Comparative effi cacy and optimal duration of f irst-line antibiotic regimens for acute otitis media in children and adolescents:a systematic review and network meta-analysis of 89 randomized clinical trials
2024-04-12MinSeoKimJaeHanKimSeohyunRyuSeungWonLeeDongKeonYonEunyoungKimAiKoyanagiElenaDragiotiJaeIlShinLeeSmith
Min Seo Kim·Jae Han Kim·Seohyun Ryu·Seung Won Lee·Dong Keon Yon·Eunyoung Kim·Ai Koyanagi·Elena Dragioti,·Jae Il Shin·Lee Smith
Abstract Introduction Antibiotic use for acute otitis media (AOM) is one of the major sources of antimicrobial resistance.However,the eff ective minimal antibiotic duration for AOM remains unclear.Moreover,guidelines often recommend broad ranges(5-10 days) of antibiotic use,yet the clinical impact of such a wide window has not been assessed.Methods We systematically searched PubMed/MEDLINE,Embase,Scopus,Web of Science,and Cochrane Library from database inception to 6 October 2021.Network meta-analysis was conducted on randomized controlled trials that assessed antibiotic treatment for AOM in children (PROSPERO CRD42020196107).Results For amoxicillin and amoxicillin-clavulanate,7-day regimens were noninferior to 10-day regimens in clinical responses [amoxicillin: risk ratio (RR) 0.919 (95% CI 0.820-1.031),amoxicillin-clavulanate: RR 1.108 (0.957-1.282)],except for ≤ 2 years.For the third-generation cephalosporins,7-day and 10-day regimens had similar clinical responses compared to placebo [7-day: RR 1.420 (1.190-1.694),10-day: RR 1.238 (1.125-1.362) compared to placebo].However,5-day regimens of amoxicillin-clavulanate and third-generation cephalosporins were inferior to 10-day regimens.Compared to amoxicillin,a shorter treatment duration was tolerable with amoxicillin-clavulanate.Conclusions Our f indings indicated that 10 days of antibiotic use may be unnecessarily long,while the treatment duration should be longer than 5 days.Otherwise,5-day regimens would be suffi cient for a modest treatment goal.Our f indings revealed that the current wide range of recommended antibiotic durations may have inf luenced the clinical outcome of AOM,and a narrower antibiotic duration window should be re-established.
Keywords Amoxicillin-potassium·Amoxicillin·Antibacterial agents·Cephalosporins·Duration of therapy
Introduction
Approximately 10% of the population experiences acute otitis media (AOM),with more than half of the cases occurring in children [1].Streptococcus pneumoniae,Haemophilus inf luenzae,andMoraxella catarrhalisare the main causative pathogenic bacteria [2],and colonization of these bacteria in the middle ear can cause severe pain,fever,and hearing loss,aff ecting the quality of life of both patients and their caregivers [1].
Owing to its acute nature,AOM is frequently treated with antibiotic treatment as per the clinical guidelines [3-6] and is the leading cause of medical consultation and antibiotic prescription [1,7].Although the extended duration of antibiotic use is a critical factor for the emergence of antimicrobialresistant bacteria [8],the minimal and acceptable duration for antibiotic treatment remains uncertain.Numerous trials have been conducted to shorten the duration of antibiotic use,but their results have been largely inconsistent [9,10].
The network meta-analysis (NMA) enables comparisons of multiple arms and indirect comparisons when a head-tohead comparison is absent and thereby has strengths over pairwise meta-analysis in identifying optimal treatment duration from diverse arrays of regimens and duration protocols [11].We conducted the f irst NMA of randomized controlled trials(RCTs) to investigate the proper duration and comparative effi cacy of antibiotic regimens as f irst-line antibiotic treatment for AOM in children and adolescents.The results of this NMA should be implicated only after physicians deem watchful waiting is insuffi cient and antibiotic therapy is needed.Since NMA warrants careful interpretation when establishing a comparison between one intervention and the other,we used a minimally contextualized approach that focuses on eff ect estimates and certainty of evidence to classify interventions into distinct categories [12].
