Effects of friction stir processing and nano-hydroxyapatite on the microstructure,hardness,degradation rate and in-vitro bioactivity of WE43 alloy for biomedical applications
2024-04-18BoWuFarazilaYusofFuguoLiHuanMiaoBushroaMohdRidhaBinMuhamadIrfanAnjumBadruddinMahmoudIbrahim
Bo Wu ,Farazila Yusof,c,∗ ,Fuguo Li ,Huan Miao ,A.R.Bushroa ,Mohd Ridha Bin Muhamad ,Irfan Anjum Badruddin,Mahmoud Z.Ibrahim
a Department of Mechanical Engineering, Faculty of Engineering, Universiti Malaya, Kuala Lumpur 50603, Malaysia
b Centre of Advanced Manufacturing and Material Processing (AMMP Centre), Universiti Malaya, Kuala Lumpur 50603, Malaysia
c Center of Foundation studies in Science, Universiti Malaya, Kuala Lumpur 50603, Malaysia
d State Key Laboratory of Solidification Processing, School of Materials Science and Engineering, Northwestern Polytechnical University, Xi’an 710072, China
e Mechanical Engineering Department, College of Engineering, King Khalid University, Abha 61421, Saudi Arabia
fDesign and Production Engineering, Faculty of Engineering, Ain Shams University, Cairo 11517, Egypt
Abstract Nowadays,magnesium alloys are emerging in biomedical implants for their similar properties to natural bones.However,the rapid degradation of magnesium alloys in biological media hinders successful implantation.Refinement of microstructure,as well as reinforcement particles can significantly improve the degradation rate.In this work,multi-pass friction stir processing (FSP) was proposed to synthesize WE43/nano-hydroxyapatite (nHA) surface composite,the microstructure,reinforced particle distribution,micro-hardness,corrosion behavior and in-vitro bioactivity were studied.The subsequent FSP passes of WE43 alloy and WE43/nHA composite refined the grain size which was reduced by 94.29% and 95.92% (2.63 and 1.88 μm,respectively) compared to base metal after three passes.This resulted in increasing the microhardness by 120% (90.86 HV0.1) and 135% (105.59 HV0.1) for the WE43 and WE43-nHA,respectively.It is found that increasing FSP passes improved the uniform distribution of nHA particles within the composite matrix which led to improved corrosion resistance and less degradation rate.The corrosion rate of the FSPed WE43/nHA composite after three passes was reduced by 38.2% (4.13 mm/year) and the degradation rate was reduced by 69.7% (2.87 mm/y).This is attributed to secondary phase (Mg24Y5 and Mg41Nd5) particle fragmentation and redistribution,as well as a homogeneous distribution of nHA.Additionally,the growing Ca-P and Mg(OH)2 layer formed on the surface represented a protective layer that reduced the degradation rate.The wettability test revealed a relatively hydrophilic surface with water contact angle of 49.1 ± 2.2° compared to 71.2 ± 2.1° for base metal.Also,biomineralization test showed that apatite layer grew after immersion 7d in simulated body fluid with atomic ratio of Ca/P 1.60 approaching the stoichiometric ratio (1.67) indicating superior bioactivity of FSPed WE43/nHA composite after three passes.These results raise that the grain refinement by FSP and introduction of nHA particles significantly improved the degradation rate and in-vitro bioactivity of WE43 alloy for biomedical applications.
Keywords: Friction stir processing;Magnesium-based composite;Nano-hydroxyapatite;Corrosion behavior;In-vitro bioactivity.
1.Introduction
As a new generation biodegradable material,magnesium(Mg) alloys have attracted extra attention for biomedical implants such as cardiovascular stent,bone plates and screws because of their similar density and elastic modulus to human bones,which reduce the adverse effects of stress shielding[1,2].Moreover,Mg alloys can be entirely degraded in vivo without additional surgeries to remove the remaining remnants [3].Despite biodegradable Mg alloys are favorable for biomedical implants,the application of Mg-based biomaterials is hampered by a number of challenges.The principal challenge is their poor corrosion resistance and rapid degradation in biological medium.Furthermore,the rapid degradation rate can result in a loss of strength,and possibility failure before the bone has sufficiently healed[4].The uncontrollable degradation rate in biological environment restrains their medical application as implant materials.
The mechanical properties and degradation behavior of Mg alloys can be improved through alloying,coating,and composite fabrication[5–10].Several magnesium alloys have been developed such as AZ,ZK and WE-series.The most common alloy considered for biomedical applications is AZ91.However,this alloy contains aluminum which has a potential risk of toxicity and creates aggregates in tissue that leads to the formation of pathological cells in Alzheimer’s disease [11].WE-series is alloyed with rare earth (RE) elements– such as yttrium (Y) and neodymium (Nd)– to enhance the mechanical properties and REs form a protective layer on the surface that improves the corrosion resistance and reduce the degradation rate in biological mediums [12].Moreover,the used Res in WE-series is non-toxic and does not impose any health risks.
