment modalities were recorded from the medical record for analysis as well.
Patients were classified according to 3 BMI classes: BMI < 25 kg/m2(lean), BMI 25-29.9 kg/m2(overweight), and BMI ≥ 30 kg/m2(obese). BMI was individually recorded from each EHR at the nearest timepoint from initial date of HCC diagnosis. Provider documentation, again through manual chart review was used in concert with confirmatory laboratory testing to assess for the presence of co-morbid metabolic risk factors including diabetes, dyslipidemia, coronary artery disease and hypertension. Patients were classified as either cirrhotic or non-cirrhotic according to criteria published previously by Mittalet al[17] and externally validated by our group[17,18]. The underlying etiology of CLD was determined by review of hepatology provider notes and supportive clinical testing. A patient with combined chronic hepatitis C (CHC) and alcohol abuse was categorized as CHC and we captured whether a sustained virologic response (SVR) was known to have occurred. Laboratory testing for a model for end-stage liver disease (MELD) calculation closest to the time of HCC diagnosis was recorded. The presence or absence of liver-related complications (ascites, hepatic encephalopathy, varices, and spontaneous bacterial peritonitis was collected through the last documentation in the EHR.
Patient survival was established from cancer registries and medical records. For patients who are still alive or died with an unknown date of death, the date of last contact available in the medical record was used to define the time of censoring for the survival analysis. Each participating site had local Institutional Review Board approval to conduct the study.
Statistical analyses
BMI group differences of patient and tumor characteristics were compared using analysis of variance, Kruskal-Wallis test, chi-square test, and Fisher's Exact test, as appropriate. Cirrhotic and non-cirrhotic cases were analyzed separately. Survival curves among BMI classes was estimated through the Kaplan-Meier method. Subgroup analyses by Milan criteria as well as etiology of CLD were conducted and included a subgroup of patients with BMI ≥ 35 kg/m2. To better evaluate potential survival differences, multivariable Cox regression models were conducted to assess risk factors for survival with HR and 95%CI presented. Risk factors included gender, race, diabetes, alcohol use, etiology of CLD, AFP, Milan criteria, screening within 2 years before HCC diagnosis, liver transplantation, and BMI categories. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute, Cary, NC, United States).
RESULTS
Study population
A total of 2548 patients with HCC were included in the analysis of which 11.2% (n= 286) were classified as non-cirrhotic (Figure 1). The three main BMI categories: Lean (n= 754), overweight (n= 861), and obese (n= 933) represented 29.6%, 33.8%, and 36.6% of the total population overall. Within each BMI class, the non-cirrhotic patients accounted for 15% (n= 100), 12% (n= 94), and 11% (n= 92), respectively. Underweight patients with a BMI < 18.5 kg/m2(n= 52) were included in the lean cohort. Of the obese cohort, 42% (n= 396) had a BMI ≥ 35 kg/m2.
