Observation of corneal cell in diabetic patients using in vivo confocal microscopy
2024-04-28
Abstract
•In vivo confocal microscopy of the cornea is a non-invasive, rapid, and comprehensive technique for real-time, dynamic observation of all layers of the cornea. Confocal microscopy allows the examination of the morphology and cell density in the different layers of the cornea through direct visualization. With the increasing prevalence of diabetes, ocular complications have become common and have garnered more interest and in-depth research from clinical and scientific communities. This paper provides a comprehensive review of research progress made using in vivo confocal microscopy to observe various layers of cornea tissue in diabetic patients.
•KEYWORDS:confocal microscopy; diabetes; corneal epithelium; stromal cells; endothelial cells
INTRODUCTION
Diabetes is one of the most prevalent systemic diseases worldwide, and its global prevalence has been increasing continuously in recent decades[1-2]. It was reported that approximately 382 million people had type 2 diabetes mellitus (T2DM) in 2013[3], and it is estimated that by 2035 diabetes will affect approximately 592 million[4]rising to 640 million by 2040[5]. In 2022, the “Clinical Guidelines for the Prevention and Treatment of Elderly Type 2 Diabetes in China” introduced a new diagnostic criterion of glycated hemoglobin (HbA1c) ≥6.5% for diabetes, resulting in an increased T2DM detection rate of 0.5% to 1.9%[6]. Diabetic ocular complications extend beyond diabetic retinopathy (DR), with conditions such as dry eye syndrome, delayed wound healing, and neurotrophic keratopathy becoming increasingly common[7-9].Invivoconfocal microscopy has been steadily advancing since its inception in the 1980s, with the development of devices like the Heidelberg Retina Tomograph-III (HRT-III) that utilises internally reflected light to collect data and produce high-resolution images of corneal cell layers at 600 to 800-fold magnification over an area up to 400 μm×400 μm, with a lateral image resolution of 1-2 μm and axial image resolution of 5-10 μm[10-11]. Due to its advantages of high resolution, magnification, and real-time capabilities,invivoconfocal microscopy plays a major role in the examination of corneal tissue and disease diagnosis in ophthalmology patients[12]. This paper provides a comprehensive review and summary of observation of the corneal cell layers in diabetic patients usinginvivoconfocal microscopy.
SUBJECTSANDMETHODS
A detailed search was conducted on PubMed using a combination of search terms including “cornea confocal”, “diabetes”, “cornea”, “epithelial”, “stromal cell”, “endothelial”, “keratocyte”, “subbasal nerve” and “Langerhans”. The search was not limited to any specific type of article and was restricted to English-language publications. The most relevant results were manually screened and categorized by Meng LR.
EthicalApprovalThe study was approved by the Ethics Committee of the First Medical Center of the General Hospital of the PLA (No.S2023-199-01).
RESULTS
CornealEpithelialCellsThe epithelial cell layer is the outermost layer of the cornea, with a thickness of about 55 μm. It is composed of 4-5 layers of non-keratinised squamous epithelial cells. By confocal microscopy, three types of epithelial cells can be discerned from the outermost to the innermost layer[13]. Superficial epithelial cells are loosely arranged, with a flat shape, and a diameter of 40-50 μm. They have highly reflective cytoplasm and a bright nucleus of 10 μm[14]. Wing cells have a polygonal shape with a diameter of 20-30 μm. They have a thin and highly reflective plasma membrane and low reflectivity in the cytoplasm. Basal epithelial cells have small, roughly round cell bodies, with a diameter of 8-10 μm. These are densely arranged, intermediate in brightness, with high reflectivity at thecell membrane, and have pale grey or minimally reflective cytoplasm[15-16]. Superficial epithelial cells have microvilli and microfolds, which maximise nutrient metabolism, prevent the intrusion of microbes and ocular fluids into the stromal layer, and help retain tears, keeping the cornea relatively dehydrated. Physical, chemical, and biological stimuli can lead to changes in cell morphology and decreased density and function, and systemic metabolic diseases can also damage these epithelial cells[17].