Another approach is grain refinement that has significant influence on mechanical properties and corrosion resistance[13].Additionally,the Mg alloy can be reinforced with bioceramics that can further promote mechanical properties,corrosion resistance and bioactivity [14,15].Friction stir processing (FSP) is an efficient technique to refine the microstructure and produce Mg matrix composites reinforced by bioceramics particles with uniform and homogeneous distribution [16–18].Multi-pass FSP can further refine the microstructure and improve the uniform distribution of reinforcement particles which enhances the mechanical properties of Mg matrix composite [19–23].The incorporation of bioceramic particles such as SiC [24],ZnO [25],Al2O3[26],TiC[27],phosphate glasses (bioactive glasses) [28],akermanite[29],SiO2[30],tri-calcium phosphate (TCP) [31,32]and hydroxyapatite (Ca10(PO4)6OH2,HA) [33,34]into Mg matrix has been considered to fabricate composites that exhibit enhanced corrosion resistance and strength.Comparing its safety,degradability and biomineralization,although some other materials have unique properties and applications,HA stands out as the most widely biocompatible ceramic material for medical applications due to its superior bioactivity required for direct tissue integration and adhesion,biocompatibility,and chemical similarity to natural bone [35–37].Recent studies have shown that the addition of HA particles induces nucleation and prevents grain growth.El-Sayed et al.[31]observed the Mg-HA composites exhibited superior corrosion resistance compared to Mg-Al2O3and Mg-TCP when subjected to the same FSP pass.This enhanced corrosion resistance was ascribed to the lower solubility constant of HA in various aqueous environments.Ahangari et al.[38]utilized electrophoretic deposition method to introduce HA particles on AZ31 alloy.However,this approach did not meet the requirements of successful implant.Hanas et al.[39]produced fine-grained AZ31/HA metal matrix composite by two passes of FSP,using rotational speed and traverse spend of 1500 rpm and 60 mm/min,respectively.But the agglomeration of HA particles was observed in the microstructure of AZ31/HA composite which cause inhomogeneous mechanical properties and corrosion behavior.Yousefpour et al.[23]investigated the microstructure evolution of AZ91/HA composite manufactured by three FSP passes.The distribution of particles in the AZ91/HA composite was greatly impacted by FSP pass.The grain size was reduced as the number of FSP increased,and HA particles were dispersed more evenly,leading to enhanced hardness and strength.However,they lacked in evaluating how the FSP pass affects corrosion resistance and bioactivity behavior.In contrast to the findings reported by Liu et al.[40],the grain size of ZK60 could not be reduced more with the increase of FSP passes.This liamation was attributed to the dissolution of precipitates after first pass.Consequently,the pinning effects of these precipitates on the mobility of grain boundaries were significantly diminished during subsequent passes.As a result,grain growth was promoted during dynamic recrystallization.Due to precipitates and grain size have important effect on the surface,they found that the increase in the number of FSP passes deteriorated the corrosion resistance.Ahmadkhaniha et al.[41]reported the influence of reinforced HA particles on microstructure and bio-corrosion behavior of pure Mg matrix.They revealed that the dynamic recrystallization triggered by severe plastic deformation caused the grain to change from coarse to fine homogenous structure.However,the corrosion resistance of the specimens with HA particles deteriorated and was reduced by 20% compared to the specimens without HA particles.Ho et al.[42]focused on the effect of micron HA (mHA,10 μm) content on thein-vitrobio-corrosion behavior of synthesized AZ31/mHA composites by FSP,the findings demonstrated that the addition of mHA led to enhanced corrosion resistance for AZ31/mHA composites compared to untreated AZ31.As nano-sized HA (nHA)has a significantly higher surface area compared to mHA due to its smaller particle size,which can lead to increased reactivity and bioactivity.However,the effect of adding nHA particles and multi-pass FSP on the properties is inconsistent.For new generation biomedical implant WE43 alloy,the studies on how microstructural changes affect the corrosion properties and bioactivity of WE43 alloy are not clear enough.
In the present work,FSP was proposed to refine the microstructure of WE43 alloy and synthesize WE43/nHA composite to improve the corrosion resistance and degradation rate for biodegradable implant applications.The influence of FSP passes and nHA particles on microstructure,hardness,corrosion resistance,degradation rate andin-vitrobioactivity of the FSPed samples were investigated.
2.Experimental procedure
2.1. Materials and FSP system
The chemical composition of hot rolled WE43 alloy is shown in Table 1.The plates were cut to a dimension of 200×100×3.5 mm.The nHA particle size was 20–60 nm in width and 60–150 nm in length.Fig.1 shows the SEM micrograph of HA particle.
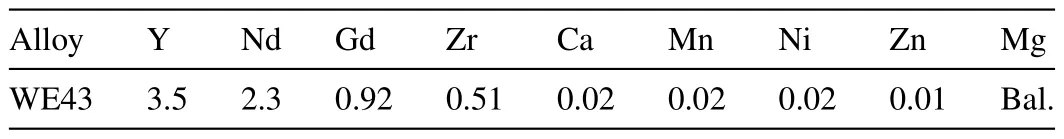
Table 1 Chemical composition of WE43 alloy (wt%).

Fig.1.The SEM image of nHA particles.
Grooves of 1 mm in width and 2 mm in depth were machined at the center of plate,then filled with nHA particles to fabricate WE43/nHA composites using one,two and three FSP passes namely as FSPed WE43/nHA-1P,WE43/nHA-2P,WE43/nHA-3P,respectively.The volume fraction of nHA particles used was kept constant at 16 vol% of the base metal.FSP was performed on a vertical CNC machine (MITSUI SEIKI VT3A,Japan) using a tungsten carbide (WC) tool with shoulder diameter of 12 mm,and a taper cylindrical pin (3 mm top diameter,5 mm bottom diameter,and 3 mm height).For each sample,one FSP pass was performed using a modified pinless tool to seal the groove surface to prevent the dispersal of nHA particles followed by the remaining FSP passes using the pin tool,Fig.2(a) depicts each stage of the FSP.The FSP passes were completely overlapped in the same direction to ensure uniform and homogeneous distribution of nHA particles.
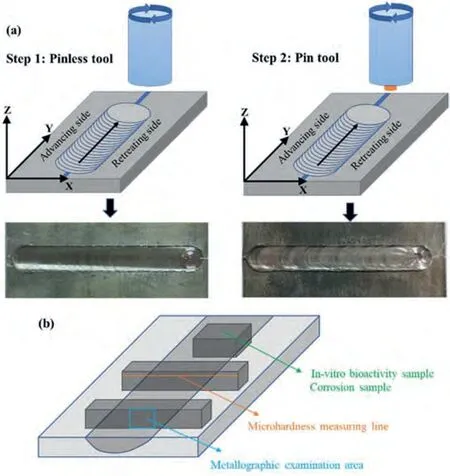
Fig.2.(a) The schematic view of the FSP and (b) the positions of the sample selected for test.
Based on previous studies [43],the rotational and traverse speeds were chosen as 1250 rpm and 30 mm/min,respectively.The WE43 alloy without adding nHA particles(namely FSPed WE43-1P,WE43-2P,WE43-3P) were also carried out for comparison purposes.
