Study on synergistic leaching of potassium and phosphorus from potassium feldspar and solid waste phosphogypsum via coupling reactions
2024-04-22ChaoLiShizhaoWangYunshanWangXuebinAnGangYangYongSun
Chao Li ,Shizhao Wang,*,Yunshan Wang ,Xuebin An ,Gang Yang,*,Yong Sun
1 School of Chemical Engineering and Technology,Hebei University of Technology,Tianjin 300130,China
2 National Engineering Research Center of Green Recycling for Strategic Metal Resources,Chinese Academy of Sciences,Beijing 100190,China
3 School of Engineering,Edith Cowan University,270 Joondalup Drive,Joondalup,WA 6027,Australia
4 Key Laboratory of Carbonaceous Wastes Processing and Process Intensification of Zhejiang Province,The University of Nottingham Ningbo China,Ningbo 315100,China
Keywords: Phosphogypsum Potassium feldspar Coupling reaction Leaching Waste treatment Kinetics
ABSTRACT To achieve the resource utilization of solid waste phosphogypsum(PG) and tackle the problem of utilizing potassium feldspar (PF),a coupled synergistic process between PG and PF is proposed in this paper.The study investigates the features of P and F in PG,and explores the decomposition of PF using hydrofluoric acid(HF)in the sulfuric acid system for K leaching and leaching of P and F in PG.The impact factors such as sulfuric acid concentration,reaction temperature,reaction time,material ratio (PG/PF),liquid–solid ratio,PF particle size,and PF calcination temperature on the leaching of P and K is systematically investigated in this paper.The results show that under optimal conditions,the leaching rate of K and P reach more than 93% and 96%,respectively.Kinetics study using shrinking core model (SCM) indicates two significant stages with internal diffusion predominantly controlling the leaching of K.The apparent activation energies of these two stages are 11.92 kJ∙mol-1 and 11.55 kJ∙mol-1,respectively.
1.Introduction
Phosphogypsum(PG),with main component as CaSO4∙2H2O,is a solid by-product generated from wet phosphoric acid production process.The annual stockpiling is estimated to be exceeding 80 million tonnes in China[1].The current status qua of bulk utilization of PG mainly relies on the preparation of building materials,geotechnical building materials,and chemical raw materialsetc[2–8].However,due to the presence of hazardous impurities such as P,F,and other organic matter (such as 2-methoxyethyl acetate) in PG,these substances will not only greatly impede the utilization of PG in the forementioned approaches,but also leads to stockpiling in colossal quantity along Yangzi River[9].More importantly,those harmful and hazardous substances impose adverse environmental impact and jeopardize the health &wellbeing of local residences.Taking the F as an example,it is estimated to be approximately 400000 tonnes being discharged annually (with considering over 0.5%in PG on mass basis).The ionic F might evaporate if it encounters at relative high temperature.In addition,the leachate of PG,which is acidic,could potentially pollute aquatic system and cause acute,chronic diseases if it is not properly handled[10].
Albeit the potassium feldspar (PF) is a K rich resource [11],its chemical stable Al-Si-O tetrahedral ionic bond structure significantly prevents the leaching of K cations out of the matrix of PF using strong inorganic acids (i.e.,sulfuric acid,hydrochloric acid,etc.)[12].Although hydrofluoric acid(HF)can destroy the structure of PF,its notorious pricy cost and underlining environmental impacts makes it much less alluring for K leaching on large-scale PF processing.
In order to tackle the problem of utilization of industrial solid waste-PG and K-rich natural resources of PF,a process that considering intrinsic chemical features both of PG and PF rich in K is proposed in this work.By successfully combining PG (including F element) with PF (rich in K element) together with the aid of sulfuric acid,the proposed synergic reactions are found to achieve K leaching and elementary recovery (F in form of HF could be recycled in the process for multiple times).To depict some more appealing features of this proposed process,Table 1 summarizes the existing widely deployed PF and PG utilization methods.
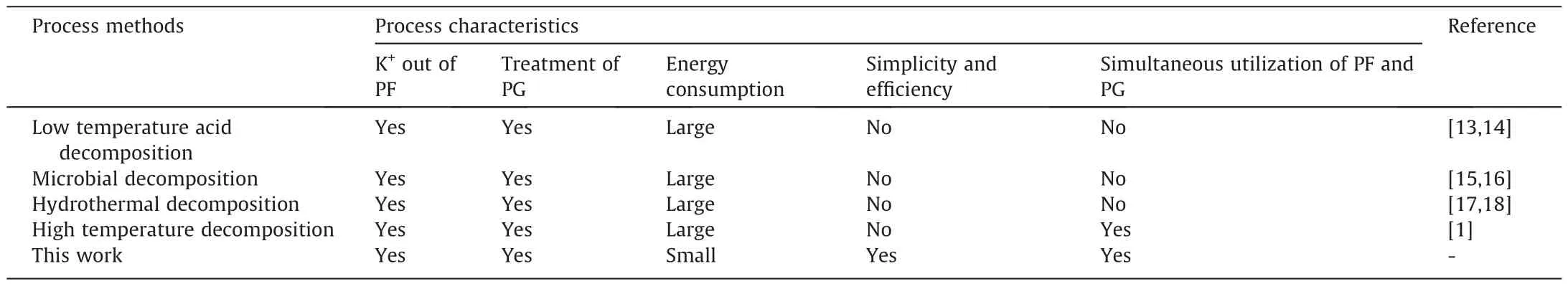
Table 1 Comparison of utilization methods of PG and PF
Through this proposed process,the removal of P and F from PG,and leaching of K from PF could be ideally achievedsimultaneously,which perfectly aligns with circular economy and the philosophy of energy cascade and integrated material utilizations.The reports of using synergic reactions for simultaneous purification of PG and decomposition of PF,to the best of our knowledge,has not been reported before.