Methods
We performed a systematic review and NMA in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses for Network Meta-Analyses guidelines[13].The study was registered with PROSPERO (registration: CRD42020196107).Screening,data extraction,and methodological appraisal of the included studies were conducted by two independent investigators (JHK and SR).
Search strategy and selection criteria
We systematically searched PubMed/MEDLINE,Embase,Scopus,Web of Science,and Cochrane Library from their inception to 6 October 2021 without any language restrictions.The search keywords included “acute”,“otitis media”,and “antibiotic treatment”;full lists of search terms are presented in the Supplementary materials on page six.To identify eligible articles,two investigators(JHK and SR) independently screened titles,abstracts,and full texts and manually searched the references of the relevant studies.Any disagreement was resolved through consultation with the other authors (MSK and JIS).
The following inclusion criteria were applied: an RCT that investigated antibiotic treatment in patients with AOM,trials that included patients under 19 years of age or provided data on this age group as a subgroup,trials that reported clinical or microbial response as an outcome,and trials of currently recommended antibiotics in guidelines[amoxicillin (AMX),amoxicillin-clavulanate (AMX-CL),cephalosporins (CEP),and macrolides (MAC)] [3-6].
The following exclusion criteria were applied: studies that included patients only 19 years of age or older,that investigated antibiotics that are not currently recommended,that compared antibiotics of the same generation for the same duration (e.g.,a third-generation CEPvs.another third-generation CEP both administered in seven-day regimens),that investigated chronic otitis media only,and studies with ambiguity in the treatment duration.Furthermore,conference and meeting abstracts,editorials,guidelines,and reviews were excluded.
Data extraction
Two investigators (JHK and SR) independently extracted data from each eligible trial,and any conflicts were resolved by consensus.The following data were extracted:PMID,name of the f irst author,publication year,country where the trial was conducted,details of the trial (study design,trial-specif ic inclusion/exclusion criteria,number of patients,and age range),details of the treatment (type of antibiotics,duration,dosage of treatment,follow-up date,and def initions of treatment response),and trial results(clinical or microbial response,pathogen-specif ic microbial response,and number of patients who experienced adverse events).
Clinical response was the primary outcome,def ined as the complete resolution of the initial signs or symptoms at the test-of-cure visit.The microbial response was the secondary outcome,def ined as the eradication of the initial pathogen or bacterial concentration below a certain level at the test-of-cure visit.Adverse events were reported in each trial,including rash,diarrhea,and vomiting.Trialspecif ic def initions of clinical and microbial success and assessment dates are described in the Supplementary materials pages 7-25.
Assessment of study and evidence quality
We evaluated the risk of bias based on the revised Cochrane assessment tool [14],which is broadly used in randomized trials.The following domains were assessed for each included study: bias arising from the randomization process,bias due to deviations from intended interventions,bias due to missing outcome data,bias in the measurement of the outcome,bias in the selection of the reported result,and overall bias.All factors were judged to have either low,some concerns,or high risk of bias.
To evaluate the certainty of the evidence,we applied the Grading of Recommendations,Assessments,Developments,and Evaluation (GRADE) approach [15].The certainty of the evidence for meta-analysis was categorized as very low,low,moderate,or high by the following four steps of the GRADE approach.First,we calculated direct and indirect treatment estimates for each comparison of the evidence network;second,the quality was rated for each direct and indirect eff ect estimate;third,we calculated the estimate of NMA for each comparison of the evidence network,and fourth,we rated the quality of each NMA estimate [1 5].Furthermore,we classif ied the interventions as follows in compliance with the recent international guidelines: not convincingly diff erent from the placebo (category 0),more eff ective than placebo (category 1),or more eff ective than at least one category 1 intervention (category 2) [12].