2.2. Microstructure characterization and phase identification
Coupons of 10×3×3 mm3were cut perpendicular to the FSP direction for microstructural observations,as shown in Fig.2(b).The strips were ground using emery papers from#600 to #5000 grit and polished till mirror-like surface using diamond paste,then etched by 5 g picric acid,10 ml acetic acid,80 ml ethanol and 10 ml deionized water for 10 s.The microstructure was examined by optical microscope(OM,OLYMPUS,Japan) and scanning electron microscope(SEM,ZEISS Sigma 300,German),while energy-dispersive spectroscopy (EDS,OXFORD Xplore) was used for elemental distribution.The grain size was determined by the linear intercept method with Nano measurer 1.2 software.X-ray diffraction (XRD,Bruker D8 Advance,Germany) with Cu Kαradiation (2θrange was 10° to 90° with 3° min-1scanning rate) was used to identify the formed phases by JADE analysis software.
2.3. Microhardness measurement
The microhardness of the base metal and the prepared samples was measured using Vickers microhardness (HMV-2 series,SHIMADZU,Japan) using a load of 100 g and 5 s dwell time.Microhardness profile measurements were taken along the cross section of a plane parallel to the surface along the stirring zone,thermomechanical affected zone,heat affected zone and base metal,as shown in Fig.2(b).The distance between successive points was kept constant at 0.5 mm.
2.4. Corrosion evaluation
2.4.1.Electrochemical test
The electrochemical corrosion test was carried out in simulated body fluid (SBF) solution at 37 ± 0.5 °C.The SBF solution was prepared according to a standard protocol (NaCl(5.403 g),NaHCO3(0.504 g),K2HPO4·3H2O (0.23 g),KCl(0.225 g),MgCl2·6H2O (0.331 g),CaCl2(0.293 g) and Na2SO4(0.072 g) dissolved in deionized water).Samples of 10×10 mm2size 10×10 mm2were cut from the stirring zone,as shown in Fig.2(b),then attached with aluminium tape and mounted in an epoxy mould so that only the surface of the FSPed is exposed.The samples were served as the working electrode,while Ag/AgCl and platinum strip were set as the reference and counter electrodes,respectively.The potentiodynamic polarization was performed from-2.0 to 2.0 V with a scanning rate of 10 mV/s using BioLogic SP-150 single channel potentiostat.With the assistance of the EC-Lab software,the values of corrosion potential (Ecorr),corrosion current density (Icorr) were used to get the corrosion rate(mm/year) from Eq.(1) [44].
whereKis a unit conversion constant (3.272 for mm/y),EWis the equivalent weight of the alloy (12.76 atomic weight),ρis the alloy density (1.8 g/cm3) andArefers to the sample surface area of the sample (cm2).
2.4.2.Immersion test
The degradation behavior of prepared samples was assessed through weight loss during 1,3,and 7 days of immersion in SBF at 37 ± 0.5 °C.Before and after immersion,the weight of the specimens was measured using a precision balance with an accuracy of 0.1 mg.After each designated immersion period,ultrasonic cleaning in ethanol and distilled water followed by a chromic acid solution was used to remove surface corrosion products.The weight loss (W,mg/cm2) and degradation rate (mm/year) were calculated by Eqs.(2) and(3) [31,45].
whereK=8.67×104is conversion factor (mm/year),Arefers to the sample surface area (cm2),Tis immersion time(h) andDis the density (1.8 g/cm3).
2.5. In-vitro bioactivity test
2.5.1.Contact angle measurement
Wettability test was performed by measuring the contact angle using optical contact angle goniometer (Data physics OCA20,Germany).All samples were polished to #2000 grade.A drop of distilled water was placed on the surface for about 10 s to stabilize before recording.The measurement was carried out under ambient conditions and five contact angle measurements were obtained on each testing surface to minimize errors.The surface energy (Es) was calculated from the contact angles using the following Eq.(4) [46].
whereθis the contact angle,Evlis the surface energy between water and air under ambient condition (72.8 mJ/m2at 20 °C).
2.5.2.Biomineralization evaluation
The samples were cut from stirring zone with size of 10×10 mm2,then polished and cleaned with ethanol in ultrasonic bath for 5 min before they were immersed in SBF for 1,3,and 7 days.All samples were mounted in epoxy and vertically placed where the surface of the samples was parallel to the gravity direction in 30 ml of SBF solution at 37 ± 0.5 °C (the volume ratio of SBF to sample surface area ratio was greater than 1:10),to prevent the impact of gravity on mineralization behavior.The SBF solution was replaced every 24 h in order to keep the pH at 7.4.Then,the surface morphology observation was conducted by SEM and the elemental composition of the apatite layer was conducted by EDS analysis.
3.Results and discussion
3.1. Microstructure characterization
Fig.3 shows the optical microstructure of FSPed WE43 alloys at stirring zone.It is clearly shown that with an increase in the FSP pass,the grain size of the FSPed WE43 alloys and the FSPed WE43/ nHA composite became finer due to the strong shear deformation and frictional heat that cause the occurrence of dynamic recrystallisation[47].Dynamic recrystallisation is a recrystallisation process that occurs during high temperature deformation,where the nucleation and growth of new grains occurs during the deformation process due to the local temperature rise at stirring zone [48].

Fig.3.The microstructure of FSPed WE43 samples at stirring zone for (a,d) one,(b,e) two,and (c,f) three passes at different magnification.
Fig.4 shows the microstructure of FSPed WE43/nHA composites at stirring zone.It can be seen severe agglomeration of nHA particles of FSPed WE43/nHA-1P composite.Due to inadequate plastic flow of reinforced nHA particles between WE43 substrates,aggregation of nanoparticles for first pass is inevitable which restricts the homogeneous distribution of nHA.With increasing FSP passes,the plastic flow of material was enhanced and severe deformation reduces the quantity and size of the agglomeration,thus leads to a more homogeneous distribution of nHA particles [31].The average grain size of the FSPed WE43/nHA composite is lower than that of the FSPed WE43 alloys for the same FSP pass as nHA particles were pinned to grain boundaries,which hampers grain border migration and subsequent grain growth [20,49],Fig.5.This is crucial for dynamic recrystallization and grain refining.After the third pass,the FSPed WE43 alloy and WE43/nHA composite have the smallest grain size (2.63 and 1.88 μm),which were reduced by 94.29%and 95.92%compared to base metal.Ho et al.[42]synthesized AZ31/mHA composites by 10 μm mHA.After 10 passes of FSP the grain size reduced from 7.7 μm to 3.5 μm,about 54.55% reduction.