2.Experimental
2.1.Experimental materials and equipment
The PF used in this paper is from Nanyang City,Henan Province,China,PG from Yichang City,Hubei Province,China,sulfuric acid is analytically pure,and the water used is deionized water.The experimental device is shown in Fig.1.
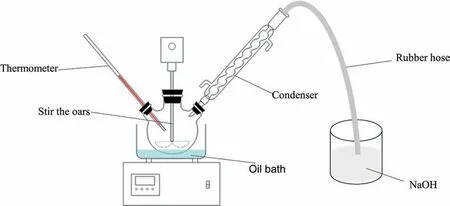
Fig.1.Experimental device.
2.1.1.Raw material pretreatment
Crushing,grinding and screening of PF to obtain mineral powder with different particle sizes.The suitable particle sizes of mineral powder are selected and roasted at different temperatures in Muffle furnace.The appropriate temperature is determined by analysis,and the most suitable mineral powder is selected as the experimental raw material.After grinding and screening,the PG is obtained by 200 mesh (75 μm) powder as the experimental raw material.
2.1.2.Raw material composition analysis
The chemical composition of PG is analyzed by XRF,as shown in Table 2.Its main components include Ca,S,Si,and other components include F,P,K,etc.The XRD analysis of PG is shown in Fig.2(a).Its main crystalline phases are CaSO4∙2H2O and SiO2,while F,P fail to show clear crystalline phases.The electron microscopic analysis of PG is shown in Fig.2(c).It can be determined that the macromolecule in PG is CaSO4∙2H2O,and the particles attached to the surface are some heterophase particles.The SEM-EDS analysis of PG is shown in Fig.3(b),and the results showed that the particles attached to the surface were heterophase F,P and Si,which corresponded to the results of XRF.

Fig.2.Characterization of PF and PG.(a) XRD pattern of PG,(b) XRD pattern of PF,(c) SEM image of PG,(d) SEM image of PF.
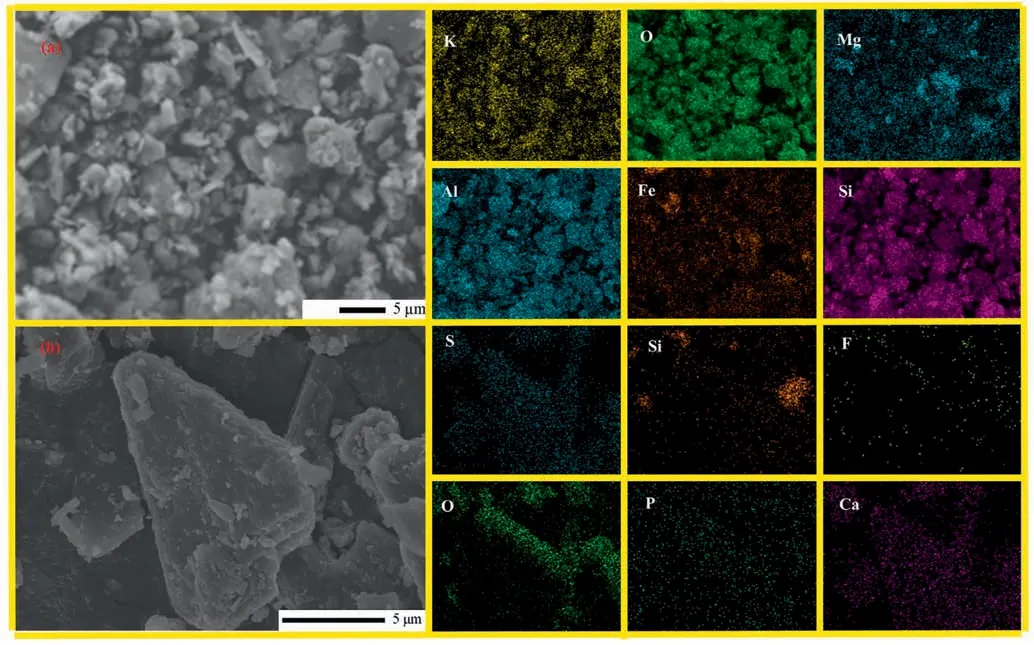
Fig.3.SEM-EDS mapping analysis.(a) PF,(b) PG.

Table 2 Chemical composition of PG determined by XRF
XRF analysis of PF with different particle sizes is shown in Table 3.The results show that the main elements of PF are Si,Al,K and Fe.XRD analysis was conducted on the PF with a particle size of 76 μm,as shown in Fig.2 (b).The results show that the main components are SiO2and KAlSi3O8.Electron microscopic analysis of PF is shown in Fig.2(d),and it can be determined that the macromolecule is KAlSi3O8.As shown in Fig.3(a),SEM-EDS analysis show that in addition to K,Al,Si and O,there were also some Fe and Mg,which was consistent with the results of XRF.

