Selective separation of pyrene from mixed polycyclic aromatic hydrocarbons by a hexahedral metal-organic cage
2024-04-06YaLiangLaiJuanSuLeXiongWuDongLuoXueZhiWangXianChaoZhouChuangWeiZhouXiaoPingZhouDanLi
Ya-Liang Lai,Juan Su,Le-Xiong Wu,Dong Luo,Xue-Zhi Wang,Xian-Chao Zhou,Chuang-Wei Zhou,Xiao-Ping Zhou,Dan Li
College of Chemistry and Materials Science,Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications,Jinan University,Guangzhou 510632,China
Keywords: Polycyclic aromatic hydrocarbons Metal-organic cage Host-guest chemistry Selective separation
ABSTRACT Polycyclic aromatic hydrocarbons (PAHs) play an important role in the industry,and the development of new materials for the selective separation of PAHs is of great significance.In this work,we report a hexahedral metal-organic cage with low symmetry by subcomponent self-assembly.In this cage,the eight ZnII centers adopt an interesting ΛΛ/ΔΔΔΔΔΔ or ΛΛΛΛΛΛ/ΔΔ configuration.This cage with a cavity volume of 520 ˚A3 can bind anthracene,phenanthrene,and pyrene to form 1:1 host-guest complexes,while the bigger triphenylene,chrysene,perylene,and coronene cannot be encapsulated.The binding constant Ka of pyrene is about 1.110×103 (mol/L)-1,which is more than an order of magnitude larger than that of anthracene and phenanthrene (111 (mol/L)-1,277 (mol/L)-1,respectively).X-ray structure studies reveal that the pyrene is located in the cavity and stabilized by multiple C–H∙∙∙π interactions.After separation from a mixture of PAHs,pyrene with >96.1% purity can be obtained.This work provides a useful method for the first time for the selective separation of pyrene from PAHs mixture by utilizing a metal-organic cage as the material,making it a useful tool for purifying and separating specific compounds from complex mixtures.
Polycyclic aromatic hydrocarbons (PAHs) are a class of compounds formed by the fusion of two or more benzene rings in a linear,angular,or cluster arrangement [1,2].Pyrene,one of the important components of PAHs,has been widely used in various areas due to its rigid structure,excellent fluorescence properties,and electron-rich conjugation properties [3–6].In industry,pyrene is mainly derived from the destructive distillation of coal tar,and the separation and purification process usually consumes huge energy.Therefore,it is emergent to develop new technologies for the selective separation of pyrene from the mixture of PAHs with high efficiency.
Metal-organic frameworks (MOFs) are crystalline porous materials with framework structures,which are formed by the selfassembly of organic ligands and metal ions through coordination bonds [7,8].In contrast to MOFs,metal-organic cages (MOCs) are molecular materials with isolated cage-like structures,which are also constructed by metal ions and organic ligands [9,10].However,most of the MOCs have good solvent solubility [11–13],and can be characterized by the methods utilized in traditional solution chemistry and used as molecular materials in solution state for application in separation [14–18],catalysis [19–21],chiral recognition [22,23],fluorescence sensing [24,25],stabilization of active substances [26,27] and drug delivery [28,29].Due to their rich structures with tunable cavities,MOCs can effectively bind PAHs through weak interactions (e.g., π-πstacking and C–H∙∙∙πinteractions) in solution [30,31].Although many MOCs can encapsulate the PAHs,the limited examples that can specifically recognize PAHs to achieve selective separation have been reported [17,32,33].Recently,Mukherjeeet al.reported a water-stable [Pd4L2]8+molecular vessel capable of selectively separating phenanthrene from a mixture of anthracene and phenanthrene in water [33].Nitschkeet al.reported the selective encapsulation and separation of coronene from a mixture of PAHsviaphase transfer of a tetrahedral MOC[32].In addition,some MOFs have also been used as materials for the separation of PAHs [34,35].However,to the best of our knowledge,the selective separation of pyrene from PAHs has not yet been achieved by using MOCs or MOFs as separating materials.