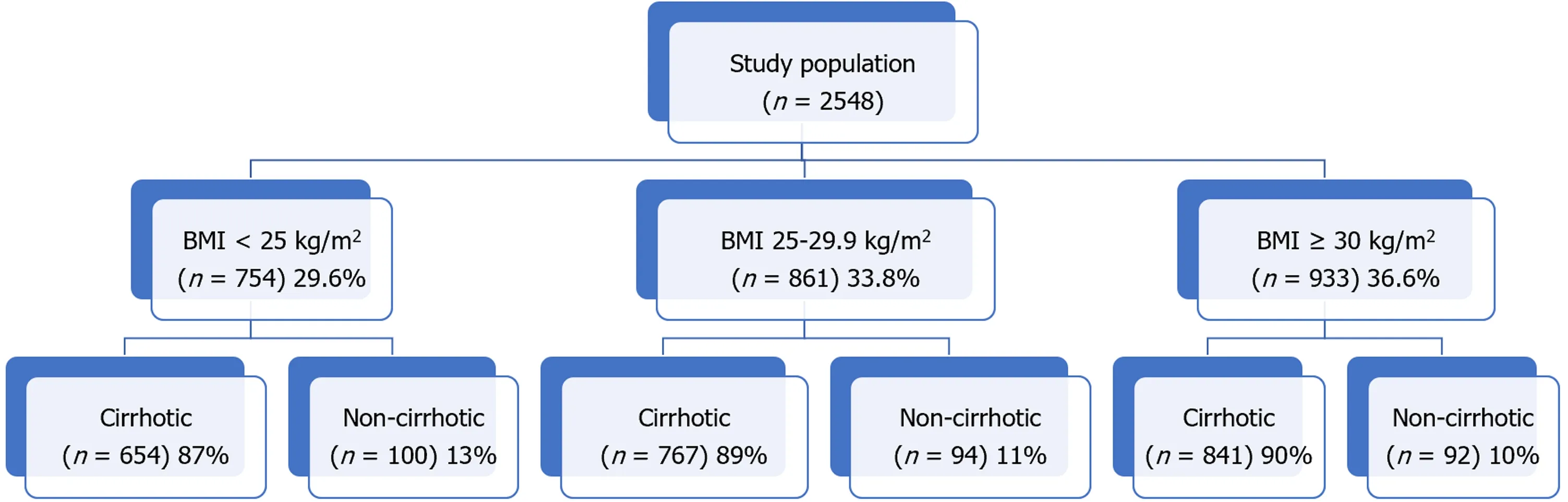
Figure 1 Study cohort by body mass index class. BMI: Body mass index class.
Clinical features of cirrhotic HCC patients
Out of 2262 patients with cirrhosis and HCC, 654 (29%) were lean, 767 (34%) were overweight, and 841 (37%) were obese (Table 1). The mean age at HCC diagnosis for cirrhotic patients was 62 years and did not differ among the three BMI classes (P= 0.43). Although women represented a minority of HCC cases overall (21%), they were overrepresented in the obese cohort accounting for 26% of cases. By comparison, men accounted for a higher percentage of cases in the lean (80%) and overweight (85%) groups (P< 0.001). Lean patients with HCC were less frequently white or Hispanic and more frequently Black or Asian. As expected, the rate of diabetes, dyslipidemia, hypertension, and coronary artery disease was highest in the obese cohort compared to the overweight and lean groups (P< 0.001 for each risk factor; Table 1). There were no significant or clinical differences in laboratory tests or MELD-Na score across the three groups.

Table 1 Patient characteristics for cirrhotic hepatocellular carcinoma, n (%)
Lean patients with HCC had the highest frequency of alcohol abuse (53%), followed by overweight (50%) and obese patients (38%,P< 0.001). As anticipated, NAFLD was the etiology of cirrhosis in 27% of obese patients with HCC and accounted for 14% and 5% in the overweight and lean groups, (P< 0.001). Correspondingly, CHC accounted for 49%, 62%, and 71% of cases across the three BMI classes (P< 0.001). SVR rates were similar across the 3 CHC BMI groups, ranging from 34% to 41% (P= 0.07). The prevalence of ALD as the only etiology of liver disease was also similar across the 3 BMI classes (13%-15%,P= 0.51).
There were no differences in the distribution of Child-Pugh classes, presence of ascites or portal vein thrombosis across the 3 groups. In contrast, the presence of encephalopathy (36%vs33%vs26%,P< 0.001) and varices (51%vs48%vs40%,P< 0.001) were significantly higher in the obese relative to the overweight and lean groups.
Tumor characteristics among BMI classes in cirrhotic population
The lean HCC cohort presented with significantly larger tumors than the overweight and lean cohorts (mean 5.1vs4.2vs4.2 cm,P< 0.001). An AFP level > 200 ng/mL was also more frequently encountered in the lean HCC group in comparison to the other two groups (36%vs23%vs26%,P< 0.001). The lean cohort presented with more aggressive tumors as evidenced by the lowest rate of single tumors (35%vs43%vs46%) and highest rate of vascular invasion or extrahepatic spread (30%vs22%vs22%,P< 0.001 for overall clinical tumor stage). Predictably, the lean cohort was least likely to fall within Milan criteria (44%vs52%vs53%,P= 0.003) and to undergo liver transplantation (9%vs18%vs18%,P< 0.001). Lastly, the lean HCC group was most likely to be diagnosed as part of a symptom workup (48%vs42%vs38%,P= 0.003) and least likely by screening (46%vs49%vs55%,P= 0.007), compared to the overweight and obese groups (Table 2).