Statistical analysis
We performed frequentist NMA,a method for comparing the effi cacy of multiple interventions simultaneously by pooling direct and indirect comparisons [16].Treatment-specif ic eff ects and diff erences between them were estimated with respect to the clinical and microbial responses.Regarding the clinical responses,we only used data of “cured” and“failed” when a trial reported the outcome as “cured”,“improved”,and “failed” since “improved” does not imply complete remission of initial symptoms.We used the “netmeta” package ofRsoftware to construct the Moore-Penrose pseudoinverse matrix and calculated the f itted values of the network model using a weighted least squares approach[17].We applied a random-eff ects model given that the expected heterogeneity between studies was less likely to be introduced by chance.
We estimated the pooled risk ratio (RR) and the corresponding 95% conf idence intervals (CIs) of the trials.Each treatment regimen was ranked by the surface under the cumulative ranking curve [18],which ranged from 0 to 1.The design-by-treatment interaction random-eff ects model was used to evaluate the inconsistency between the direct and indirect evidence.A signif icant inconsistency was suggested when the analysis for consistency reportedP< 0.05 or the credibility interval excluded the null [19].We assessed inconsistency by comparing direct and indirect evidence,where available,by producing a network heat plot.
Then the beautiful lady said: Go forth14 at once, and do not return to say good-by to your mother, for these things must be done quickly, and the Shoes of Swiftness themselves will carry you to the land of the Three Gray Sisters --for they know the measure of that way
We performed a sensitivity analysis for children aged ≤ 2 years for clinical responses,as current guidelines inferred longer treatment durations for them [3 ].We produced comparison-adjusted funnel plots to explore publication bias or other small-study eff ects.All analyses were conducted usingRsoftware (version 3.6.0) and Stata (Stata Corp,College Station,TX,USA,version 15.0).All statistical tests were two sided,and statistical signif icance was set atP< 0.05.
Results
From the systematic database search,we identif ied 6717 candidate articles after removing duplicates,of which 272 were retained after title and abstract screening.After full-text screening,89 RCTs were included,with a total of 24,091 patients (median 119 patients per trial,interquartile range 61.5-181.5,range 7-294) (Fig.1).The 89 trials were included in the NMA of clinical response,and 22 were included in the NMA of microbial response.The list of included trials and their characteristics are summarized in Supplementary materials page 7-29.Inconsistency,which represents the discordance between direct and indirect comparisons,was evaluated for clinical response,microbial response,and adverse rate,and none was subject to global inconsistency.However,high heterogeneity (I 2=71.5%)and potential publication bias (Egger’s testP=0.01) were detected for adverse events (Supplementary materials page 50-55).
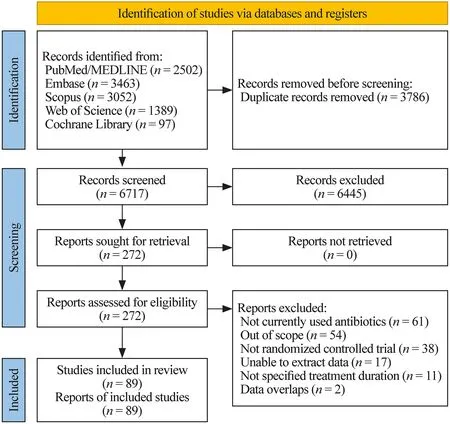
Fig.1 Study diagram
Clinical responses
The network plot of clinical response (Fig.2 a) showed 22 nodes(21 antibiotic therapy regimens and placebo) and 41 comparisons.The 7-day/10-day regimens of AMX-CL,7-day/10-day regimens of third-generation CEP,and 3-day regimen of MAC were classif ied as category 2 (Table 1).For children and adolescents,all antibiotic therapy regimens were signif icantly more eff ective than the placebo,except for the single/2-day/7-day regimens of AMX,a 5-day regimen of f irst-generation CEP,and a 5-day regimen of AMX-CL (Fig.3 a).For children aged ≤ 2 years,all antibiotic therapy regimens were signif icantly more eff ective than the placebo,except for the 7-day regimen of AMX and 5-day/7-day regimens of AMX-CL (Fig.3 b).Moreover,there were no signif icant diff erences in the overall adverse events between any antibiotic therapy regimen and placebo (Supplementary materials page 51).