Studies have shown that diabetes, a multi-system disorder, affects the morphology and metabolism of the cornea to some extent. Confocal microscopy can reveal ocular surface abnormalities that may not be detected in the early stages by other ophthalmic examination devices such as the slit lamp[18-19].Invivoconfocal microscopy of the cornea can detect early thinning of the epithelial cell layer that occurs in diabetic patients[14]. Moreover, there is an increase in thickness and irregularity of the epithelial basement membrane, often accompanied by a significant reduction in the number of basal cells with less dense and irregular arrangements[18, 20](as shown in Figure 1). The reduced adhesion of epithelial cells in diabetic patients is widely thought to be associated with the generation and accumulation of advanced glycation end products (AGEs) in the late stage of diabetes[21-22]. Epithelial cell damage associated with diabetes is commonly observed in dry eye syndrome, superficial punctuate keratopathy, persistent epithelial defects, delayed wound healing, and even in recurrent epithelial erosion. A study by Shihetal[21]suggests that this damage worsens progressively with the duration of diabetes. Akhtaretal[23]reported that the number of basal cells in the epithelial basement membrane increases with age in the normal population, but this age-related correlation is weakened in patients with diabetes. In rodent models, researchers have detected a decrease in the expression of pro-epithelial healing factors related to the cornea in diabetic animals compared to controls[24-26],e.g., TGFβ3, epithelial growth factor, and ciliary neurotrophic factor, while the expression of NFκB, which promotes inflammation and affects cell development is increased.
SubepithelialNervesandLangerhansCellsDry eye syndrome is the most common ocular manifestation of diabetic eye disease. It is characterized by reduced tear secretion and associated eye discomfort, leading to decreased corneal sensitivity. In the early stages, corneal nerve degeneration can be detected by confocal microscopy examination[7, 25]. The cornea is the most densely innervated tissue in the human body, with approximately 7 000 nociceptive receptors per square millimeter. The corneal nerves originate from the ophthalmic branch of the trigeminal nerve, which is the fifth cranial nerve[27-28]. The corneal nerves originate in the deep stroma, penetrate the peripheral cornea, and radiate towards the central cornea, before terminating beneath the basal epithelial and Bowman’s layers[29]. The morphology and trajectory of the sub-basal nerve fibers in the cornea can be visualized by confocal microscopy. They appear as highly reflective linear structures, forming Y-shaped bifurcations or H-shaped nerve fiber bundles arranged radially in the cornea[25]. Their diameter fluctuates between 0.52 μm and 4.60 μm and converges towards the central cornea[14], forming a spiral or vortex pattern at the densest area. Manual, semi-automated (Image J with Neuron J plugin), or fully automated (ACC metrics) software tools are available for quantitative measurements of sub-basal nerves in the cornea epithelium[30]. Several studies have shown that diabetic patients have lower sub-basal nerve density, fewer branches, and increased tortuosity (as shown in Figure 1)[31-34]. In a study by Chaoetal[35], patients with type 2 diabetes had significantly decreased central corneal nerve fiber density compared to healthy controls and pre-diabetic individuals. The study also found a significant correlation between HbA1c levels, corneal nerve fiber density, and body mass index. Furthermore, it is expected that 47%-64% of diabetic patients will develop corneal nerve damage prior to peripheral neuropathy[36]. Confocal microscopy detects changes in corneal nerve plexus density earlier than electrophysiological tests can detect peripheral neuropathy, and the severity of both types of nerve damage is correlated. A cross-sectional study conducted from June 2019 to August 2020[37], involving 129 patients with type 2 diabetes, found similar results. The study revealed that corneal nerve fiber damage occurs earlier than DR in diabetic patients.
