Boosting kinetic separation of ethylene and ethane on microporous materials via crystal size control
2024-04-22YixuanMaCongYuLifengYangRiminYouYawenBoQihanGongHuabinXingXiliCui
Yixuan Ma,Cong Yu,Lifeng Yang,Rimin You,Yawen Bo,Qihan Gong,*,Huabin Xing,3,4,Xili Cui,3,4,*
1 Key Laboratory of Biomass Chemical Engineering of Ministry of Education,College of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310012,China
2 Fundamental Science &Advanced Technology Lab,PetroChina Petrochemical Research Institute,China National Petroleum Corporation,Beijing 102200,China
3 ZJU-Hangzhou Global Scientific and Technological Innovation Center,Zhejiang University,Hangzhou 311200,China
4 Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering,Hangzhou 310027,China
Keywords: Adsorption Adsorbent Ethylene Binary mixture Crystal size control Kinetic separation
ABSTRACT The adsorptive separation of C2H4 and C2H6,as an alternative to distillation units consuming high energy,is a promising yet challenging research.The great similarity in the molecular size of C2H4 and C2H6 brings challenges to the regulation of adsorbents to realize efficient dynamic separation.Herein,we reported the enhancement of the kinetic separation of C2H4/C2H6 by controlling the crystal size of ZnAtzPO4(Atz=3-amino-1,2,4-triazole)to amplify the diffusion difference of C2H4 and C2H6.Through adjusting the synthesis temperature,reactant concentration,and ligands/metal ions molar ratio,ZnAtzPO4 crystals with different sizes were obtained.Both single-component kinetic adsorption tests and binary-component dynamic breakthrough experiments confirmed the enhancement of the dynamic separation of C2H4/C2H6 with the increase in the crystal size of ZnAtzPO4.The separation selectivity of C2H4/C2H6 increased from 1.3 to 98.5 with the increase in the crystal size of ZnAtzPO4.This work demonstrated the role of morphology and size control of adsorbent crystals in the improvement of the C2H4/C2H6 kinetic separation performance.
1.Introduction
Ethylene(C2H4)is a key feedstock to many chemicals and polymers [1].During the production of ethylene from the steamcracking reaction of carbon-based feedstocks,ethane (C2H6) is one of the main impurities.C2H6must be removed from C2H4to produce high-pure C2H4for the polymer production process.The present industrial method to enrich C2H4from C2H4/C2H6mixtures mostly relied on cryogenic distillation,requiring a large energy consumption,ca.0.3% of global energy[2].Under the global urgent obligation of carbon neutralization,all kinds of energy-saving transformations of chemical plants will be a long-term significant reform,including the introduction of adsorption separation technology[3–8].And for the adsorptive separation of C2H4/C2H6mixtures,the exploration of efficient adsorbents is of great significance.