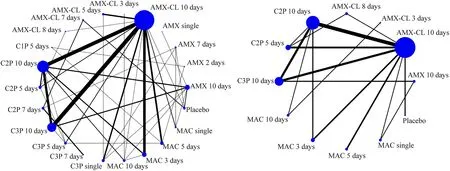
Fig.2 Network plots of a clinical response and b microbial response.The network plot summarizes investigational drug pairs of included clinical trials for a given outcome.The size of nodes is proportional to the total number of trials for each regimen,and the width of lines is proportional to the number of trials that compare each pair of antibiotic regimens.AMX amoxicillin,AMX-CL amoxicillin-clavulanate,CnP nth-generation cephalosporins,MAC macrolides
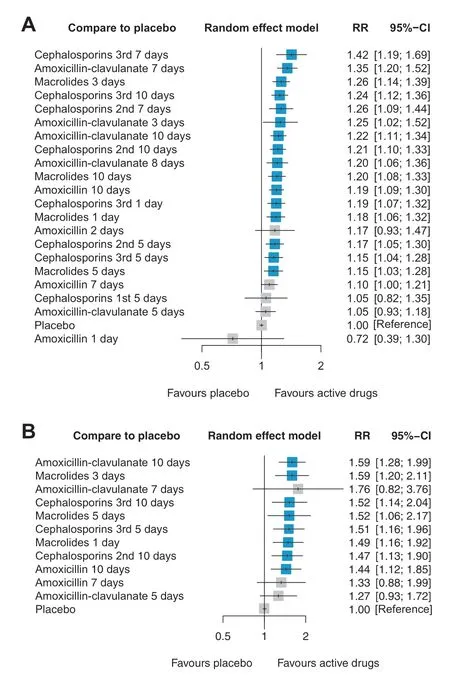
Fig.3 Forest plots for network meta-analysis of antibiotics regimens compared with the placebo for clinical response in a children of all ages (≤ 18 years),and b children of ≤ 2 years.Regimens are ordered in eff ect size and regimens that are signif icantly more eff ective than placebo in terms of clinical response are marked in the blue box.The greater RR indicates better clinical responses with the corresponding regimen compared to that with placebo.RR risk ratio,CI conf idence interval
Head-to-head comparison of treatment durations for clinical responses
For AMX,there was no signif icant diff erence between the eff ects of the 7-day and 10-day regimens [RR 0.919 (95%CI 0.820-1.031),quality of evidence: low] (Table 2).For AMX-CL,there was no signif icant diff erence between the eff ects of 7-day and 10-day regimens [RR 1.108 (95% CI 0.957-1.282),quality of evidence: moderate],while a 5-day regimen showed a reduced eff ect compared to 10-day regimens [RR 0.860 (95% CI 0.800-0.925),quality of evidence:high] (Table 2).For third-generation CEP,a 5-day regimen showed a signif icantly lower eff ect than a 10-day regimen[RR 0.932 (95% CI 0.879-0.988),quality of evidence: high](Table 2).Notably,both 7-day and 10-day regimens of third-generation CEP were included in category 2,of which the quality of evidence was moderate in both,but a comparison between them was not available (Table 1,Fig.2 a).For MAC,there was no signif icant diff erence in the eff ects between the 3-day and 10-day regimens [RR 1.052 (95% CI 0.988-1.120),quality of evidence: moderate] (Table 2).The 3-day regimen was classif ied as category 2,while the 10-day regimen was classif ied as category 1 (Table 1).