Additionally, significant numbers of white, high-reflective, and irregularly shaped dendritic cells can be observed accompanying the sub-basal nerves. These are Langerhans cells, the only antigen-presenting immune cells derived from the myeloid lineage in the corneal epithelium, and their density decreases from the cornea periphery towards the center[32, 38]. In the peripheral region of the cornea, the lower area has the highest density of Langerhans cells, followed by the upper and nasal areas, while the temporal area has the lowest density[39]. Cell protrusions are often absent or are short and sparse in the central region, indicating the presence of immature dendritic cells[38]. For differentiation purposes, Langerhans are classified into 3 categories[40-41]: Level 1 indicates no dendrites, Level 2 indicates the presence of small dendrites with a length not exceeding the maximum diameter of the cell body, and Level 3 indicates the presence of dendrites with a length exceeding the maximum diameter of the cell body. Healthy corneas depend on the normal function and interaction between epithelial cells, sensory neurons, and innate immune cells[17]. Diabetes disrupts the interaction and interdependence of these three cell types. The structural basis of the ocular surface neuroimmune functional unit is the interaction between the subepithelial basal nerve and Langerhans cells within the epithelium, which is sensitive to interference from microbial infections, hyperglycemia, and lack of reactive oxygen species.
Stimuli such as hypoxia, inflammation, or high blood sugar can induce the migration, aggregation, and activation of Langerhans cells[42].In diabetic patients, corneal Langerhans cells demonstrate increased maturation and proliferation[43](as shown by the arrow in Figure 1D). Research by D’Onofrioetal[8, 44-45]has shown that reductions in subepithelial basal nerves are associated with DR and renal impairment in different types of diabetes, including type 1 diabetes, type 2 diabetes, and latent autoimmune diabetes in adults. As DR progresses, the damage to the subepithelial basal nerve worsens, accompanied by significant reductions in subepithelial basal nerve fiber density in parallel with the progression of renal dysfunction, and an increase in the activation and total number of Langerhans cells. Age, weight, height, body mass index, HbA1c, total cholesterol, low-density lipoprotein cholesterol, and triglycerides have all been identified as risk factors for corneal nerve loss[46]. Corneal nerve parameters are negatively correlated with HbA1c levels and duration of diabetes[47-48].Furthermore, a significant finding was the positive correlation between corneal nerve fiber length, tear film height, and tear film break-up time[37]. This conclusion was further validated through quantitative analysis, demonstrating the close association between corneal nerve fibers and ocular conditions. These findings provide valuable guidance for predicting DR and ocular surface diseases through the assessment of cornea sub-basal nerve fiber damage[49].
StromalCellsThe corneal stromal layer comprises approximately 90% of the corneal volume. It is approximately 500 μm thick and contains keratocytes and collagen fibrils in an amorphous matrix composed of proteoglycans, ions, and extracellular substances[1]. Collagen fibre bundles, known as stromal fibril lamellae, have a regular arrangement that ensures high transparency of the stroma. These corneal collagen fibrils and the amorphous matrix appear as a featureless dark reflective background by confocal microscopy. Keratocyte nuclei are 5-30 μm in diameter, and these cells are weakly reflective and form an interconnected network. The anterior stromal cells are bean-shaped, while the posterior stromal cells are elliptical. The cell density is the highest in the anterior stroma, located 50 100 um behind the Bowman’s membrane[14], and gradually decreases from the middle to the posterior stroma. Large stromal fibers are present in the matrix. When cornea stromal inflammation is induced by external factors, stromal cells become activated. Confocal microscopy reveals irregular and intertwined cell contours, appearing polygonal or crab claw-shaped, with highly reflective cell bodies and indistinct cell nuclei. Effects on stromal cells are not only age-related[50-51], but persistent hyperglycemic stimulation also affects their morphology and metabolism. Increased cornea stromal thickness with decreased density can be observed in diabetic patients by confocal microscopy (as shown in Figure 2). Additionally, keratocyte density is significantly correlated with the duration of diabetes and HbA1c levels[51-52]. Kaltenieceetal[51], in their evaluation of stromal cells in 86 type 1 and type 2 diabetic patients and 21 age-matched controls by confocal microscopy,found reduced keratocyte density in the anterior, middle, and posterior stroma of diabetic patients, and a correlation between the number of stromal cells and damage to the subbasal nerve. Furthermore, a study conducted by Iqbaletal[53]examined the alterations in corneal tissue in diabetic patients following weight loss surgery. The study revealed that diabetic patients who had previously suffered damage to their stromal cells and nerves experienced a certain level of restoration in both the stroma and nerves after undergoing the weight loss procedure. This implies that there is a potential for ameliorating corneal damage in diabetic patients by effectively managing their body mass index.