Fig.4.The microstructure of FSPed WE43/nHA composite at stirring zone for (a,d) one,(b,e) two,and (c,f) three passes at different magnification.
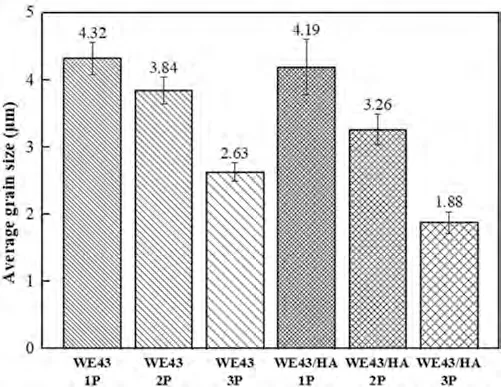
Fig.5.The average grain size for FSPed WE43 alloys and WE43/nHA composites.
This indicates that the particle size has a significant influence on grain refinement.Both mHA and nHA particles are pinned to grain boundaries,but their size,nucleation,and effect on grain growth are different.In terms of grain refinement,nHA is more effective than mHA.As nano-sized HA(nHA) has a notably higher surface area compared to mHA due to its smaller particle size.During dynamic recrystallization,decreasing the particle size from mHA to nHA dramatically increases the number of particles,thereby increasing the number of preferential sites for nucleation recrystallization.Meanwhile,enhancing the pinning effect of particles and augments fragmentation of pre-existing grains.The introduction of nHA particles and consequent FSP passes have a significant effect on the grain refinement and particle distribution.The present finding is consistent with previous research on the effect of FSP pass on reinforced particle distribution [50,51].
Figs.6 and 7 depict SEM micrographs coupled with EDS mapping and point EDS spectrum for FSPed WE43-3P and FSPed WE43/nHA-3P samples at stirring zone,respectively.The SEM micrographs,Figs.6(a) and 7(a),show a highly refined microstructure,which is mainly due to the severe plastic deformation.In addition,REs (Y,Nd,and Gd) are detected in both cases as verified by the EDS mapping in Figs.6(c-f) and 7(c-g).Mg24Y5and Mg41Nd5are intermetallic phases with the size of 1∼2 μm,Fig.6(a),which can effectively suppress the dislocation movement and grain boundary sliding and play a role in grain refinement of WE43 during FSP because of second phase pinned to grain boundaries during deformation[52].The second phase was refined by FSP,which led to the refinement ofα-Mg matrix with a uniform grain size and effective redistribution of the second phase.The yellow circle in Fig.7(a)indicates that HA particles were distributed within stirring zone for FSPed WE43/nHA-3P composite.Calcium and phosphorus were detected from FSPed WE43/nHA-3P composite with weight percentage of 2.68% and 3.99%,respectively.

Fig.6.(a) SEM micrograph,(b) EDS spectrum,and (c-f) EDS mapping of FSPed WE43-3P alloy.

Fig.7.(a) SEM micrograph,(b) EDS point spectrum (the yellow circle in Fig.7(a)),and (c-g) EDS mapping of FSPed WE43/nHA-3P composite.
Due to the strong tendency to segregate at grain boundaries,REs can contribute to the pinning effect and the solute drag effect and significantly inhibit the dynamic recrystallization of grains,so the FSPed WE43/nHA-3P composite has the smallest grain size[53].Fig.7 shows a homogeneous distribution of nHA particles in WE43 throughout the stirring zone.This is because nHA particles can hinder the development of grains through the Zener pinning effect during three FSP passes of continuously stirring and rubbing [54].Therefore,increasing FSP passes will break up agglomerated particles into tiny agglomerates and separate particles.
3.2. Phase identification
XRD patterns of the FSPed WE43 alloys and FSPed WE43/nHA composites are presented in Fig.8.Diffraction peaks corresponding toα-Mg,Mg24Y5,and Mg41Nd5phases can be observed for all samples.Interestingly,the relative peak intensities ofα-Mg phase is increasing,while Mg24Y5,and Mg41Nd5phases are decreasing for FSPed samples compared to base metal because after FSP,the phases were broken into tiny grains with the size of 1∼2μm and the volume fraction of these phases were nearly 0.85% (Mg24Y5)and 0.93% (Mg41Nd5).For base metal,the diffraction peaks ofα-Mg included higher peak of (101) and relatively lower peak of(002)which indicated no obvious preferential orientation.These patterns are comparable to those of the computer simulated random orientated of Mg alloy [55].
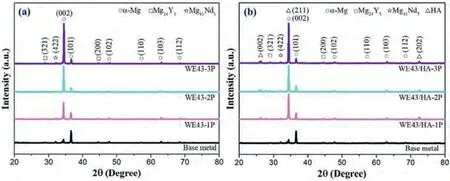
Fig.8.XRD patterns of base metal and (a) FSPed WE43 and (b) FSPed WE43/nHA samples.
For FSPed samples,the peak intensity of (101) significantly decreased and the peak of(002)exhibited an extremely high intensity.Indicating that plane (002) is perpendicular to the surface plane.For the hexagonal Mg alloys,it is common for the (002) plane on the rolling or extrusion flat plane [56].The FSP is similar to rolling in that the stirring tool passes over the plate under compressive stress,inducing rotation of the grains in the stirring zone and parallel to the surface of the workpiece at high temperature [57].
During FSP,secondary phase particles are redistributed because of high temperature plastic deformation,Mg24Y5and Mg41Nd5promote recrystallization and nucleation between particles and Mg matrix interfaces.With increasing FSP passes,it induced preferential orientation and formed shear deformation planes of (002).As seen from Fig.8,the (002) peak of FSPed WE43 samples has both a shifted and widened appearance when compared to the base metal.However,the range of variation of the (002) peak ofα-Mg for FSPed WE43/nHA composite is relatively moderate than FSPed WE43 samples from one pass to three passes.This result may be due to the presence of nHA particles,which constrain material flow.For FSPed WE43/nHA composites,HA diffraction peaks were observed including (002),(211) and(202).The intensities of the (002) and (202) of HA diffraction peaks are weak and constant,while for (211) the peak fluctuates considerably.