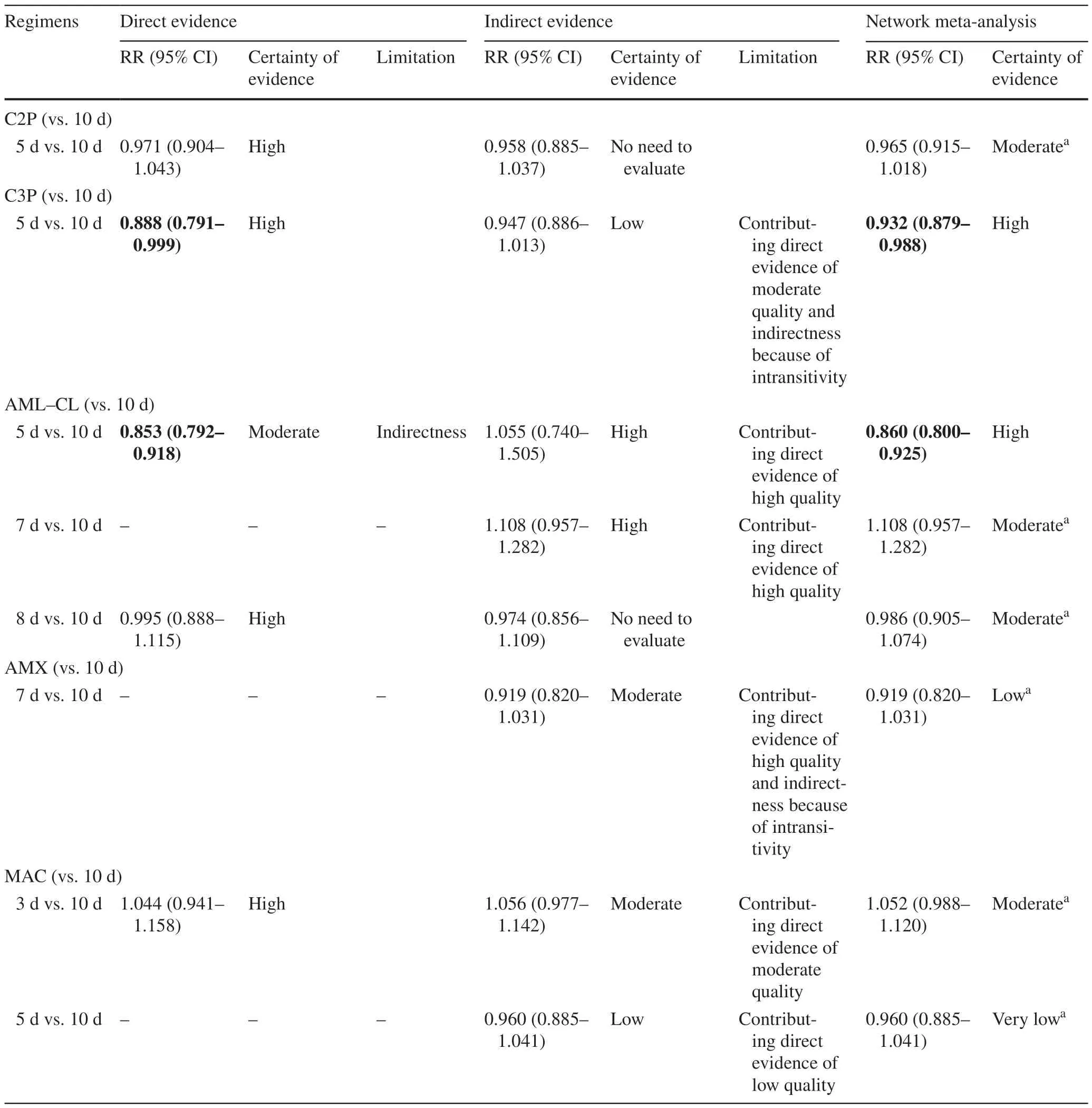
Table 2 Quality ratings for comparison of drugs recommended by international guidelines (clinical response) and optimal treatment duration to warrant successful clinical response in children (≤ 18 years)
Microbial responses
The network plot of the microbial response (Fig.2 b) showed 12 nodes (11 antibiotic therapy regimens and placebo) and 16 comparisons.No antibiotic regimen was classif ied as category 2 for microbial response (Table 1).All antibiotic therapy regimens were more eff ective than the placebo with statistical signif icance,except for single/f ive-day regimens of MAC (Supplementary materials page 46).