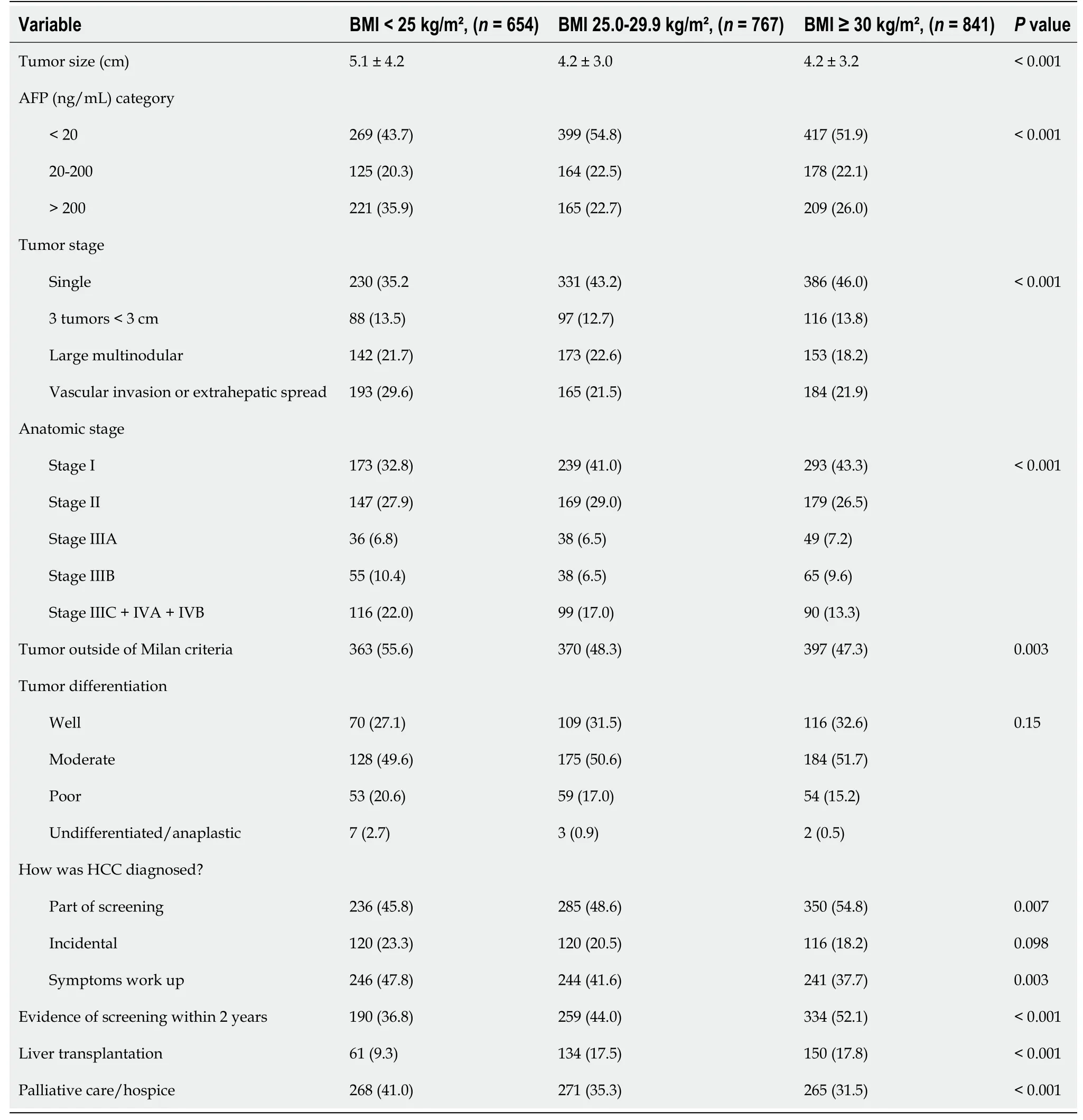
Table 2 Tumor, diagnosis, and treatment characteristics for cirrhotic hepatocellular carcinoma, n (%)
Patient survival by BMI classification
Median survival in the lean HCC cohort was 1.28 years (P< 0.0001, 95%CI 1.03-1.44) and was significantly lower compared to the overweight; 2.13 years (95%CI 1.79-2.59) and obese; 2.14 years (95%CI 1.83-2.51) cohorts (Figure 2A). The reduction in overall survival for lean cirrhotic HCC patients did not persist upon multivariate analysis (P= 0.36; Table 3). Although there was no difference in survival by BMI class when adjusting for patients with HCCs within Milan criteria (P= 0.35), there was a significantly increased mortality for lean cohort patients with HCCs outside Milan criteria compared to the other 2 BMI groups (P< 0.0001; Figure 3). This observation remained significant on multivariate analysis as patients with tumors within Milan criteria had a significant survival benefit (HR = 0.59, 95%CI 0.48-0.72,P< 0.0001) as did those patients undergoing liver transplantation (HR = 0.10, 95%CI 0.06-0.17,P< 0.0001). A SVR from CHC infection was also associated with a survival benefit (HR = 0.27, 95%CI 0.21-0.35,P< 0.0001) while an AFP > 200 ng/mL (HR = 1.93, 95%CI 1.61-2.32,P< 0.0001) and tumor size (cm) (HR = 1.08, 95%CI 1.05-1.12) were associated with worse survival (Table 3).

Figure 2 Patient survival. A: Patient survival with hepatocellular carcinoma according to body mass index class (BMI); B: Overall survival (OS) across four BMI groups by liver disease etiology: Hepatitis C virus; C: OS across four BMI groups by liver disease etiology: Alcohol; D: OS across four BMI groups by liver disease etiology: Nonalcoholic fatty liver disease. BMI: Body mass index class.

Figure 3 Patient survival after hepatocellular carcinoma diagnosis by body mass index class adjusted for Milan criteria. BMI: Body mass index class.

Table 3 Multivariable cox regression model of cirrhotic patient and tumor risk factors associated with survival after hepatocellular carcinoma diagnosis