3.3. Microhardness measurement
Fig.9 shows 2D contour colored maps and line graphs of the microhardness profile of FSPed WE43 alloy and the FSPed WE43/nHA composite,while the average microhardness of the FSPed samples is shown in Fig.10.Both 2D contour colored maps and line graphs of microhardness profile for FSPed WE43 alloys and FSPed WE43/nHA composites showed similar trends.The hardness at the stirring zone was the highest,followed by thermomechanical affected zone and then base metal.
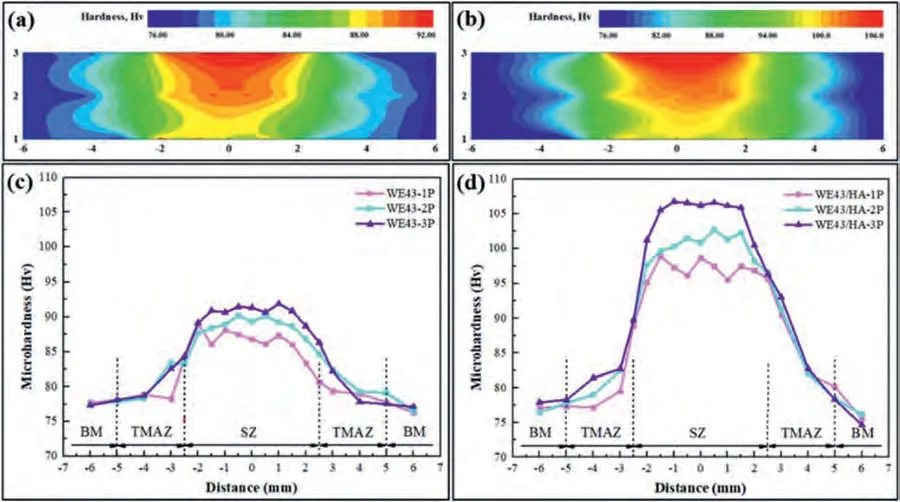
Fig.9.(a,b) Microhardness contour mapping and (c,d) line graph of microhardness profile of FSPed WE43 and WE43/nHA samples.
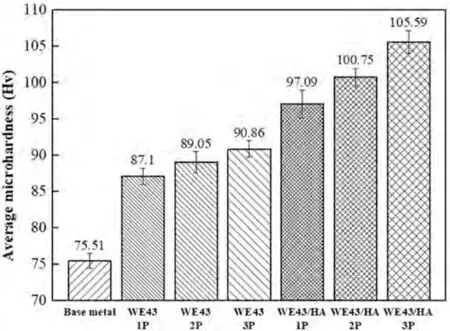
Fig.10.The average microhardness of base metal and FSPed samples at stirring zone.
Under the same FSP pass,the microhardness of FSPed WE43 alloys is lower than that of FSPed WE43/nHA composites,and all samples showed this trend.Simultaneously,it can be observed that the fluctuation of the microhardness curve for FSPed WE43/nHA composites is higher than that of FSPed WE43 alloy,which is mainly attributed to the introduction of reinforced nHA particles and grain refinement.Since the distribution of nHA particles is influenced by the number of passes,the distribution of microhardness values is therefore also affected.The microhardness at the stirring zone fluctuates considerably for FSPed WE43/nHA-1P composite due to the agglomeration of the reinforcement particles and their inhomogeneous distribution.In Fig.9(d),the microhardness of FSPed WE43/nHA-3P shows relatively stable fluctuation values,which indicates that nHA particles are relatively uniformly dispersed in the WE43 matrix,which is consistent with the microstructural observations.
The microhardness of the FSPed samples varied significantly between the different zones and continued to increase at the stirring zone after the one,two and three passes.With the increase in the FSP pass,the microhardness values for the FSPed WE43 alloys and FSPed WE43/nHA composites increased significantly due to grain size reduction.The grain size in the stirring zone becomes smaller,and the refinement effect obviously occurs.Therefore,the main cause for the increase in hardness is the strengthening of the grain boundary.Because of the substantial microstructure homogeneity and refinement,the heterogeneous nucleation of Mg24Y5and Mg41Nd5effectively suppressed the dislocation movement and grain boundary sliding,which played a role in grain refinement.The intermetallic phases Mg24Y5and Mg41Nd5are uniformly distributed throughoutα-Mg as small fragments.Therefore,the microhardness of SZ is typically more consistent and higher than that of the base metal.
3.4. Corrosion test
3.4.1.Electrochemical test
Fig.11 shows the potentiodynamic polarization curves for base metal,FSPed WE43 alloys,and FSPed WE43/nHA composites.The values ofEcorr,Icorr,βa,βc,and corrosion rate were calculated,whereβarefers to the slope of the anodic branch,andβcrefers to the slope of the cathodic branch.Table 2 lists the results of the potentiodynamic polarization test for base metal and FSPed samples.TheEcorrandIcorrreflect the corrosion tendency of the sample.The higher theIcorrvalue,the faster the corrosion reaction and the degree of deeper the corrosion [58].Icorrhas the ability to measure the corrosion rate from a dynamic point of view.The presence of a largerIcorrvalue and a more negativeEcorrvalue are indicators for low corrosion resistance [20,59,60].The value ofEcorrin all FSPed WE43 alloys and WE43/nHA composites are higher than base metal.Notably,theIcorrfor FSPed samples were significantly lower than base metal,indicating improved corrosion resistance.These results could indicate that the corrosion resistance of FSPed samples was enhanced by increasing the FSP pass and introduction of reinforced nHA particles.