Table 3 Chemical composition of PF determined by XRF
2.2.Experimental methods
2.2.1.Experimental process
The experimental process is shown in Fig.4.(i) PF powder with particle size less than 76 μm was roasted in Muffle furnace at 600°C for 1 h.(ii)The roasted PF powder was mixed with PG with particle size less than 76 μm in a certain proportion,and put into the grinder,grinding for 5–6 min to make it evenly mixed.(iii)The mixed mineral powder and a certain concentration of sulfuric acid are added to the three-neck pancake in a certain proportion,and the oil bath begins to stir and heat,undissolved silicon tetrafluoride gas is absorbed by NaOH during the reaction.(iv)The solid–liquid separation is realized through filtration after different designated reaction time.The filter cake is washed with hot water slurry and filtered again to ensure that there are no soluble ions in the filter cake.(v) After solid–liquid separation,the slag phase was put into the oven and dried at 50 °C for 10 h.
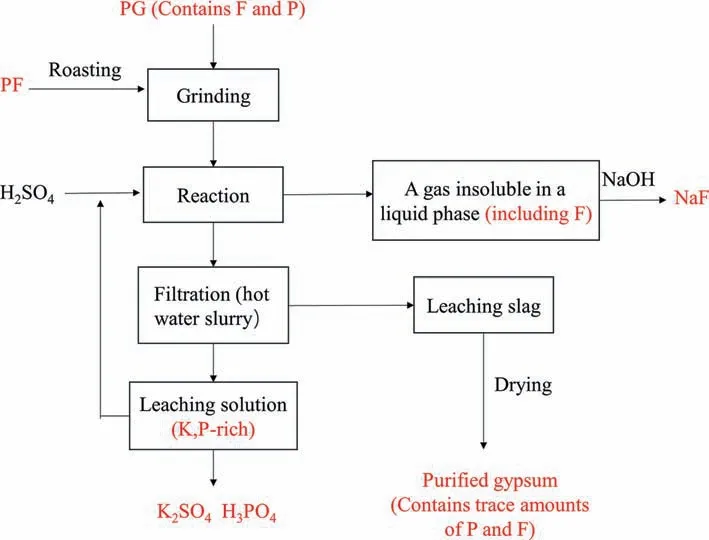
Fig.4.Experimental procedure.
2.2.2.Analysis and characterization
Using X-ray fluorescence spectrometers(XRF)(PANalytical B.V.,Netherlands),the solid phase sample is analyzed to determine its chemical composition by the company’s AXIOS fluorescence analyzer.
The content of potassium in liquid phase was determined by atomic absorption spectrophotometer (4530F Shanghai Yidian Analytical Instrument Co.,Ltd.).
The content of phosphorus in liquid phase was determined according to GB/T 1871.1—1995.
Using X-ray diffractometry (XRD) (PANalytical B.V.,Netherlands) Company,Empyrean type) the solid phase sample is analyzed to determine its structure and composition.The diffractometer uses graphite filtered Cu Kα radiation,the acceleration voltage is 40 kV,the tube current is 30 mA,and the data are obtained in the scanning range of 5°–90° and the scanning time is 5.55 min.
The surface morphology of the samples was observed and analyzed with a scanning electron microscope(SEM)(JSM-6700F thermal field emission scanning electron microscope)at an accelerated voltage of 5 kV.Mineral dissociation analyzer (MLA) was used to analyze the element content and relative element distribution of the samples by electron backscattering diffraction(EBSD) and Xray spectroscopy.
The surface of PG was analyzed using X-ray photoelectron spectroscopy(XPS)(Thermo Fisher Scientific,Model ESCALAB 250Xi,Al Kα excitation source).
The leaching rates of potassium and phosphorus are calculated as shown in Eqs.(1) and (2).
In the formula,XandYare the leaching rates of potassium and phosphorus elements,M1represents the content of elements in mineral powder before the reaction,M2represents the content of elements in slag phase after the reaction,andm1represents the content of elements in the leaching solution.
3.Results and Discussion
3.1.The occurrence forms of fluorine and phosphorus in PG
Fig.2 shows that XRD is difficult to analyze the occurrence form of F in PG,therefore EBSD-EDS and XPS analysis are used to determine the occurrence form of F in PG.As shown in Fig.5,F in PG mainly exists in the form of K2SiF6,Na2SiF6,CaF2,Ca5(PO4)3F and AlF3,which can be identified through different contrast.Among them,as shown in Fig.5(a),and(d),a part of K2SiF6exists in combination with CaSO4∙2H2O,while another portion exists independently.In Fig.5(c),Na2SiF6mainly exists combination with K2SiF6.In Fig.5(b),CaF2primarily exists on its own.In Fig.5(e),Ca5(PO4)3F,which did not participate in the wet-process phosphoric acid reaction,mainly exists combination with CaSO4∙2H2O.In Fig.5(f),AlF3mainly exists in combination with CaSO4∙2H2O[19,20].The analysis results of Si and F elements in PG are shown in Fig.6.Fig.6(a) shows the XPS pattern of Si element,which displays three peaks after peak fitting.By comparing NIST XPS database,it is found that the substances corresponding to 104.6 eV,103.4 eV and 102.36 eV are K2SiF6,SiO2and CaSiO3respectively.The XPS pattern of F is shown in Fig.6(b),which displays four peaks after peak fitting.By comparing NIST XPS database,it is found that the substances corresponding to 686.6 eV,686 eV,and 687.8 eV were K2SiF6,Na2SiF6and AlF3,respectively.The database search also revealed that 684.6 eV corresponds to two substances,CaF2and Ca5(PO4)3F [21].By combining the analysis of EBSD-EDS and XPS,it is possible to determine the chemical speciation of F in PG.The occurrence state of residual P in PG is shown in Fig.5(e).Part of it comes from unreacted Ca5(PO4)3F,while the other part originates from the presence of calcium hydrogen phosphate during the wet process phosphoric acid.Calcium hydrogen phosphate has a similar unit cell parameter to that of calcium sulfate dihydrate,which gradually enters some calcium sulfate crystal cells during the crystallization process,forming eutectic phosphorus[22].The above analysis provides a theoretical basis for the removal of P and F in PG and the subsequent decomposition of PF.