A final survival analysis was performed after stratifying by the three most common etiologies of CLD: Hepatitis C virus (HCV), alcohol, and NAFLD (Figure 2B-D). For each etiology, the obese HCC cohort was further subdivided into Class I obese (BMI: 30-34.9 kg/m2) and Class II & III (BMI ≥ 35 kg/m2). Median survival was significantly lower for the lean HCC cohort with underlying HCV (1.42 years,P= 0.01, 95%CI 1.2-1.79) and alcohol (0.7 years,P= 0.0007, 95%CI 0.21-1.15) compared to the three other BMI groups. The lean NAFLD-related HCC cohort patients were predictably low in number (n= 23) and their median survival, while lower at 1.44 years (95%CI: 0.39-) did not reach statistical significance compared to the overweight (2.95 years, 95%CI 1.5-4.14), obese class I (1.99 years 1.42-) and obese class II (2.23 years, 95%CI 1.54-2.87) (Poverall = 0.84 among 4 BMI classes).
Patient and tumor characteristics among BMI classes in non-cirrhotic population
The 286 patients with HCC but without cirrhosis were evaluated according to BMI classification (Supplementary Table 1). Patients with non-cirrhotic HCC presented at a mean age of 66, 69, and 67 years old in the lean, overweight, and obese cohorts respectively (P= 0.22). Interestingly, women accounted for 30% of the non-cirrhotic HCC cohort (compared to 21% in the cirrhotic HCC cohort) though there was no significant difference in gender distribution across the three BMI strata. As observed in cirrhotic HCC (Table 1), the non-cirrhotic obese group was more often white than in the overweight or lean groups (88%vs79%vs72%, respectively) and less often Black (9%vs10%vs21%, respectively,P= 0.015). As expected, 78% of the cases had Aspartate aminotransferase to platelet ratio index scores < 1.0% and 76% had no record of undergoing HCC screening. No CLD etiology could be ascertained in 48% (137/286) of the cases, while the remaining were either NAFLD or viral hepatitis. HCC tumor size on presentation tended to be larger in the lean cohort (9.2 cm) compared to the overweight (7.6 cm) and obese (7.7 cm) cohorts respectively (P= 0.06) though there was no difference among the BMI classes in clinical tumor stage with 51% of the tumors presenting as single lesions and 23% presenting with vascular invasion or extrahepatic spread. Median survival in non-cirrhotic HCC patients in the lean (2.95 years, 95%CI 1.12-6.52), overweight (2.14 years 95%CI 0.96-2.96), and obese (2.77 years 95%CI 1.33-3.17) cohorts was not significantly different.