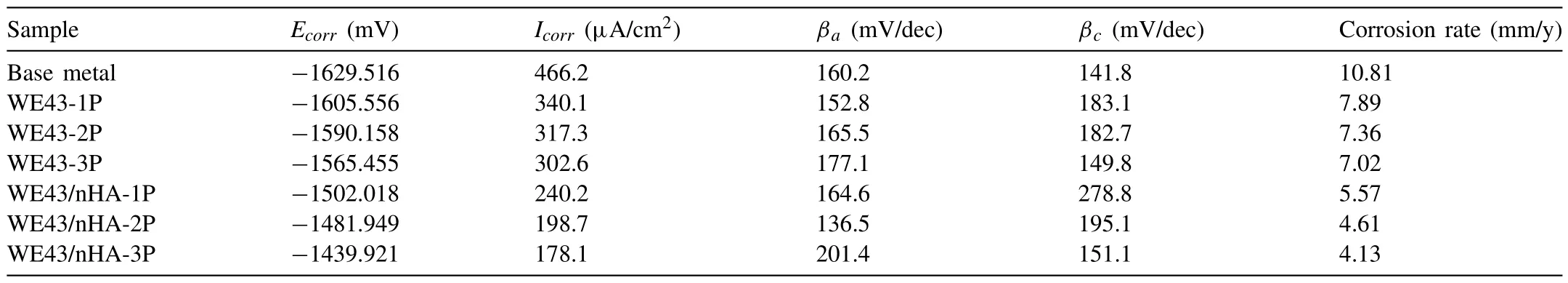
Table 2 The results of potentiodynamic polarization test for base metal and FSPed samples.

Fig.11.Potentiodynamic polarization curves for base metal and (a) FSPed WE43 alloys,(b) FSPed WE43/nHA composites.
The cathodic reaction is the generation of H2,while the anodic reaction is the dissolution of Mg on the contact surface of the sample with the SBF solution,according to Eqs.(5)and(6) [29].
Ultimately,protective layers of MgO and Mg(OH)2were generated on the surface according to Eqs.(7) and (8) [34].
With increasing FSP pass from one pass to three passes,the tendency ofEcorrchanged,but the value ofIcorrand corrosion rate were still becoming lower.Because of the grain size was remarkably refined after FSP especially three passes,also secondary phase particles was uniformly redistributed and with more homogeneity of nHA particles [20].The formation of Mg(OH)2serves as a protective layer that reduce the corrosion and degradation rate.
After the addition of nHA particles,localized corrosion may occur at the aggregation of nHA particles,multi-pass FSP not only enable nHA to be more uniform distributed,but also reduce the possibility of localized corrosion,thus resulted in more uniform corrosion occurring for FSPed samples compared to base metal and hence improved the corrosion resistance [61].Moreover,theIcorrof FSPed WE43/nHA composites was significantly reduced and theEcorrof the FSPed WE43/nHA composites moved in the positive direction compared with the FSPed WE43 alloys,which indicated FSPed WE43/nHA composites have better corrosion resistance compared with FSPed WE43 alloys.
The FSPed WE43/nHA-3P composite had the lowestIcorr(178.1 μA/cm2) and corrosion rate (4.13 mm/y),decreased by 38.2% compared to base metal.This is regraded to the refined microstructure which lead to larger grain boundary density and local grain boundary misorientation that accelerate the formation rate of dense Mg(OH)2protective layer.Also,the nHA caused the increase of interfaces that helps apatite nucleation and growth,and rapidly forms a compact apatite layer which forms a barrier and promotes the corrosion resistance [62]
3.4.2.Degradation in immersion test
The weight loss after 1d,3d,and 7d for the base metal and FSPed samples is shown in Fig.12(a)and(b).The base metal experienced more weight loss compared to the FSPed WE43 alloy and FSPed WE43/nHA composites.As the FSP pass from one pass to three passes increased,the weight loss for FSPed samples gradually decreased.FSPed WE43/nHA-3P showed the lowest weight loss.Comparing the FSPed WE43 alloys and FSPed WE43/nHA composites at the same pass,it was found that the FSPed WE43/nHA composites had lower weight loss than the FSPed WE43 alloy.

Fig.12.Weight loss and corrosion rate of base metal and (a,c) FSPed WE43 alloys,(b,d) FSPed WE43/nHA composites in SBF.
The degradation rate calculated by Eq.(3)based on weight loss after 1d,3d and 7d of immersion is shown in Fig.12(c)and (d).The results demonstrate that FSP enhanced the corrosion resistance of the WE43 alloy.The degradation rate of the FSPed WE43/nHA composites was found to be lower than that of FSPed WE43 alloys under the same FSP pass,which can prove that the corrosion resistance has improved due to enhanced biomineralization due to the addition of nHA.The corrosion rates for 7d immersion of FSPed WE43-3P and FSPed WE43/nHA-3P were 6.02 and 2.87 mm/y,reduction of 36.4% and 69.7% compared to base metal.During degradation,Mg(OH)2is formed due to the consumption of hydrogen ions and the release of OH-ions.Because the apatite layer and the relatively more stable Mg(OH)2layer were formed on the surface during the immersion period.These layers usually have lower solubility in SBF and provide protection to the immersion surface.Meanwhile,with the increase of FSP passes,the degradation rates for 7d immersion of FSPed WE43/nHA-1P and FSPed WE43/nHA-3P were 3.73 and 2.87 mm/y.This means that FSP passes had a positive impact on the degradation rate.By increasing the number of FSP passes,the combined effect of better nano-size and more uniform distribution of nHA can promote uniform corrosion of the immersion surface layer and thus reduce localized corrosion.
The degradation rate derived from the weight loss of the samples from immersion test shows similarity trend to the corrosion rate determined by the electrochemical test.Zengin et al.[63]provided a direct comparison between these two methods and they reported that the degradation rate computed from weight loss was higher than corrosion rate from electrochemical test as the results from electrochemical test were influenced by the hydrogen evolution in the anodic region on the film-free zone which affects the absolute value of the corrosion rate.Therefore,different methods can be used to understand the corrosion susceptibility of Mg alloys [64,65].In the present paper,FSPed WE43/nHA composite samples have relatively reduced corrosion rates in both tests.
3.5. In-vitro bioactivity test
3.5.1.Wettability test
Fig.13 displays the experimental contact angle measurement and the calculated surface energy for base metal and FSPed samples.According to the observed contact angles,FSPed samples decreased the contact angle and increased the surface energy.The contact angle for all FSPed samples is less than 58°which means they are hydrophilic.A hydrophilic surface facilitates the absorption of specific proteins,cell attachment and proliferation kinetics which can subsequently form a strong bonding between the implant and tissue [66,67].The results demonstrate that FSP can boost the surface energy and enhance the hydrophilic of WE43 alloy.