Herein,we report a hexahedral metal-organic cage,formulated as [(ZnII8L6)(OTf)16] (denoted as1,Scheme 1),which is constructed by subcomponent self-assembly of TAPB (1,2,4,5-tetrakis-(4-aminolphenyl)benzene),2-formylpyridine,and Zn(OTf)2.In cage1,the eight ZnIIcenters adopt unusualΛΛ/ΔΔΔΔΔΔorΛΛΛΛΛΛ/ΔΔconfiguration,which leads to low symmetry for a hexahedron [36].Cage1contains an electron-rich hydrophobic inner cavity with a volume of 520 ˚A3,which can act as a potential host for PAHs.We selected seven PAHs (anthracene,phenanthrene,pyrene,triphenylene,chrysene,perylene,and coronene) to study their host-guest chemistry due to the suitable free molecular volumes (ranging of 262–392 ˚A3).Interestingly,MOC1can bind anthracene,phenanthrene,and pyrene to form 1:1 host-guest complexes,respectively,while triphenylene,chrysene,perylene,and coronene cannot be encapsulated by1due to their big molecular volumes.Moreover,MOC1shows the best affinity to pyrene.The binding constantKais about 1.110×103(mol/L)-1,which is more than an order of magnitude larger than for anthracene and phenanthrene (111 (mol/L)-1,277 (mol/L)-1,respectively).X-ray structure of the host-guest complex pyrene⊂1display that the pyrene is located in the cavity and stabilized by multi C–H∙∙∙πinteractions.Further studies showed that MOC1could selectively encapsulate pyrene from a mixture of seven PAHs.The pyrene with 96.1% purity can be obtained by a separation cycle.For the first time,the selective separation of pyrene from the PAHs mixture is achieved by the design of MOC with a suitable cavity.
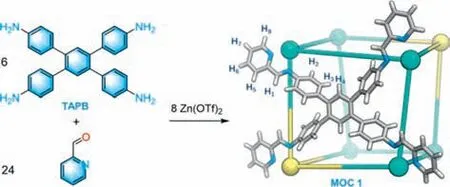
Scheme 1.Schematic diagram of self-assembly of MOC 1.
TAPB was synthesized by following a reported procedure (see experimental section in Supporting information for details) [37].The reaction of TAPB,2-formylpyridine,and Zn(OTf)2in a 6:24:8 molar ratio in CH3CN solution at 70 °C for 8 h afforded MOC1(Scheme 1).MOC1was characterized by nuclear magnetic resonance (NMR),electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS),thermal gravimetric analyzer (TGA),N2adsorption,powder X-ray diffraction (PXRD),and single-crystal X-ray diffraction (SCXRD).
The1H NMR spectrum of MOC1in CD3CN displayed three distinct ligand environments,reflecting the presence of three signals on the imine (H1) and phenyl (H2,H3,H4,Fig.1a).There exist three sets of signals in the NMR spectrum of1,which may be due to the configurational difference of the eight ZnIIvertices leading to a low symmetric structure.The1H–1H correlation spectroscopy(COSY) data also supported this result (Fig.S3 in Supporting information).Two-dimensional (2D)1H–1H diffusion-ordered spectroscopy (DOSY) studies showed that the1H signals of MOC1in CD3CN mainly belonged to a single species with a logDof about-9.38 (Fig.1a).The hydrodynamic radius was calculated to be∼14.9 ˚Aviathe Stokes-Einstein equation [38].
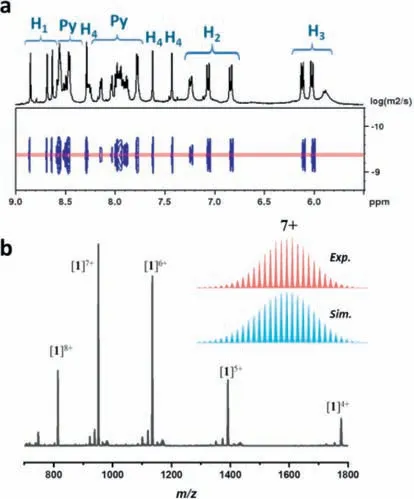
Fig.1.(a) Partial 1H NMR and DOSY spectra of MOC 1 in CD3CN.(b) Mass spectrum of MOC 1 in CH3CN.Insets show the calculated (blue) and experimental (red)isotopic patterns of the +7 peak at m/z=951.1409 corresponding to the species of[1–9OTf]7+.
Mass spectrometry is a powerful method to study the structure of supramolecular cages [39].The composition of MOC1was precisely determined by ESI-TOF-MS in CH3CN.The most prominent peak atm/z=951.1409 was corresponding to the[1–9OTf]7+(calcd.951.1399) species.Other signs atm/z=813.6223,1134.4832,1391.1659,and 1776.1921 correspond to [1–8OTf]8+(calcd.813.6219),[1–10OTf]6+(calcd.1134.4825),[1–11OTf]5+(calcd.1391.1667),and [1–12OTf]4+(calcd.1776.1924),respectively(Fig.1b).The experimental isotopic patterns of these peaks closely match their theoretical patterns (Fig.1b and Fig.S4 in Supporting information),which confirms the ZnII8L6composition of MOC1.The MS results also manifest that MOC1is stable in the CH3CN solution.