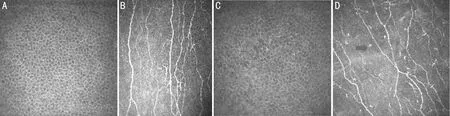
Figure 1 Confocal microscopy images of epithelial basal cells and subepithelial nerve fibres in normal cornea and diabetic corneas.
EndothelialCellsEndothelial cells are located in the innermost layer of the cornea. This 4-6 μm layer is composed of hexagonal or polygonal cells of 20 μm diameter. Under the confocal microscope, these cells appear as bright cell bodies with dark cell boundaries, making it difficult to discern the nuclei. Endothelial cells play an active role in transporting fluid against an osmotic gradient to the anterior chamber using specialized Na+-K+-ATPase pumps[54], maintaining corneal transparency and stromal hydration balance[55]. The density of endothelial cells is approximately 3 000-4 000 cells/mm2at birth and gradually decreases to around 2 500 cells/mm2in adulthood. As individuals age, this density declines at an average rate of about 0.6% per year[56-57]. Furthermore, factors such as race, trauma, inflammation, intraocular surgery, and certain systemic diseases like diabetes can also influence the structure and function of the endothelial layer[58-59].
Diabetic patients are prone to corneal endothelial cell damage as a result of prolonged exposure to hyperglycaemia. Extensive literature[18, 60-63]has explored this phenomenon and revealed that diabetic patients exhibit thickening of the cornea, reduced density of endothelial cells, and increased cell area. Furthermore, there is a decrease in the number of hexagonal cells (as shown in Figure 3). Research aimed at elucidating the pathophysiological mechanisms underlying the detrimental effects of hyperglycaemia on corneal endothelium has shown that this condition results in a notable decline in the expression and functionality of the Na+/K+-ATPase[64], damage to F-actin filaments[65-66], osmotic injury resulting from excessive accumulation of sorbitol, and oxidative DNA damage caused by the accumulation of AGE[67]. In murine models, Chenetal[68]have explored the effects of high blood sugar on the induction of endoplasmic reticulum stress. This stress leads to corneal edema and dysfunction of endothelial cells. The authors proposed that targeting mitochondrial inhibition of endoplasmic reticulum stress could be of therapeutic benefit for these conditions.
Furthermore, higher levels of HbA1c are correlated with more pronounced endothelial injury[69-70]. In a cross-sectional study involving 100 diabetic patients and 92 non-diabetic individuals, patients with HbA1c levels >7% exhibited a significantly lower proportion of hexagonal endothelial cells compared to those with HbA1c levels ≤ 7%[69]. Additionally, as the severity of DR progresses, there is a worsening of endothelial cell damage[71-72].In patients with cataracts who have undergone phacoemulsification surgery, a longer duration of diabetes is correlated with more severe endothelial damage, as visualized by confocal microscopy. Furthermore, a prolonged recovery period may be necessary for these patients[73-76].

Figure 3 Confocal microscopy images of endothelial cells in normal and diabetic corneas showing larger and unevenly distributed cell areas with a lower proportion of hexagonal cells in the latter. A: The endothelial cells in the normal cornea are hexagonal and evenly distributed; B: The endothelial cells in diabetic cornea shows a significant and uneven increase in area compared to that in normal cornea, with a decrease in the proportion of hexagonal cells.
DISCUSSION
In conclusion, the incidence of ocular diseases caused by diabetes is on the rise. These conditions present significant challenges to the ocular health and overall comfort of individuals living with diabetes. Therefore, it is crucial to prioritize the eye health of diabetes patients and implement appropriate preventive and treatment measures to reduce the occurrence and progression of eye diseases. Since its introduction in the 1980s,invivoconfocal microscopy of the cornea, particularly the HRT-III, has revolutionized the early detection of corneal nerve and cell damage[10]. Confocal microscopy plays a crucial role in assisting ophthalmologists in the early detection and prevention of cornea diseases as well as in the improvement of clinical treatment, especially in individuals with diabetes. Overall, confocal microscopy is a valuable tool to better understand and manage ocular disease caused by diabetes by providing detailed visual information and precise measurements.