However,the intrinsically small and similar molecular sizes[9,10] of C2H4(0.328 nm × 0.418 nm × 0.484 nm) and C2H6(0.3 81 nm×0.408 nm×0.482 nm)make it challenging to explore efficient adsorbents with high C2H4/C2H6selectivity.Many researchers have explored various types of materials to selectively adsorb C2H4from C2H4/C2H6mixture [10–36].So far,the design strategy of adsorbents with efficient separation performance can be mainly classified into two categories.For the first class of adsorbent design,transition-metal ions and unsaturated metal sites were introduced into porous materials,such as Fe-MOF-74 [12] and PAF-1-SO3Ag [22],performing efficient separation by thermodynamic effect.The IAST (ideal adsorbed solution theory) separation selectivity of C2H4/C2H6on Fe-MOF-74 and PAF-1-SO3Ag were as high as 13–18 and 27,respectively.However,the preferentially interact of PAF-1-SO3Ag with the π-electrons of C2H4molecules resulted in relatively high adsorption energy (106 kJ∙mol-1) [17].The second class of design strategy was controlling the pore size and pore structure of adsorbents to realize kinetic separation based on the diffusion rate.In an ideal scenario,approximately selective molecular sieving of C2H4and C2H6based on large diffusion rates was illustrated by UTSA-280 (pore size 0.3–0.4 nm) [19] and Mggallate (pore size 0.356 nm) [26].Other adsorbents such as ITQ-55 [16],Cu(OPTz) [22] showed potential separation performance by kinetic effect.The selectivity for kinetic separation of C2H4/C2H6was up to 50 on ITQ-55 with heart-shaped cages and framework flexibility.Recently,our group [17] reported an ultramicroporous metal–organic framework [Zn3(Atz)3(PO4)]∞(ZnAtzPO4,Atz=3-amino-1,2,4-triazole) for efficient separation of C2H4and C2H6by exploiting equilibrium-kinetic synergetic effect.The ZnAtzPO4adsorbent showed high C2H4/C2H6selectivity and low adsorption heat(17.3 to–30.0 kJ∙mol-1).Overall,the strategy of controlling and tuning the pore structure of adsorbents to enhance the diffusion difference between C2H4and C2H6is promising.Furthermore,it also should be noted that the crystal size and orientation are also important factors affecting the diffusion of gases.However,there is a lack of studies on controlling the morphology and size of adsorbent crystals to enhance the kinetic separation of C2H4and C2H6.
In this work,we focus on the enhancement of the kinetic separation of C2H4/C2H6by controlling the crystal size of ZnAtzPO4to amplify the diffusion difference of C2H4and C2H6(Fig.1).Through adjusting the synthesis temperature,reactant concentration,and ligands/metal ions molar ratio,ZnAtzPO4crystals with different sizes (8.8–43.5 μm) were obtained.Single-component kinetic adsorption tests and binary-component dynamic breakthrough experiments confirmed the increase of C2H4/C2H6separation selectivity,from 1.3 to 98.5,with the increase of the crystal size of ZnAtzPO4.

Fig.1.Schematic representation of the concept of this work.The strategy of crystal size enlargement to acquire longer gas diffusion paths for enhanced C2H4/C2H6 kinetic separation.
2.Experimental
2.1.Chemicals
All the chemicals were purchased from open commercial markets and used as received without any additional process except 3-amino-1,2,4-triazole (Atz,96%),which was purchased from Macklin and further purified by recrystallization.3Zn(OH)2∙2ZnCO3(97%)was purchased from Alfa Aesar.Phosphoric acid(85%(mass)in H2O),ammonium hydroxide(30% (mass))were purchased from Macklin.N-butanol (analytical reagent) was purchased from Sinopharm Chemical Reagent Co.,Ltd.(China).
2.2.Materials
Samples of ZnAtzPO4were synthesized according to the report[17] with some modifications.
2.2.1.Synthesis of ZnAtzPO4-M1
A mixture containing phosphoric acid (0.035 g),ammonium hydroxide (490 μl),3Zn(OH)2∙2ZnCO3(1.2 g),Atz (4.8 g),H2O(24 ml),andn-butanol (12 ml) were added in a Teflon tube(50 ml),then sealed and fixed in a homogeneous reactor (Wanshsin),setting the temperature inside as 160 °C.After waiting for 4 d,the Teflon tube was cooled to room temperature naturally.Filter the white powdery solids out of the mixed phase in the tube,then washed the product with methanol and water successively,and dried in air for 10 min around.Lastly,heated the product at 100 °C under vacuum for 8 h to activate the sample.
2.2.2.Synthesis of ZnAtzPO4-M2 and ZnAtzPO4-M3
A mixture containing phosphoric acid (0.035 g),ammonium hydroxide (480 μl),3Zn(OH)2∙2ZnCO3(0.9 g),Atz (3.6 g),H2O(24 ml),andn-butanol (12 ml) were added in a Teflon tube(50 ml),then sealed and fixed in a homogeneous reactor (Wanshsin) with set temperature of 200 °C for 24 h (200 °C and 6 h for ZnAtzPO4-M3).After that,the Teflon tube was cooled to room temperature naturally.Other processes were the same as above.