Fig.5.Analysis of PG by EBSD-EDS.
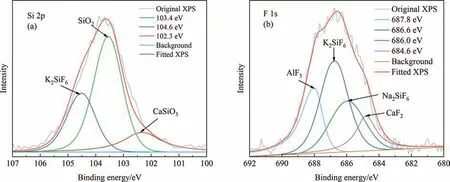
Fig.6.Analysis of PG by XPS.(a) Si,(b) F.
Through the analysis of EBSD-EDS and XPS,it is evident that P primarily exists in the form of insoluble P and eutectic phosphorus,while F predominantly exists in the form of insoluble fluorides.Conventional washing and lime neutralization methods face difficulties in effectively removing these insoluble P and F compounds.However,in this study,the sulfuric acid system employed not only enables the efficient removal of P and F,but also facilitates the effective utilization of F.
3.2.Effect of process parameters
In this paper,the effects of process parameters such as material ratio (PGvs.PF),sulfuric acid concentration (%,mass),reaction temperature (°C),reaction time (min),liquid–solid ratio,stirring rate (r∙min-1),calcination temperature of PF (°C),particle size of PF (μm)and other factors on the leaching process of P and K were systematically investigated.
3.2.1.Effect of reaction time
The effect of reaction time is shown in Fig.7.With the extension of time,the leaching rates of K and P are gradually increased.The leaching rate of K undergoes a significant change at around 10 min,where within the first 10 min,the primary process is the leaching of K from the PG.After 10 min,the main mechanism shifts to the leaching of K through the decomposition of PF.There are two main reasons for this transition.Firstly,the K in the PG is present in the form of K2SiF6,which has a less stable structure compared to PF.Secondly,during the initial stages of the reaction,the amount of HF generated in the system is low,which is insufficient to induce the decomposition of PF.After 10 min,the fluorides in the PG (including K2SiF6in PG) are largely decomposed,and the generated HF facilitates the decomposition of PF [23].The rapid attainment of equilibrium in the leaching of P is attributed to the ability to disrupt the crystal lattice structure of calcium sulfate dihydrate and release eutectic phosphorus within a relatively short time frame,under specific conditions of temperature and sulfuric acid concentration [24],the appropriate reaction time is 200–240 min.
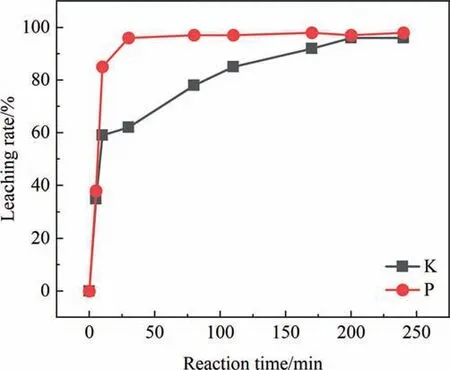
Fig.7.Effect of reaction time on leaching rate of K and P.
3.2.2.Effect of the material ratio (PG vs.PF)
The effect of the ratio of PGvs.PF on K leaching is shown in Fig.8.The results show that when the material ratio increases from 3 to 10,the K leaching rate increases from 64.4% to 95.8%.When the material ratio is relatively small,the leaching rate of K is low,and the HF generated in the system is not enough to decompose PF.With the increase of the material ratio and the increase of HF,the leaching rate of K can be significantly improved.When the material ratio is greater than 10,the leaching rate of K hardly changed,indicating that the leaching rate of K reached equilibrium[25].In addition,the study shows that the material ratio has little effect on the P leaching rate in this process,and the appropriate material ratio is 10–12.

Fig.8.Effect of the ratio of PG vs. PF on leaching rate of K and P.