Thermogravimetric analysis (TGA) showed that MOC1was stable to about 350°C (Fig.S5 in Supporting information).Unfortunately,crystals of MOC1were not stable in air,and they changed to amorphous after exposure to air due to lost the guest solvent molecules,which was confirmed by PXRD of MOC1(Fig.S6 in Supporting information).Such a phenomenon is normal for MOCs.N2adsorption experiment was also carried out.The result showed that MOC1was not a good porous solid material (Fig.S7 in Supporting information).
Yellow block crystals of MOC1suitable for single crystal X-ray diffraction (SCXRD) analysis were obtained by diffusing ethyl acetate vapor into its CH3CN solution.SCXRD revealed that1crystallizes in the space groupP21/n,featuring an 8-nuclear hexahedral cage structure (Fig.2a).Six ligands as faces bind eight ZnIIions to form the hexahedral cage.Interestingly,the ZnIIcenters adoptΛΛΔΔΔΔΔΔorΛΛΛΛΛΛΔΔconfigurations (Figs.2c and d),and both two enantiomers co-existed in the crystal structure to produce the racemate1(Fig.2a).Notably,MOC1can be reduced to an irregular hexahedral ball-stick structure by treating the ZnIIcenters as nodes (Fig.2b).The twoΛΛorΔΔZnIIcenters were located on the body diagonal of the hexahedron,which probably lead to the form of a hexahedral cage with a symmetry ofD3.The low symmetry of1was consistent with the result of its1H NMR in CD3CN [40].Although a lot of hexahedral MOCs have been reported [41–43],to the best of our knowledge,such vertex configurations are observed for the first time.The metalto-metal distances of ZnIIcenters forming adjacent vertices range from 10.332 ˚A to 13.601 ˚A,further suggesting the low symmetry in1.The maximum dimension is 28.543 ˚A (H∙∙∙H distance),which is in good agreement with the results of DOSY.MOC1has an electron-rich hydrophobic cavity with a volume of 520 ˚A3estimated by VOIDOO [44],which is probably an ideal host for encapsulation and separation of PAHs.
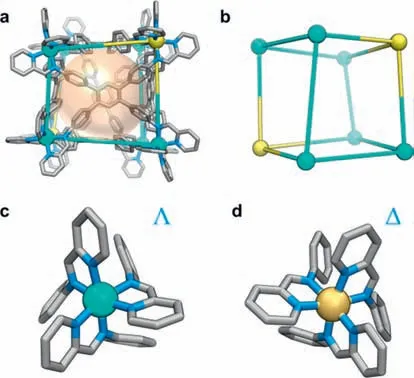
Fig.2.X-ray Crystal structure of MOC 1: (a) Hexahedral cage structure,(b) topology structure,and coordination in (c) Λ and (d)Δ absolute configurations for octahedral zinc centers.The ZnII centers with Δ and Λ configurations were colored orange-yellow and cyan,respectively.gray,C;blue,N.The anions and hydrogen atoms were omitted for clarity.
As shown in Fig.3,seven PAHs were chosen to study the hostguest chemistry with MOC1.Before the experimental study,we calculated their molecular volume by using Materials Studio 2018.The free volumes of PAHs were generated by using the accessible Connolly surface calculation.As shown in Fig.3,the molecular volumes of these PAHs range from 262 ˚A3to 392 ˚A3and are smaller than the volume of1(520 ˚A3).Interestingly,the molecular volume of pyrene is 287 ˚A3,and its packing coefficient (ratio of guest volume to host volume) in the cavity of1will be 55.2%.According to Rebek’s rule [30,45,46],the best binding will be reached for pyrene.In addition,the two-dimensional sizes of these seven PAH molecules were also calculated (Table S2 in Supporting information).The range of the minimum size of each molecule was 7.388–11.901 ˚A,which is smaller than the maximum cavity size of the cage (Zn-Zn distances,13.601 ˚A),suggesting that1can probably trap these PAHs.
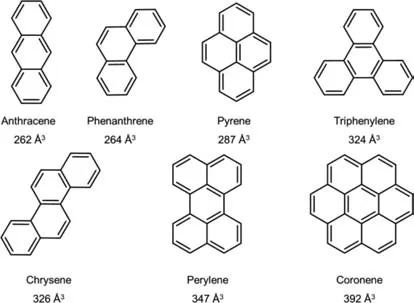
Fig.3.PAH molecules and their volumes.Materials Studio 2018 was used for the calculation.The Connolly radius vdW,scale factor,and grid interval were set as 1.0 ˚A,1.26 and 0.7 ˚A,respectively.