2.2.3.Preparation of ZnAtzPO4-M0
Nano-sized materials (ZnAtzPO4-M0) were producedviagrinding micron crystals (ZnAtzPO4-M1) synthesized previously.
2.3.Characterization methods
X-ray powder diffraction (PXRD) data were collected on a Shimadzu XRD-600 diffractometer (Cu Kα λ=0.1540598 nm),with operating power of 40 kV and 30 mA.The scan speed and the range of 2θ were set as 4.0(°)∙min-1and from 5°to 50°.The morphology of materials was analyzed using a field emission scanning electron microscope(FE-SEM,SU-8010,Hitachi,Japan).The particle sizes of each sample were measured by Nano Measurer 1.2 on a large number of SEM images of materials.
2.4.Adsorption experiments
The time-dependent adsorption profiles of C2H4and C2H6on the samples were measured by BEL-SORP-max II equipment at 30 kPa,0 °C.For the test at the set pressure,a fixed amount of gas was introduced into the sample chamber,then the equipment monitored the pressure in chamber until it declined to stable[37].
2.5.Breakthrough experiments
The breakthrough experiments were performed on a selfassembly dynamic device (Fig.S1,in Supplementary Material).The ZnAtzPO4samples (after degassed overnight at 100 °C with a moderate N2flow) were packed in a stainless steel column(4.6 mm inner diameter × 50 mm).The binary mixture of C2H4/C2H6(50/50,volume ratio,the initial concentration of C2H4or C2H6isC0) was introduced at 0 °C.The outlet (the concentration of C2H4or C2H6at outlet isCA) from the column was monitored by gas chromatography (490 Micro GC,Agilent Technologies,USA) with a thermal conductivity detector.
2.6.Calculations of diffusion time constants
The time-dependent adsorption profiles of C2H4and C2H6were measured on Belsorp-max II analyzer.The diffusion time constants(Dc/r2,s-1) were calculated by the following micropore diffusion model[38,39].Eq.(1)was used to calculate the diffusion time constants of gases on ZnAtzPO4-M2 and ZnAtzPO4-M3,and Eq.(2)was used on ZnAtzPO4-M0 and ZnAtzPO4-M1 due to the lack of sorption data (qt/qe) below 0.3 resulted by the fast diffusion of gases.
wheremt/m∞is the fractional adsorption uptake,Dc(m2∙s-1) is the intracrystalline diffusivity of gas molecules in porous media,rc(m)is the crystal radius,t(s) is the time.
3.Results and Discussion
3.1.Crystal size control of ZnAtzPO4
The networks of ZnAtzPO4is constructed by the twodimensional layers of Zn2+and deprotonated Atz which are pillared byanions[17].As shown in Fig.2(a),the key characteristic of pore structure in ZnAtzPO4was the pocket-like space interconnected by narrow bottleneck (0.382 nm),which is close to the molecular size of C2H6.In this work,we focus on the investigation of the influence of the ZnAtzPO4crystal size on the dynamic diffusion behavior of C2H4and C2H6,which play a key role on the separation performance of ZnAtzPO4.To modulate the crystal size of ZnAtzPO4,the detailed effects of temperature,reactant concentration,reaction time and ligands/metal ions molar ratio were investigated respectively.Among all those parameters of synthesis condition,the effects of temperature and reaction time were the most important.Therefore,we mainly discuss the influence of synthetic temperature and reaction time to the crystal size and structure.Firstly,in order to discuss the crystal size of ZnAtzPO4quantitatively,we choose the diamond shape that closest to the crystal shape as the model,and take the side length as shown in Fig.2(b)as the crystal size.As shown in the microscope graph from Fig.2(c),with the synthetic temperature in a range of 140–200 °C and the reaction time in a range of 6 h–6 d,the crystal size changed from nanometer to micrometer.When the temperature is 160 °C,the crystal size increased first and then decreased with time,so as to the temperature of 180°C and 200°C.For the synthetic temperatures of 160,180,and 200 °C,the maximum crystal size at each temperature is 25 μm (160 °C),29 μm (180 °C),and 44 μm(200 °C).In addition,the effects of reactant concentration and ligands/metal ions molar ratio were shown in Figs.S2 and S3.Overall,by changing the synthesis conditions,we could modulate the crystal size of ZnAtzPO4controllably.