3.2.3.Effect of sulfuric acid concentration
The effect of sulfuric acid concentration is shown in Fig.9.The study shows that,in the process of sulfuric acid concentration from 5% to 30%(mass),the leaching rates of K and P increase with the increase of sulfuric acid concentration.When the concentration of sulfuric acid is too low,the low leaching rate of K is because the decomposition of PF also requires the participation of sulfuric acid.In addition,the F in PG exists in the form of K2SiF6,Na2SiF6,CaF2,Ca5(PO4)3F and AlF3.The conversion of CaF2,Ca5(PO4)3F and AlF3to HF requires the participation of sulfuric acid,and sulfuric acid can also promote the production of HF by K2SiF6and Na2SiF6.The low leaching rate of P is attributed to the insufficient sulfuric acid concentration which is not enough to break the lattice of calcium sulfate dihydrate,thus preventing the doping impurities of co-crystallized phosphorus from being released.Additionally,the leaching of P in Ca5(PO4)3F and the transformation of F also require the involvement of sulfuric acid.When the concentration of sulfuric acid reaches 70%(mass),the leaching rate of K decreases significantly.This is due to the fact that concentrated sulfuric acid primarily exists in molecular form in water,while hydrogen ions in water are mostly freely mobile electrons.As the sulfuric acid concentration increases,the proportion of water decreases,leading to a reduction in the number of freely mobile hydrogen ions in water.Consequently,the acidity of the system becomes weaker,resulting in a decrease in the availability of hydrogen ions to combine with fluoride ions to form HF.As a result,the leaching rate of K decreases.The appropriate concentration of sulfuric acid is 30%–50%(mass).
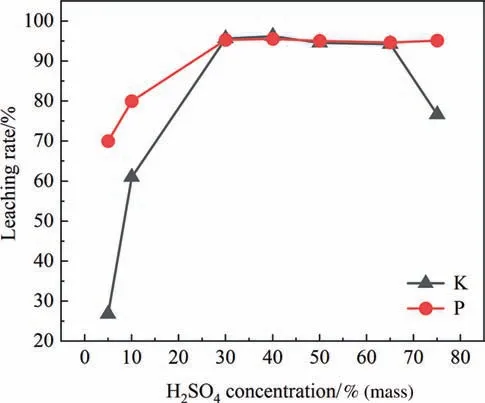
Fig.9.Effect of H2SO4 concentration on leaching rate of K and P.
3.2.4.Effect of reaction temperature
The effect of reaction temperature is shown in Fig.10.With the increase of temperature,the leaching rates of K and P both increase significantly first[26],and the appropriate reaction temperature is 110–120 °C.When the temperature is too low,the low leaching rate of K is because K2SiF6and Na2SiF6in PG cannot be hydrolyzed,and HF produced in the system is not enough to decompose most PF.When the temperature increases,the leaching rate of K increases significantly.On the one hand,it is because K2SiF6and Na2SiF6begin to undergo hydrolysis and produce more HF.On the other hand,the increase in temperature promotes the progress of the reaction [27].In addition,at a higher temperature,the viscosity of the reaction system solution decreases and the diffusion coefficient increases,which is more conducive to the diffusion of the generated HF to the surface of PF or the interior of the particles,thus promoting the decomposition of PF[28].For the leaching rate of P,on one hand,the increase in temperature promotes the reaction of fluorapatite.On the other hand,P is released from the lattice.From Fig.9 and Fig.10,it can be observed that when the temperature is below 70 °C or the sulfuric acid concentration is below 5%,the leaching rate is below 70%.Additionally,since a sulfuric acid concentration below 5% cannot disrupt the lattice of dihydrate calcium sulfate,it is difficult to break down the lattice of dihydrate calcium sulfate below 70 °C [24].
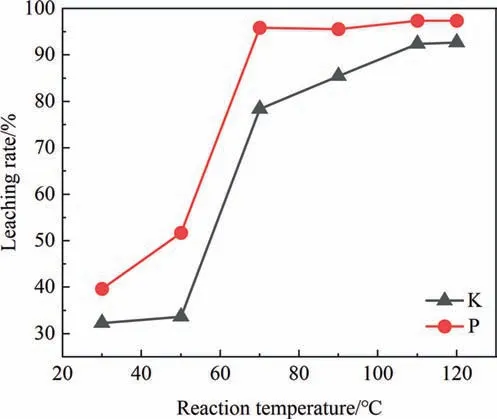
Fig.10.Effect of reaction temperature on leaching rate of K and P.
3.2.5.Effect of the liquid–solid ratio
The effect of the liquid–solid ratio is shown in Fig.11.With the increase of the liquid–solid ratio,the leaching rates of both K and P increase simultaneously.The diffusion resistance and solution viscosity in the system will be reduced,which promotes the leaching of both K and P [29].The appropriate liquid–solid ratio is 8–10.

Fig.11.Effect of the ratio of liquid to solid on leaching rate of K and P.
3.2.6.Effect of stirring rate
The effect of stirring rate is shown in Fig.12.With the increase of stirring rate,the leaching rates of K and P increase gradually.When the stirring rate is too low,the particles are easy to aggregate in the solution,thus hindering the reaction.With an increase of stirring rate,the reaction rate is enhanced as a result of improved diffusivity [30],the appropriate stirring rate is 300–500 r∙min-1.
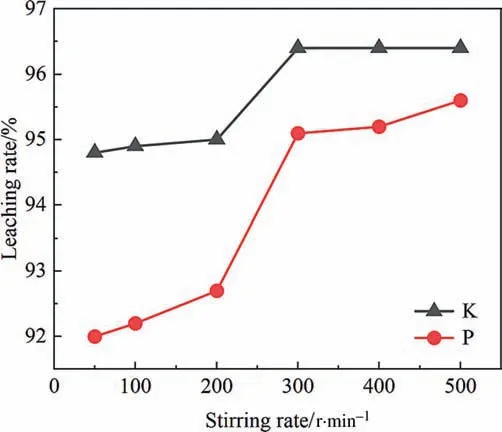
Fig.12.Effect of stirring rate on leaching rate of K and P.