We performed subgroup analyses for causative pathogens of AOM,includingHaemophilus inf luenzae,Moraxella catarrhalis,Streptococcus pneumoniae,andStreptococcus pyrogens
(Supplementary materials page 56-59).Most regimens were consistently eff ective forMoraxella catarrhalisandStreptococcus pneumoniaebut not forHaemophilus inf ulenzae.However,these results seemed to be aff ected by a lack of statistical power due to the small sample size forHaemophilus inf ulenzae,rather than the absence of actual eff ectiveness.
Head-to-head comparison of treatment durations for microbial responses


Table 3 Quality ratings for comparison of drugs recommended by international guidelines (microbial response) and optimal treatment duration to warrant successful microbial response in children (≤ 18 years)
Risk of bias assessment and quality of evidence
The risk of bias graph,summary plot,and detailed information are displayed in the Supplementary materials page 60-151.Of the 89 trials,20 were ranked as having “some concerns” or “high” risk of bias.There may be two sources of bias.First,only 25 of 89 were double-blinded trials,while others were open,single-blinded,or did not provide related information.Second,some trials had a small number of participants,which may have inf luenced the eff ect size of the antibiotic therapy regimen.The quality of evidence assessment based on the GRADE approach is shown in Tables 1,2,and 3.
Discussion
We broadly categorized all antibiotic regimens currently recommended for AOM in children and adolescents and evaluated the comparative effi cacy and certainty of evidence underlying each regimen.Our results suggested that the 7-day regimens of AMX,AMX-CL,MAC,and likely third-generation CEP were not inferior to the 10-day regimens of their classes in terms of clinical responses,which may indicate that the treatment duration could be shorter than the commonly recommended 10-day regimens.Microbial responses and adverse rates did not diff er substantially among the antibiotic regimens with varying durations.Of note,this study does not oppose the common practice of watchful waiting but rather addresses clinical situations where watchful waiting is insuffi cient and antibiotic treatment should be initiated.
Antimicrobial resistance has been a concern for decades because of its dreadful impact on public health and healthrelated expenditure [20,21].Clinical conditions around AOM seem to be closely related to this problem.The latest Global Burden of Diseases report revealed thatStreptococcus pneumoniae(a major pathogen of AOM) and β-lactams(currently recommended f irst-line antibiotics for AOM) are one of the leading problematic pathogens and antibiotics for antimicrobial resistance,respectively [22].Given that the overuse of antibiotics is a major driver of antimicrobial resistance,our f inding that the duration of antibiotic treatment for AOM could be shortened provides meaningful implications.
The guidelines from the United States,Europe,and developing countries most often recommended AMX as a f irst-line antibiotic therapy mainly because of its low cost and narrow antibacterial spectrum [3,5,23].Although we agreed with the eff ectiveness of AMX compared to placebo,our f indings suggested that AMX-CL could be a preferred option in limited conditions,given that AMX-CL (category 2) was associated with a signif ciantly better clinical response than AMX (category 1) (Table 1).Moreover,among moderate to high certainty of evidence (GRADE),clinical responses from 3-and 7-day regimens of AMX-CL were not inferior to 7-and 10-day regimens of AMX (Table 1 and Fig.3 a),possibly indicating that reduced duration could be achieved with AMX-CL.However,f irst-line antibiotics should not be solely determined by their effi cacy.The clinical decision should adhere to extensive multifactorial risk-benef it and cost-eff ective assessments,as well as consideration for local patterns of microbial prevalence and antimicrobial resistance.