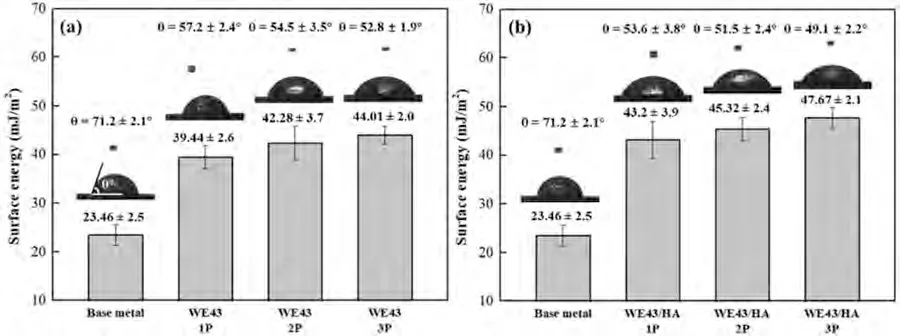
Fig.13.Calculated surface energies and photograph of the water droplets on base metal and (a) FSPed WE43 alloys,(b) FSPed WE43/nHA composite.
With increasing FSP pass from one pass to three passes,the surface energies of FSPed WE43 alloys and FSPed WE43/nHA composite show an increasing trend.Because all samples were mechanically polished to the same level before testing,the effect of surface roughness is negligible.Therefore,the surface wettability improvement can be only attributed to the decrease of grain size because of increasing FSP pass.It is obvious that the presence of nHA particles promotes surface wettability because HA forms the initial stable nucleus and then grows up.Some nucleation sites that occur in a solid-state transformation process are heterogeneous nucleation on the surface of the metal matrix [68].The nucleation and crystal formation of apatite during the mineralization process is highly dependent on the wettability of the surface,particularly under low pH conditions [69].Successful implant-tissue integration is heavily contingent on such a wetting-favored surface.Therefore,the surface wettability not only affects the rate of formation HA appetite,but also influences the quality of the mineralization.
3.5.2.Biomineralization
Fig.14 displays the surface morphologies and corresponding EDS of base metal and FSPed WE43-3P sample after immersion in SBF for 1d and 7d.After 1d immersion,cracks appeared at the surface of both base metal and FSPed WE43-3P samples.This occurred due to the generation of hydrogen gas that was generated during the immersion period [70].Also,the dry crack film of corrosion products was due to the dehydration of samples after immersion.The cracked structure for FSPed WE43-3P sample was relatively uniform and flat,while large pieces of precipitates swelled and showed different layers at the surface of base metal,as shown in the yellow dotted circle in the Fig.14(a).Studies have shown that the corrosion products of RE and Mg alloys in SBF solutions are mainly Mg(OH)2compound and calcium phosphate minerals [71].Mg ions were released in substantial quantities during immersion and contributed to the creation of the mineral phase.
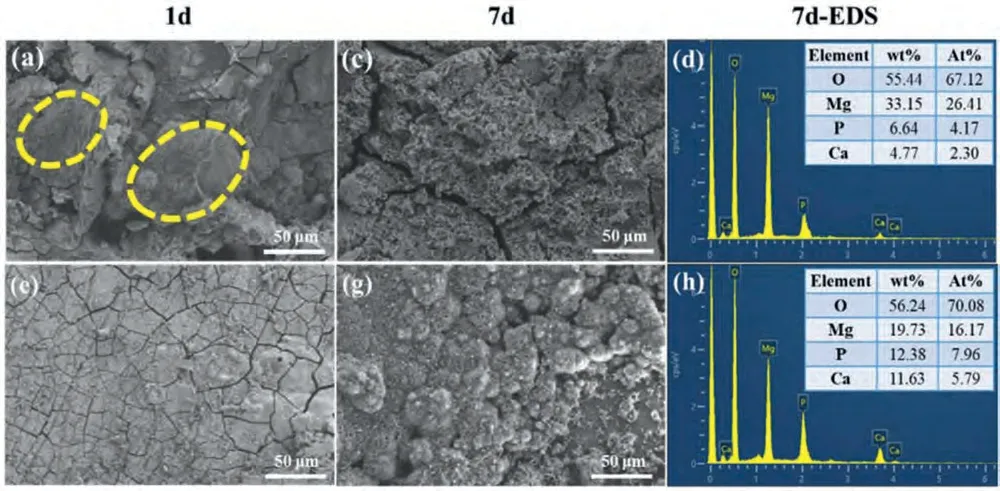
Fig.14.Surface morphologies and corresponding EDS of (a,e) base metal and (c,g) FSPed WE43-3P alloy after immersion for 1d and 7d
After 7d immersion,the surface of base metal in Fig.14(c)can be seen corrosion products as well as corrosion pits,especially obvious at the crack propagation boundary.Because the cracks propagation among the fragmented precipitate became large gaps which might induce localized corrosion under the precipitates.For the FSPed WE43-3P sample,small cracks became larger after 7d immersion.The surface was compactly packed with irregular clustered globular shape precipitates.
From corresponding EDS of base metal and FSPed WE43-3P samples in Fig.14,the amount of Mg decreased dramatically from 43.86 wt% (1d) to 19.73 wt% (7d).Different from base metal,the amounts of O,Ca and P increased considerably from 1d to 7d for FSPed WE43-3P alloy.The low degradation rate of FSPed WE43-3P alloy resulted in formation of the high Ca/P ration-based apatite layer after biomineralization.This indicates the growth of apatite layer which revealed the enhanced bioactivity of FSPed WE43-3P
Fig.15 shows the surface morphologies and corresponding EDS of FSPed WE43/nHA composite after immersion in SBF for 1d and 7d Thick and consistent apatite layer was formed on the surface after 1d of immersion.It always exhibited a spherical morphology as the immersion time increased from 1d to 7d Because of the incorporation of nano HA particles,the apatite layer on FSPed WE43/nHA composites was more readily apparent.The fragmented precipitates built up at the surface and their size at 7d was larger than that after 1d Moreover,the combination effect of small grain size and nHA particles led to rapid biomineralization for FSPed WE43/nHA composites compared to FSPed WE43 alloys.