Fig.2.(a)The pore channel of ZnAtzPO4 and connolly surface.(b)The schematic picture and crystal size defination of ZnAtzPO4 crystal,and the crystal size was defined as the longest length.(c)Microscope images of ZnAtzPO4 synthesized with the extension of time at different temperatures.Marked in red were the largest ZnAtzPO4 synthesized at corresponding temperature,with the sizes noted.
The study above laid an important foundation for further preparation of ZnAtzPO4samples with different scales of crystal size.According to above results,the control methods of ZnAtzPO4crystal size were formed,and samples with crystal size from 9 to 44 μm were produced.To carry on C2H4/C2H6separation study,we selected three representative samples of 9,25,44 μm termed as ZnAtzPO4-M1,ZnAtzPO4-M2,ZnAtzPO4-M3 respectively,as shown in the SEM images in Fig.3.It’s worth noting that the crystal size of each sample was the mean value of statistical data from about 100 crystals from the SEM images (see Table S2 for detailed data).The results showed that the precise size of ZnAtzPO4-M1,ZnAtzPO4-M2 and ZnAtzPO4-M3 was 8.8,20.7 and 43.5 μm,respectively (Fig.3).In addition,nano-size crystals (ZnAtzPO4-M0) were producedviadownsizing micron crystals synthesized previously.As shown in Figs.3(e) and S9,all ZnAtzPO4samples with different crystal sizes showed good crystallinity and thermal stability.

Fig.3.SEM images of(a)ZnAtzPO4-M0,(b)ZnAtzPO4-M1,(c)ZnAtzPO4-M2,(d)ZnAtzPO4-M3 crystals.Due to the preparation method of grinding,the sizes of ZnAtzPO4-M0 crystals on specific axes were unknown.(e) PXRD patterns of ZnAtzPO4-M0,ZnAtzPO4-M1,ZnAtzPO4-M2 and ZnAtzPO4-M3.
3.2.Sorption properties
To evaluate the kinetic behavior of C2H4and C2H6adsorption on ZnAtzPO4with different crystal sizes,the time-dependent adsorption profiles were measured at 0°C and 30 kPa on each sample.As shown in Fig.4(a) and (b),the profiles of C2H4adsorption on four samples almost overlapped,while those profiles of C2H6showed a large difference.This large difference in the adsorption behavior between C2H4and C2H6caused by the crystal size demonstrated the key role of crystal size modulation.Specifically,with the largest crystal size of 43.5 μm,ZnAtzPO4-M3 showed the lowest C2H6adsorption rate (Fig.4(b)).The C2H6adsorption on ZnAtzPO4-M3 took more than 130 min but still did not reach equilibrium.Compared,the smallest crystal of ZnAtzPO4-M0 exhibited much faster adsorption rate than ZnAtzPO4-M1,ZnAtzPO4-M2,and ZnAtzPO4-M3.
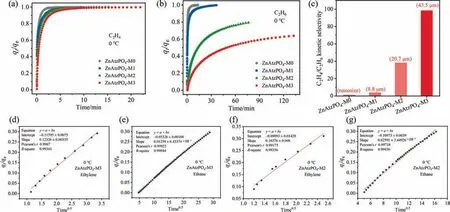
Fig.4.(a) C2H4 and (b) C2H6 time-dependent adsorption curves of ZnAtzPO4-M0,ZnAtzPO4-M1,ZnAtzPO4-M2 and ZnAtzPO4-M3 at 0 °C and 30 kPa.(c) C2H4/C2H6 kinetic selectivities of adsorption separation on ZnAtzPO4-M0,ZnAtzPO4-M1,ZnAtzPO4-M2 and ZnAtzPO4-M3 at 0°C and 30 kPa.(d)–(g)Fitting curves for C2H4 and C2H6 adsorbed by microporous diffusion model on ZnAtzPO4-M2 and ZnAtzPO4-M3.