3.2.7.Effect of particle size of PF
The effect of PF powder particle size is shown in Fig.13.With the decrease of particle size,the leaching rate of K gradually increases and then becomes stable.The reason for the improvement of K leaching rate is that the smaller the particle size,the larger the specific surface area,the smaller the internal diffusion resistance and the greater the driving force to the reaction,so the leaching rate will increase[30],the appropriate particle size is 60–80 μm.The particle size of PF powder has no appreciably effect on P leaching and does not interfere.
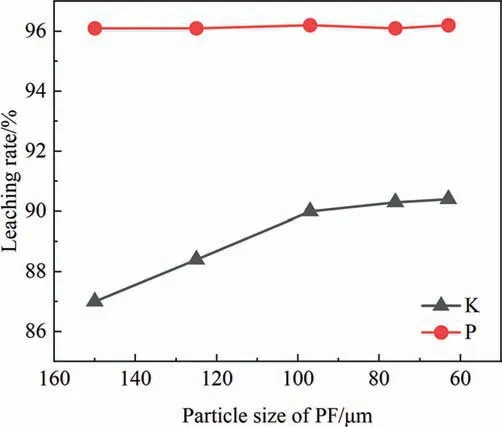
Fig.13.Effect of particle size of PF on leaching rate of K and P.
3.2.8.Effect of roasting temperature of PF
The effect of calcination temperature on PF is shown in Fig.14.When calcination temperature is 600 °C,the leaching rate of K increases significantly,which may be because calcination at 600 °C improves the activity of PF.XRD analysis at different calcination temperatures is shown in Fig.15,it can be observed that after calcination at 600 °C,some small diffraction peaks of PF become weaker.Additionally,high temperature is also conducive to the combustion of organic matter in mineral powder.Compared with 600°C,the leaching rate of K at 800°C did not increase significantly,indicating that it is meaningless to increase the temperature,and high temperature means the increase of energy consumption.Therefore,the appropriate calcination temperature of PF is 600°C,and the calcination temperature of PF has no effect on the P leaching rate,and the P leaching reaction is independent.
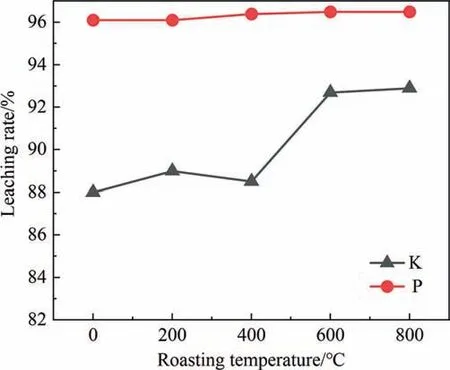
Fig.14.Effect of roasting temperature on leaching rate of K and P.
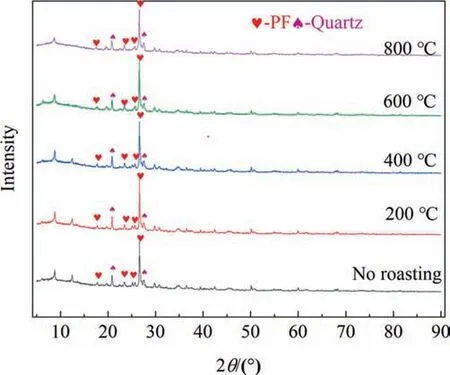
Fig.15.XRD patterns of PF at different roasting temperatures.
3.3.Comparison of pre-reaction and after reaction slag phases
XRF analysis was performed on raw material pre-reaction and slag phase after reaction,and the results were shown in Table 4 and Table 5.K decreased from 1.357% to 0.14%,P from 0.481% to 0.021%,and F from 0.864% to less than 0.01%.XPS analysis of pre-reaction raw material and after reaction slag phase Fig.16(a)and 16(b) show that the strength of pre-reaction raw material on the P 2p orbit is much greater than that of after reaction slag phase,indicating that the content of P in the slag phase is far less than that in the raw material.Fig.16(c) and (d) show that the strength of F 1s of raw material pre-reaction is also much greater than that of slag phase after reaction,and the fitting degree of the curve is also better for the raw materials pre-reaction than for the slag phase.Fig.16(e) and (f) show that there is a K 2p peak in the raw materials pre-reaction,but no obvious peak in the slag phase after reaction,indicating that the K in the raw materials has been basically decomposed.The electron microscopic analysis of raw material pre-reaction and slag phase after reaction is shown in Fig.17.The results show that many PF particles adhere to the surface of PG before reaction,and the PF particles basically decompose after reaction[19].Through the above analysis,it is shown that the process in this paper can effectively leach K from PF and effectively remove P and F from PG simultaneously,which provides favorable conditions for the subsequent application of PG.

Fig.16.(a)Analysis of P pre-reaction,(b)analysis of P after reaction,(c)analysis of F pre-reaction,(d)analysis of F after reaction,(e)analysis of K pre-reaction,(f)analysis of K after reaction.