Based on the above theoretical calculation results,the experiments of host-guest chemistry of MOC1and PAHs (Fig.3) were carried out.In a typical experiment,the MOC1was dissolved in CD3CN or CD3NO2(0.6 mL,0.002 mmol),and excess PAHs were added,respectively.The solution was equilibrated at 60°C for 3 h,and the reactants were characterized by1H NMR.
As shown in Fig.4,the1H NMR peaks of1showed significant downfield or upfield shifting in comparison to the free1were observed for the imine (H1,|Δδ|=0.05–0.10 ppm) and phenyl protons (H2|Δδ|=0.16 ppm,H3|Δδ|=0.07–0.09 ppm,H4|Δδ|=0.16–0.51 ppm) after encapsulation of pyrene.In addition,two peaks(Ha,Hb) of the bound pyrene were observed in the spectrum of pyrene⊂1(Fig.4a,Figs.S8 and S9 in Supporting information).The unidentified signal of Hc was probably overlapped with the signals of the MOC1.The1H–1H COSY spectrum showed a strong correlation between Haand Hb,suggesting that Haand Hbbelong to the encapsulation of pyrene.The obvious upfield shift of Ha,Hbin comparison to the signs of free pyrene (about 1.0 ppm),suggests that host1exhibits a slow-exchange binding process on the proton NMR timescale.The host-guest stoichiometry was found to be 1:1 by integration of the peaks of1H NMR of pyrene⊂1,suggesting that one cage molecule can host a pyrene molecule.This result is consistent with the packing coefficient (55.2%).
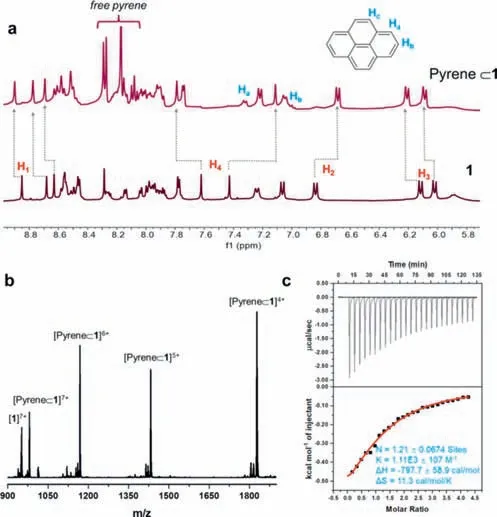
Fig.4.(a) Aromatic region of the 1H NMR spectra of free 1 (bottom),and pyrene⊂1 formed by adding 5 equiv.of pyrene to 1 (top).(b) Mass spectrometry of pyrene⊂1.(c) Binding isotherm corresponding to the ITC titration of 1 with pyrene([1]=1.0 mmol/L,and [pyrene]=20.0 mmol/L,CH3CN/CH3OH (v/v=1:1) at 30°C).
ESI-TOF-MS was further employed to confirm the formation of pyrene⊂1.As shown in Fig.4b and Fig.S10 (Supporting information),the peaks withm/zvalues of 980.1313,1168.3345,1431.7878 and 1827.0033 correspond to [pyrene⊂1–9OTf]7+(calcd.980.1421),[pyrene⊂1–10OTf]6+(calcd.1168.3278),[pyrene⊂1–11OTf]5+(calcd.1431.7202),and [pyrene⊂1–12OTf]4+(calcd.1826.9314),respectively.This result further manifests that MOC1successfully encapsulated the pyrene as the guest with a 1:1 stoichiometric ratio.
We also tested other six PAHs.Similar to pyrene,both anthracene and phenanthrene can also be encapsulated by MOC1successfully,respectively,which were documented by1H NMR and ESI-TOF-MS studies (Figs.S11-S14 in Supporting information).Due to the poor solubility of these PAHs in CD3CN,the encapsulating reactions with MOC1were carried out in CD3NO2.As shown in Figs.S15-S18 (Supporting information),the1H NMR peaks of1were not shifted after adding the triphenylene,chrysene,perylene,and coronene into its CD3NO2solution,respectively.As a comparison,we tested the encapsulating experiment of pyrene in CD3NO2.The results showed that pyrene was successfully encapsulated by1(Fig.S19 in Supporting information).These results indicate that the triphenylene,chrysene,perylene,and coronene cannot be bound by MOC1,which is probably due to their relatively bigger molecular volumes (Fig.3).