Since the rollout of the pneumococcal conjugate vaccine (PCV),the prevalent causative pathogen of AOM has dynamically changed [24].Emerging evidence supports thatHaemophilus inf luenzaehas become a predominant strain in the post-PCV era,whereas the burden of AOM caused byStreptococcus pneumoniaehas been reduced [24-26].PCV coverage reached 47.9% in 2019 globally [27] but varied widely across global regions;the percentage of countries reaching the Global Vaccine Action Plan national coverage target in 2019 was 69% in high-income areas and 24%in Sub-Saharan Africa [27].Acknowledging the large discrepancy in the regional PCV coverage,a tailored selection of f irst-line antibiotics would be necessary.AMX remains a preferred choice in PCV-covered countries or children because theHaemophilus infl uenzaestrain still has a low rate of beta-lactamase activity.In contrast,regions not covered by PCV would f ind that AMX-CL would be more suitable considering the higher prevalence ofStreptococcus pneumoniaeand its resistant strains [28].AMX-CL could also be selected for severe/persistent AOM that often requires more eff ective antibiotic treatment or when local microbial prevalence indicates possible non-susceptibility to AMX.However,AMX-CL is a broad-spectrum antibiotic and should be carefully considered as a f irst-line treatment given the ecological price of increased antibiotic resistance.
Current guidelines for AOM management in children and adolescents use nonspecif ic terms when mentioning treatment duration [3-6].The guidelines from the American Academy of Pediatrics (AAP),European,and developing countries recommended 5-10 days of antibiotic use[3,5,23].However,these recommendations are somewhat vague and not fully supported by clinical evidence [3].More importantly,a wide range in recommended duration has not been challenged for its validity,although clinical responses could diff er between 5-and 10-day antibiotic regimens.Herein,we observed that the 5-day regimens of major antibiotic therapies were inferior to 10-day regimens in clinical response,while 7-day regimens were noninferior to 10-day regimens.This may indicate that a 10-day regimen may be unnecessarily long for AOM,while the treatment duration should be longer than 5 days to achieve an equivalent treatment goal to a 10-day regimen.Otherwise,5-day regimens would be suffi cient to attain a modest treatment goal given the minimal effi cacy gap with 10-day regimens.Although no one-size-f its-all approach would be promising for AOM,reducing the window in recommended antibiotic duration would minimize the risk of antibiotic overuse and subsequent antimicrobial resistance.
The AAP and South Africa guidelines recommend a longer regimen of AMX for children aged ≤ 2 years compared to those aged > 2 years [3,29].Our sensitivity analyses(Fig.3 b) seemed to support this statement.In our analyses,both the 10-day regimens of AMX and AMX-CL showed better clinical responses than the placebo,while the 7-day regimens of AMX and AMX-CL did not (Fig.3 b).These results may be due to their immature immune system where maternal antibodies fade away,but their ability to f ight against pathogens is not yet fully established [30],resulting in higher rates of treatment failure.However,it should be noted that the small sample size of those aged ≤ 2 years may have aff ected our results and need to be re-evaluated with a larger sample size.Considering that evidence was lacking to support a shorter treatment duration,a conservative regimen of AMX or AMX-CL may be more appropriate for children aged ≤ 2 years.
The current guidelines suggest a 5-to 10-day regimen of CEP as an alternative for those with penicillin allergies or more severe AOM [3 -6].Third-generation (cefdinir,cefpodoxime,and ceftriaxone) and second-generation (cefuroxime) regimens were mentioned for use.All generations showed better clinical and microbial responses than the placebo in our analyses,except for the f irst generation,which supports the decision of guidelines to choose the second or third generation.From our results,both 7-day and 10-day regimens of third-generation CEP were classif ied into category 2 in terms of clinical response,and thus,a 7-day regimen seemed to be suffi cient.However,a head-to-head comparison between 7 days versus 10 days was not available owing to insuffi cient data (Tables 2 and 3).Meanwhile,the 5-day regimen might be too short,as head-to-head analysis indicated a signif icantly lower rate of clinical success for the 5-day regimen of third-generation CEP compared to the 10-day regimen (Table 2).Altogether,our f indings support the 7-day regimen of CEP,albeit lacking direct evidence,and we call for further research comparing the eff ects between 7-day and 10-day regimens to conf irm the noninferiority of the 7-day regimen.