Fig.17.SEM images of (a) pre-reaction and (b) after reaction material.

Table 4 XRF analysis of pre-reaction materials

Table 5 XRF analysis of materials after reaction
3.4.Mechanism of P and K leaching in coupling reaction of PF and PG
The mechanism of coupling reaction of PF and PG to leach P and K is shown in Fig.18.Based on the results of the process experiments and the reported Refs [25,31],the process is similar to the leaching process of P and K in potassium-phosphate ores.The entire process can be divided into the following two stages.
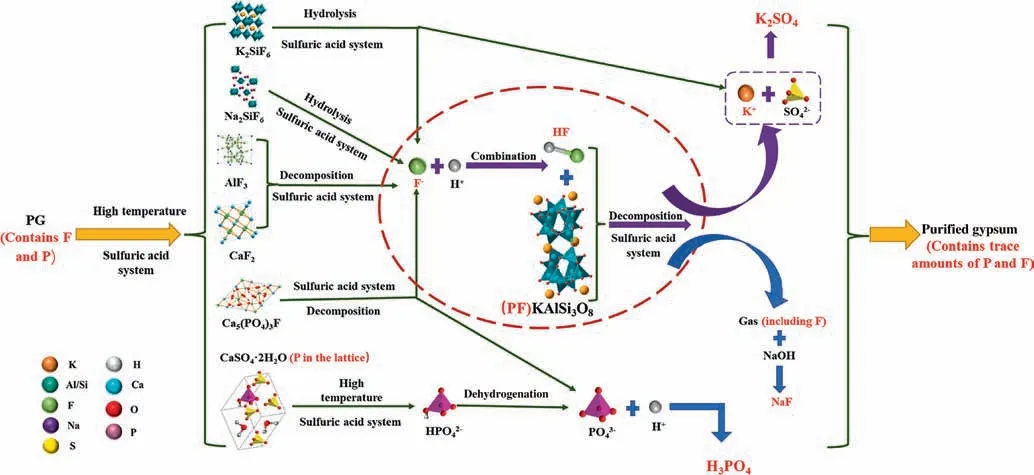
Fig.18.Leaching mechanism of the process.
In the first stage,with the increase of temperature,K2SiF6and Na2SiF6in PG begin to hydrolyze and produce HF,and the produced KF and NaF,as well as the original AlF3,Ca5(PO4)3F and CaF2in PG react with sulfuric acid to produce HF,in which Ca5(PO4)3F also produces phosphoric acid.At the same time,CaSO4∙2H2O,CaSO4∙0.5H2O,and CaSO4dissolve in sulfuric acid,releasing eutectic phosphorus from the lattice structure.
In the second stage,under the conditions of the presence of HF and a large amount of sulfuric acid,the reaction of PF dissociation can be effectively promoted in the forward direction,thereby achieving efficient dissociation of PF.
Some side reactions may occur during the leaching process.
During the decomposition reaction of PF,the F element involved in the reaction will be converted into SiF4.In this process,some HF may also enter the gas phase,and some side reactions may occur.Most of the F element will enter the gas phase in the form of SiF4.To effectively recover the F element,the gas outlet can be connected to NaOH to convert the F into NaF.
3.5.Leaching kinetics
In this system,the leaching of P and K belongs to the wet extraction process,which is a complex liquid–solid reaction process.Typically,the unreacted core model (shrinkage core model,SCM) is used for fitting analysis [32].The control steps of this model are external diffusion control,chemical reaction control,and internal diffusion control(product layer).However,the impact of external diffusion is significantly reduced in the presence of mechanical stirring in this system and can be reasonably ignored[13].Based on the principle of liquid–solid reaction and the reaction system,the rate equation of the reaction process can be expressed as follows using the SCM.
When the reaction rate is controlled by the chemical reaction,the rate equation is expressed as Eq.(20).
When the reaction rate is controlled by internal diffusion,the reaction rate equation can be casted into:
Wherexis the leaching rate of P or K (%),tis the reaction time(min.),andk(min-1) is the apparent rate constant determined by the equations.Fig.7 shows that the leaching of P reaches equilibrium rapidly because P only comes from PG,which is a single process.However,the leaching rate of K exhibits two distinct stages with different rates.During the first stage,majority K associated compounds were leached out of the matrix of PG.During the second stage,with the aid of HF,most of F associated compounds were liberated from the matrix PF.The leaching of K is a multifaceted process that involves several intricate stages.Therefore,a comprehensive elucidation of the leaching kinetics of potassium is imperative for a more holistic understanding of the process.
According to the accumulation rate of K in leachate during the first 10 min period,it can be regarded as the first stage [28].Fig.19 shows that the internal diffusion control outweighs the chemical reaction as reflected from correlation coefficient of R2,suggesting leaching reactions occurring faster than of transport.Therefore,during the first stage of leaching,internal control is the rate-controlling step of the process [31].The accompanying apparent rate constantkfrom regression were summarized in Table 6.

Fig.19.The fitting effect of the two SCM models for the first stage,(a) chemical reaction control model,(b) internal diffusion model.