DlSCUSSlON
Our analysis of over 2500 patients diagnosed with HCC over the last decade focused on the differences between overweight and obese BMI classifications relative to a cohort of lean patients. The lean group, at the time of HCC diagnosis was enriched with hepatitis C and alcohol abuse and presented with significantly larger tumors as well as more aggressive tumors which resulted in lower frequency of liver transplantation compared to the overweight and obese groups. By multivariate analysis, however, the impact of BMI classification on patient survival was eclipsed by established survival outcomes such as presenting within Milan criteria and achieving a cure of CHC infection.
Our results should be interpreted within the context of emerging evidence demonstrating the significance of sarcopenia in survival following the diagnosis of HCC. In a Japanese study of over 1200 patients with HCC who underwent computed tomography for body composition assessment; sarcopenia, intramuscular fat deposition, and high visceral adiposity, but not BMI were significant predictors of survival by multivariate analysis[19]. Progressive skeletal muscle volume loss as measured by the psoas muscle index in patients undergoing locoregional therapy for HCC has also recently been associated with poor prognosis[20,21]. We acknowledge that the use of BMI as a variable to evaluate survival in patients with HCC has its limitations. However, our findings reinforce what one would anticipate contrasting BMI classes. The lean cohort, presenting more often without prior HCC screening and with more advanced tumors as we found in our analysis, likely comprises patients with cancer related cachexia. Richet al[22] investigated the impact of cachexia defined as > 5% weight loss in the six months prior to HCC diagnosis compared to pre-cachexia (2%-5% weight loss) and stable/increased weight patients[22]. Approximately 25% of 600 patients met criteria for cachexia. Notably, BMI in the cachexia cohort was significantly lower than in the pre-cachexia and stable weight groups (25.4vs28.3vs28.5,P< 001). The authors found that cachexia was independently associated with increased mortality with a median overall survival of 11.3 months which is comparable to the 15.4 months we found in our lean cohort. Thus BMI, while not an ideal surrogate of cachexia, is still of consequence particularly when evaluated in a considerably larger cohort such as ours.
A strength of our study was including a sub-group survival analysis of BMI classes according to HCV, ALD, and NAFLD etiologies. Since 53% of the patients from the lean HCC cohort were classified as having a history of alcohol abuse, the interaction between alcohol use and HCV could have led to more aggressive tumors in the lean cohort which was comprised of 71% HCV-related HCCs. The differences in screening rates preceding HCC diagnosis among the three BMI cohorts is a natural limitation from a retrospective study and highlights the fundamental challenge in routine cirrhosis management, namely access to screening and the diagnostic accuracy of our screening methodology. A recent detailed investigation of the limitations of screening found that just over a third of patients diagnosed with HCC had regular outpatient care in the year before presenting with HCC[23]. Furthermore, the adequacy of ultrasound visualization for HCC screening was reported to be sub-optimal in nearly 20% of cirrhosis patients, particularly in obese patients with NAFLD and ALD[24]. While newer blood-based biomarkers hold promise and may improve upon ultrasound for surveillance[25,26], issues surrounding access to testing will undoubtedly persist.
CONCLUSlON
Reconsidering the use of the term “obesity paradox” in patients with advanced HCC outside the Milan criteria is a salient conclusion to draw from our study. In fact, our results reinforce the larger impact of cancer related weight loss which is at least in part a result of delayed diagnoses. The present focus on creating a robust screening apparatus for our liver disease patients at risk for HCC is of critical importance to prevent the past from repeating itself[27].
ARTlCLE HlGHLlGHTS
Research background
This study examines a large cohort of patients diagnosed with hepatocellular carcinoma (HCC) at two academic medical centers where liver transplantation is offered. Extensive data collection on patient and tumor variables were obtained to investigate the relationship between body mass index (BMI) classification and outcomes of patients with HCC.