Further,the adsorption profiles were fitted using micropore diffusion model to collect gases diffusion data,and the diffusion time constants were given asDc/r2(s-1),whereDcis the diffusion coefficient andris a characteristic length scale[40].The adsorption profiles were fitted well by the model,with theR-square above 0.9(Fig.4(d)–(g)).As shown in Table 1,the adsorption rate of two gases decreased with the increase of the crystal size of ZnAtzPO4.For example,theDc/r2of C2H6on ZnAtzPO4-M0 was 4.01 × 10–3s-1while theDc/r2of C2H6on ZnAtzPO4-M3 was 1.34 × 10–5s-1,the variation showed more than two orders of magnitude,much wider than that of C2H4(5.30 × 10–3s-1versus1.32 × 10–3s-1).These different variations in C2H4and C2H6diffusion with crystal size were caused by their difference in molecular size.To represent C2H4/C2H6diffusion difference more specifically,the kinetic selectivity of separation was calculated by the ratio ofDc/r2of two gases.As shown in Fig.4(c),the C2H4/C2H6kinetic selectivity of ZnAtzPO4-M0 (1.3),ZnAtzPO4-M1 (4.0),ZnAtzPO4-M2 (39.9),ZnAtzPO4-M3(98.5)increased with the increase of the crystal size of ZnAtzPO4-M0(nano size),ZnAtzPO4-M1(8.8 μm),ZnAtzPO4-M2(20.7 μm),ZnAtzPO4-M3(43.5 μm).That is,as the crystals became larger,the kinetic selectivity for C2H4over C2H6increased,making ZnAtzPO4-M3 advantageous for C2H4/C2H6kinetic adsorption separation.
Table 1 Diffusion time constants (Dc/) of C2H4 and C2H6 on ZnAtzPO4-M0,ZnAtzPO4-M1,ZnAtzPO4-M2,ZnAtzPO4-M3

Table 1 Diffusion time constants (Dc/) of C2H4 and C2H6 on ZnAtzPO4-M0,ZnAtzPO4-M1,ZnAtzPO4-M2,ZnAtzPO4-M3
The C2H4/C2H6kinetic adsorption selectivity on large crystals pushed us to further evaluate and compare the dynamic separation performance on ZnAtzPO4-M0,ZnAtzPO4-M1,ZnAtzPO4-M2 and ZnAtzPO4-M3 in a fixed bed.The breakthrough experiments were conducted with a flow rate of 0.75 ml∙min-1of binary gas mixtures C2H4/C2H6(50/50,volume ratio) at 0 °C.Considering the fact that the pre-column pressure of column packed with ZnAtzPO4-M0 was as large as 60 kPa,much higher than the other sample columns(5 kPa),the experimental data of ZnAtzPO4-M0 (Fig.S7) were not compared in this work.As shown in Fig.5(a),C2H6flowed out at 37.6 and 16.2 min∙g-1of the fixed bed of ZnAtzPO4-M2 and ZnAtzPO4-M3,respectively,while C2H4flowed out at the similar time(65 min∙g-1).Overall,as the crystal size increased,C2H6broke through earlier.In consequence,the adsorption capacities of C2H6in process were 0.62 and 0.12 mmol∙g-1on ZnAtzPO4-M2 and ZnAtzPO4-M3.And the uptake capacity of C2H4on ZnAtzPO4-M2 and ZnAtzPO4-M3 was about 1.56 mmol∙g-1(Fig.5(b)).It shows that the adsorption of C2H6gradually became away from equilibrium state in the practical process when the size of the adsorbent crystals increased.The dynamic C2H4/C2H6selectivity that calculated from breakthrough curves was 1.9,2.5 and 13 for ZnAtzPO4-M1,ZnAtzPO4-M2,ZnAtzPO4-M3,respectively (Fig.5(c)).These results indicated that the larger crystallites that within a certain range were more favorable for kinetic adsorption separation.The longer diffusion paths strengthened C2H4/C2H6diffusion difference,thus increasing the dynamic separation selectivity of C2H4/C2H6.