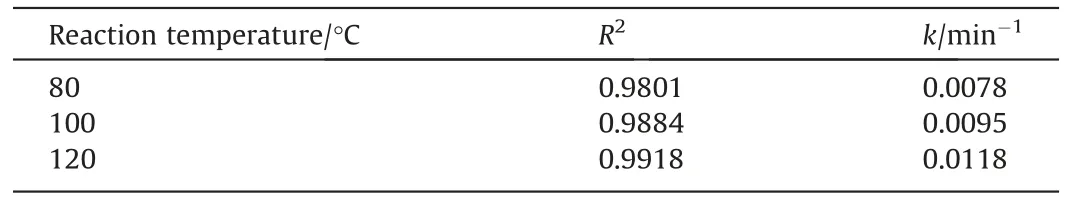
Table 6 Apparent rate constant k at different temperatures in the first stage
According to the Arrhenius equation and logarithmic equation,the relationship between reaction rate and reaction temperature is shown in Eqs.(22) and (23),respectively.
wherekis the apparent rate constant for the SCM(min-1),A0is the preexponential factor,Tis the reaction temperature(K),Ris the gas constant (8.314 J∙mol-1∙K-1),Eais the apparent activation energy(kJ∙mol-1).TheEaof 11.92 kJ∙mol-1is achieved and shown in Fig.20.In general,theEaof diffusion-controlled process is relatively small(less than 12 kJ∙mol-1).Our obtainedEaagrees with the literature report [27].

Fig.20.Linear fitting effect of Arrhenius equation for the first stage.
After 10 min of reaction,the second stage kicks in.The need for modifying the equation used in the first stage arises due to the varied boundary conditions of the first and second stages.It is imperative to customize the equation,with regard to the updated boundary conditions,in order to obtain an accurate and optimal solution of the phenomenon under consideration.The kinetics are shown in Eqs.(24) and (25) [28],respectively.
wherex1is the leaching fractions of K att1=10 min,k(min-1)is the apparent rate constant for the SCM,tis reaction time (min).
Fig.21 shows that rate was also dominated by transport during the second stage [1].The accompanying apparent rate constantkfrom regression were summarized in Table 7.

Fig.21.The fitting effect of the two SCM models for the second stage.(a) Chemical reaction control model,(b) Internal diffusion model.
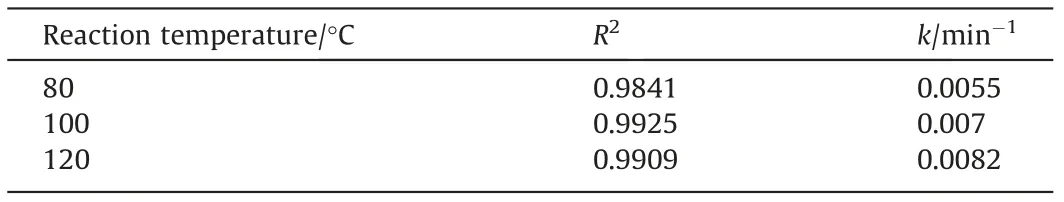
Table 7 Apparent rate constant k at different temperatures in the second stage
Similarly,the range ofEa(See Fig.22) in this work(11.55 kJ∙mol-1),agrees with the Refs[27].The internal diffusivity of both chemical reagents and resultant product plays the key role during leaching.To enhance the entire reaction rate,endeavors in improving transport should be made.
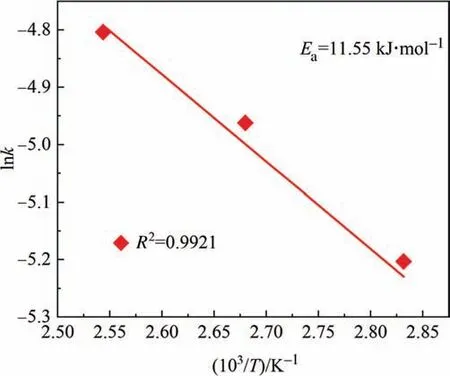
Fig.22.Linear fitting effect of Arrhenius equation for the second stage.
4.Conclusions
The findings of this research shed light on the efficient leaching of P and Kviathe proposed coupled reaction,along with the removal of P and F from PG.Various techniquesi.e.,EBSD-EDS,XPS,XRF,XRD,SEM,XPS,have been employed to characterize the reactants and products to shed lights on the possible reactions during leaching.The major influencing factors for K and P leaching were systematically investigated.At optimal condition,the leaching rate of both K and P could reach more than 93% and 96%,respectively.The kinetic study shows internal diffusion predominates with apparent activation energies of 11.92 kJ∙mol-1and 11.55 kJ∙mol-1being achieved for two different stages.This study provides a possible way for the resource utilization of huge amount of PG solid waste and difficult to use PF.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was jointly supported by the National Key Research and Development Program of China (2019YFC1905800),the National Key Research &Development Program of China(2018YFC1903500),the commercial project by Beijing Zhong Dian Hua Yuan Environment Protection Technology Co.,Ltd.(E01211200005),the Regional key projects of the science and technology service network program (STS program) of the Chinese Academy of Sciences (KFJ-STS-QYZD-153),the Ningbo Science and Technology Innovation Key Projects (2020Z099,2022Z028),the Ningbo Municipal Commonweal Key Program (2019C10033).We thank the support of Mineral Resources Analytical and Testing Center,Institute of Process Engineering,Chinese Academy of Science.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