Research motivation
The motivation for our research study is to explore how different BMI strata impact survival in patients with HCC.
Research objectives
It is apparent that a lean BMI in patients at the time of HCC diagnosis reflects advanced tumor burden but is not independently associated with worse survival.
Research methods
Patient and tumor characteristics were compared according to BMI < 25 kg/m2(lean), BMI 25-29.9 kg/m2(overweight), and BMI ≥ 30 kg/m2. The Kaplan-Meier method was used to estimate survival by BMI categories. A multivariable model was performed to investigate risk factors (including the three BMI strata) associated with survival following HCC diagnosis.
Research results
Our research demonstrates interesting differences when comparing patients across BMI categories. For example, women with HCC were more likely to be in a higher BMI classification than men. Chronic hepatitis C infection was by far the most common reason for chronic liver disease in our cohort, and achieving sustained virologic response, not unexpectedly was associated with improved survival. We did not see significant differences in the Child-Pugh class or model for end stage liver disease scores according to the three different BMI. We did not see a survival difference by BMI class in our large cohort of 286 non-cirrhotic HCC cases patients.
Research conclusions
The relevant conclusion that one can draw from this study is the importance of identifying patients early in their presentation as our results confirm well established risk factors for reduced survival in patients with HCC trump the perceived protection of the "obesity paradox".
Research perspectives
The future research in this field needs to focus on improving patient access to screening for HCC to prevent a delay in diagnosis.
ACKNOWLEDGEMENTS
The following individuals have contributed to the data extraction: Keaton R Jones, MD, Lara Dakhoul, MD and Chelsey McShane, MD (Indiana University School of Medicine), and Patrick Roche (Atrium Health).
FOOTNOTES
Author contributions:deLemos AS, Gawrieh S, and Chalasani N conceived the study, analyzed data, and contributed to draft and final manuscript preparation; Zhao J, and Nguyen HM provided statistical analysis, critical appraisal of data, and manuscript editing; Patel M, Kooken B, Mathur K, Mazhar A, Burney H, and Kettler C assisted with tumor registry data entry and maintenance of database; McCarter M assisted with statistical analysis.
Supported byin part David W Crabb Professorship Endowment at Indiana University School of Medicine and an intramural grant from the Atrium Health Center for Outcomes Research and Evaluation (CORE) (to deLemos AS).
lnstitutional review board statement:Each participating site had local Institutional Review Board approval to conduct the study.
lnformed consent statement:This study was retrospective and did not have any direct patient contact and was completely deidentified.
Conflict-of-interest statement:Dr. Gawrieh consulting: TransMedics, Pfizer, research grant support: Cirius, Galmed and Zydus. Dr. Chalasani had paid consulting activities with following companies in last 12 months: Abbvie, Madrigal, Galectin, Zydus, Boehringer-Ingelheim, and Altimmune. He and his institution receive research funding from DSM, Exact Sciences, and Galectin. The remaining authors have no conflicts of interests to declare in the last 12 months.
Data sharing statement:A data sharing agreement was established between Atrium Health and Indiana University School of Medicine for the purpose of compiling a de-identified patient registry.
STROBE statement:The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORClD number:Andrew Scott deLemos 0000-0003-1232-7986; Naga Chalasani 0000-0003-4082-3178; Samer Gawrieh 0000-0002-2056-4909.
S-Editor:Li L
L-Editor:A
P-Editor:Cai YX
杂志排行
World Journal of Hepatology的其它文章
- Update in lean metabolic dysfunction-associated steatotic liver disease
- Retrospective study of the incidence, risk factors, treatment outcomes of bacterial infections at uncommon sites in cirrhotic patients
- Palliative long-term abdominal drains vs large volume paracenteses for the management of refractory ascites in end-stage liver disease
- Comprehensive prognostic and immune analysis of sterol Oacyltransferase 1 in patients with hepatocellular carcinoma
- Prediction model for hepatitis B e antigen seroconversion in chronic hepatitis B with peginterferon-alfa treated based on a responseguided therapy strategy
- lnfluence of nonalcoholic fatty liver disease on response to antiviral treatment in patients with chronic hepatitis B: A meta-analysis