Fig.15.Surface morphologies and corresponding EDS of the (a-c) FSPed WE43/nHA-1P,(d-f) FSPed WE43/nHA-2P and (g-i) FSPed WE43/nHA-3P alloy after immersion for 1d and 7d.
After 7d immersion,different size and distribution of clustered globular shape precipitates were observed which indicates that FSP passes have a significant effect on apatite layer formation.The accumulation of thick corrosion products includes Mg(OH)2and Ca-P biominerals has occurred[72,73].The Ca-P contained layer can be deposited on the top of Mg(OH)2and gradually becomes a protective layer to prevent Mg matrix degradation.In addition,with increasing immersion time,it is generally accepted that Ca-P layer formation can be used to assess the extent of biomineralization.Indeed,the Ca-P layer plays an important role in cell viability and cell proliferation by inhibiting H2evolution.Table 3 list the elemental composition and atomic ratio of Ca/P for base metal and all FSPed samples.The atomic ratio of Ca/P for base metal was 0.55.After FSP,the atomic ratio of Ca/P was higher but still lower than 1 for FSPed WE43 samples.And it turned out to be higher than 1 for FSPed WE43/nHA composites,this inverse indicates that the phases in the precipitates changed,as the deposition of Ca and P were significant after 7d of immersion.With the increasing of FSP passes,the atomic ratio of Ca/P increased.The highest atomic ratio of Ca/P was 1.60 for FSPed WE43/nHA-1P after 7 d.The higher Ca/P mineral phase shows superior bioactivity for FSPed WE43/nHA composites.This indicates that the FSPed WE43/nHA-3P composite tends to form higher quality mineral phases and can be suitable for implant application.
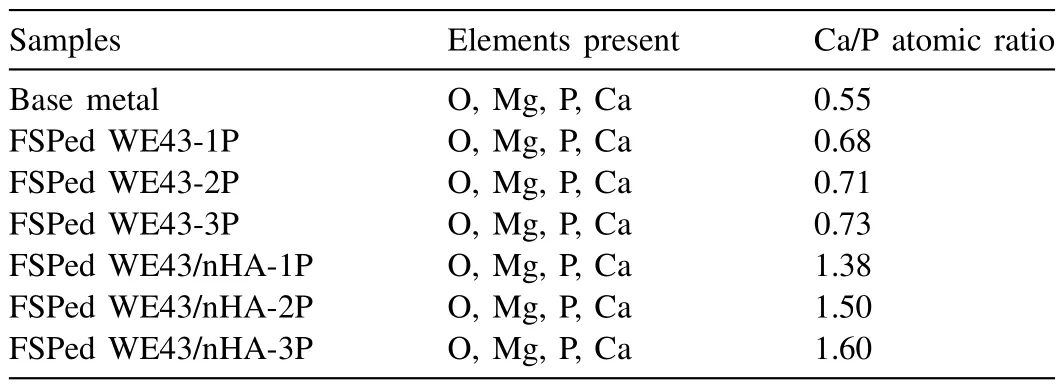
Table 3 Elemental composition and atomic ratio of Ca/P for base metal and FSPed samples.
4.Conclusions
In this research,FSPed WE43 alloys and FSPed WE43/nHA composites were produced by multiple FSP passes (one,two,and three).The effects of nHA and FSP passes on the evolution of microstructure,distribution of reinforced particles,microhardness,degradation rate andin-vitrobioactivity of WE43 Mg alloy have been investigated.It is concluded that:
(1) With increasing the number of passes,the average grain size was significantly reduced by 94.29% (2.63 μm)for FSPed WE43 after 3 passes,while it was reduced further for WE43/nHA by 95.92% (1.88 μm) due to the existence of nHA which hinders the growth of crystal structure.
(2) The addition of nHA and applying higher FSP passes led to an improvement in microhardness for FSPed WE43/nHA-3P composite (105.59 HV which is 135%higher than base metal) due to further grain refinement and more homogenous distribution of nHA.
(3) Corrosion resistance of FSPed samples was enhanced by increasing FSP pass and introduction of nHA.FSPed WE43/nHA-3P composite had the lowest corrosion rate 4.13 mm/y which decreased to 38.2% compared to base metal.
(4) The contact angle for all FSPed samples were less than 58° which means hydrophilicity.The surface energies of FSPed WE43 alloys and FSPed WE43/nHA composites show an increasing trend with increasing FSP passes.The presence of nHA particles resulted in FSPed WE43/nHA composites excellent wettability than FSPed WE43 alloys under same FSP pass.
(5) During immersion test,FSPed WE43/nHA-3P composite showed the lowest weight loss due to enhanced biomineralization with the addition of nHA.The Ca-P contained layer deposited on the top of Mg(OH)2and became a protective layer to prevent Mg matrix degradation.The degradation rate after 7d immersion was 2.87 mm/y,with a reduction of 69.7%compared to base metal.The atomic ratio of Ca/P reached 1.60 which indicates superior bioactivity for FSPed WE43/nHA-3P composite.
In the future,in addition to investigating the development of in-situ surface metal-matrix composites using different reinforcement particles,further investigations on toxicity of composites are needed to evaluate the biocompatibility in case to explore their potential as medical implant materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the University Malaya (Grant code: FRGS/1/2022/TK10/UM/02/6),and the National Natural Science Foundation of China (Grant No.51275414,No.51605387).The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the Large Groups Project under grant number RGP.2/303/44.
杂志排行
Journal of Magnesium and Alloys的其它文章
- A comprehensive review on the processing-property relationships of laser strengthened magnesium
- Recent advances in electrochemical performance of Mg-based electrochemical energy storage materials in supercapacitors: Enhancement and mechanism
- Peri-implant gas accumulation in response to magnesium-based musculoskeletal biomaterials: Reframing current evidence for preclinical research and clinical evaluation
- Influence of laser parameters on the microstructures and surface properties in laser surface modification of biomedical magnesium alloys
- Experimental and simulation research on hollow AZ31 magnesium alloy three-channel joint by hot extrusion forming with sand mandrel
- Mg/MgO interfaces as efficient hydrogen evolution cathodes causing accelerated corrosion of additive manufactured Mg alloys: A DFT analysis