Some guidelines recommend a 5-to 10-day MAC regimen for patients who have an allergy to penicillin and/or cephalosporins [4,5].Several studies reported better antibiotic coverage of AMX,AMX-CL,or CEP for common AOM pathogens compared to MAC [31-33].Consistent with previous reports,we identif ied a doubtful eff ect of MAC on microbial responses,and MAC was the only type of antibiotic classif ied into category 0 (no diff erence with placebo) for the microbial response.Therefore,practitioners may consider MAC in limited conditions where a patient is allergic to both penicillin and CEP or a macrolide-sensitive pathogen is cultured.
Findings from this study should be interpreted in light of their limitations.First,approximately 6% of the included studies were published within the last 10 years,and only one was published after the 2015 AAP guideline,which may be because consensus has been reached to some degree on antibiotic agents for AOM based on the evidence from numerous RCTs excluding the last 10 years.Considering changes in susceptibility to antibiotics or differences in susceptibility between regions,it is expected that more clear evidence should be derived from additional research.Moreover,consideration for epidemiological changes in AOM would be required in future studies such as PCV coverage and the use of objective measurement for subjective symptoms (e.g.,AOM Severity of Symptoms)since they could aff ect the effi cacy of antibiotic treatment.Second,although most of the included RCTs investigated uncomplicated AOM,some trials included small portions of patients with otorrhea and tympanic membrane perforation.Current guidelines diff erentiated complicated AOM from uncomplicated AOM [3],but such subgroup analysis was not applicable because the included trials did not discriminate between these two clinical entities.However,we excluded trials that investigated chronic otitis media only(0.37% in full texts screening;0.03% in total screening),and patients with complicated symptoms were randomized and constituted a small proportion (< 5%) within each trial.Therefore,such a case mix is less likely to have inf luenced our results.Last,we could not provide subgroup analysis for PCV status due to limited data presentation from the included RCTs.
In conclusion,the 5-day regimens of major antibiotic therapies were inferior to 10-day regimens in clinical response,while 7-day regimens were noninferior to 10-day regimens.This may indicate that 10-day regimens may be unnecessarily long for AOM,but the treatment duration should be longer than 5 days to achieve an equivalent treatment goal to a 10-day regimen.Meanwhile,the 5-day regimen would be acceptable to attain a modest treatment goal given the minimal effi cacy gap with 10-day regimens.Our f indings revealed that the current wide range of recommended antibiotic durations (5-10 days) from guidelines may have inf luenced the clinical outcome of AOM,and a narrower antibiotic duration window should be re-established based on a high bar of seven days with regional-and treatment goal-specif ic modif ications.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s12519-023-00716-8.
AcknowledgementsNot applicable.
Author contributionsMSK,JHK,and SR contributed equally to this article as co-f irst authors.MSK: conceptualization,formal analysis,writing-original draft,review and editing,visualization.JHK: conceptualization,data curation,writing-original draft,review and editing.SR: conceptualization,data curation,writing-original draft.SWL,DKY,EK,LS,AK,and ED: writing-review and editing.JIS: conceptualization,writing-review and editing,supervision.All authors had full access to the study data and the corresponding authors had the f inal responsibility for the decision to submit for publication.
FundingNot applicable.
Data availabilityThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interestNo f inancial or non-f inancial benef its have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approvalNo ethics approval was required for this publication because this study did not involve the primary data of human or animal subjects.
杂志排行
World Journal of Pediatrics的其它文章
- Insights intopostural orthostatic tachycardia syndrome afterCOVID-19 inpediatric patients
- Modeling tuberous sclerosis complex with human induced pluripotent stem cells
- Improved survival at the cost of more chronic lung disease? Current management and outcomes in extremely preterm infants born in New South Wales and the Australian Capital Territory: 2010-2020
- Development and validation of a nomogram to predict allograft survival after pediatric liver transplantation
- Perioperative complication incidence and risk factorsfor retroperitoneal neuroblastoma in children: analysis of 571 patients
- Proteomic prof iling of cerebrospinal fluid in pediatric myelin oligodendrocyte glycoprotein antibody-associated disease