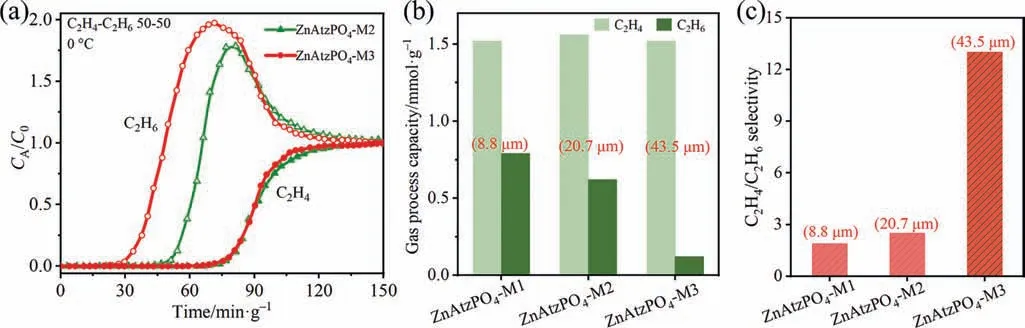
Fig.5.(a)Experimental breakthrough curves of C2H4/C2H6(50/50,volume ratio;0°C)mixtures on ZnAtzPO4-M2 and ZnAtzPO4-M3.Comparisons of C2H4 and C2H6 dynamic capacities (b) and C2H4/C2H6 experimental selectivities (c) on ZnAtzPO4-M1,ZnAtzPO4-M2 and ZnAtzPO4-M3 in the process.
4.Conclusions
In summary,we reported the improvement of kinetic C2H4/C2H6adsorption separation performance on an ultra-microporous metal–organic framework ZnAtzPO4,viaamplifying gases diffusion difference by crystal size control.We investigated the effects of synthesis temperature,reactant concentration,ligands/metal ions molar ratio,reaction time,etc.on the size of the crystals formed.In this way,samples ZnAtzPO4-M0,ZnAtzPO4-M1,ZnAtzPO4-M2,ZnAtzPO4-M3 with different crystal sizes of nanoscale,8.8,20.7 and 43.5 μm were prepared.Adsorption experiments of C2H4and C2H6on four samples were conducted.As the kinetic tests showed that the C2H6diffusion time constant declined from 4.01×10–3s-1on ZnAtzPO4-M0 to 1.34 × 10–5s-1on ZnAtzPO4-M3,and the corresponding C2H4/C2H6kinetic selectivity increased to 98.5(ZnAtzPO4-M3)from 1.3(ZnAtzPO4-M0).The breakthrough experiment results also showed that as the crystals enlarged,the dynamic C2H4/C2H6selectivity on ZnAtzPO4-M1,ZnAtzPO4-M2,ZnAtzPO4-M3 increased successively.It revealed the enhancement of the dynamic separation of C2H4/C2H6with the increase of the crystal size of ZnAtzPO4.The large difference in the adsorption behavior between C2H4and C2H6caused by the crystal size demonstrated the key role of crystal size modulation.This study provided an inspiration for the improvement of the dynamic separation of structure-similar molecules by the modulation of the crystal structure and size of adsorbents.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Key Research and Development Program of China(2022YFB3806800);the National Natural Science Foundation of China (22122811,22008209),the Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering(2021SZ-TD008).
Supplementary Material
Microscope images,adsorption isotherms,time-dependent adsorption curves,experimental breakthrough curves and crystal size data.Supplementary material to this article can be found online at https://doi.org/10.1016/j.cjche.2023.06.001.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