According to the reported procedure [32],the binding constants of pyrene,anthracene,and phenanthrene to MOC1were determined by1H NMR integration at 298 K.The binding constantsKafor anthracene,phenanthrene,and pyrene were estimated to be approximately 111 (mol/L)-1,277 (mol/L)-1and 1184 (mol/L)-1,respectively.Furthermore,isothermal titration calorimetry (ITC) was employed to measure the binding constants,which was often used to study the host-guest chemistry of coordination cages [47,48].As shown in Fig.4c,upon the addition of pyrene (20.0 mmol/L) to a CH3CN/CH3OH (v/v=1:1) solution of1(1.0 mmol/L) yielded association constants of 1110±107 (mol/L)-1,the titration fit curve showed the molar ratio of 1:1 for the host-guest complex.The results of ITC were in good agreement with the results obtained by1H NMR and mass spectrometry.TheΔHandΔSvalues were-797.7 cal/mol and 11.3 cal mol-1K-1,respectively,indicating that the complexation reaction was driven by enthalpy.The titration results showed no obvious heat change was observed by adding anthracene and phenanthrene into the CH3CN/CH3OH solution of1under the same conditions (Fig.S20 in Supporting information),respectively.These results indicated that the binding between anthracene (or phenanthrene) and MOC1was very weak,which is consistent with their small binding constants obtained by1H NMR.
To understand the mechanism of the encapsulation,we attempt to obtain the crystal structure of pyrene⊂1.Excess of pyrene was added to the CH3CN solution of MOC1and slowly diffused with ethyl acetate vapor,the crystals of pyrene⊂1suitable for X-ray diffraction analysis were obtained after about 1 week.As shown in Fig.5a,the crystal structure clearly shows that one pyrene molecules locate in the cavity of1.The shortest distance between the pyrene and1was about 2.8 ˚A (C∙∙∙H distance),which indicates that there exist C–H∙∙∙πinteractions in them.After the encapsulation of pyrene,the metal-metal distances of the adjacent vertex ZnIIcenter range from 10.336 ˚A to 13.666 ˚A,which were very close to that of guest free1,indicating that its cavity volume did not change after encapsulation of pyrene.Interestingly,after encapsulation of pyrene,MOC1can further bind ethyl acetate molecules(Fig.S21 in Supporting information).In addition,the independent gradient model of this structural model based on Hirshfeld partitioning (IGMH) [49] analysis showed that the pyrene molecule was surrounded by green isosurfaces (Fig.5b),and the calculated binding energy was -34.68 kcal/mol.These results indicate that there exist weak interactions between pyrene and the cavity of1,which probably stabilize the host-guest complex of pyrene⊂1.
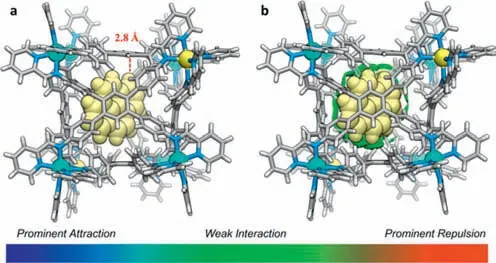
Fig.5.(a) Crystal structure of pyrene⊂1 (C–H∙∙∙π interactions were highlighted with a dashed red line).(b) Independent gradient model for the Hirshfeld partition(IGMH) analysis between pyrene and the wall of the cavity in 1.The color scale showed a range of interaction strengths: prominent attraction (blue),weak interaction (green),and prominent repulsion (red).The anions and ethyl acetate were omitted for clarity.Color labels: Zn,cyan or yellow;C,gray;N,blue;H,light gray.Pyrene was shown as a space-filling mode with yellow color.
The above experimental results suggest that MOC1has a stronger capacity in binding pyrene than other PAHs,which may be used as a material for the selective separation of pyrene from the mixture of PAHs.A mixture of PAHs (pyrene,3 equiv.,and other PAHs,1 equiv.,based on1) was added to a CH3CN solution of1(20 mL,0.001 mol/L,Figs.6a and b).After equilibration at 60 °C for 3 h,the free PAHs were removed by the filter after adding ether,in which the free PAHs were dissolved in the ether and pyrene⊂1was precipitated.The powder of pyrene⊂1was obtained by filter (Figs.6c and d).1H NMR demonstrated the pyrene was mainly selected by1to form pyrene⊂1,and no1H NMR signal of other PAH molecules was detected (Fig.S22b in Supporting information).These experimental results indicated that1selectively captured the pyrene from the mixture of PAHs.The trapped pyrene can be released by solid-liquid extraction by using ethyl acetate as the solution to obtain the empty1(Fig.6e and Fig.S22c in Supporting information).The successfully recovered pyrene from the mixture was also proved by the1H NMR spectrum,which showed that the recovered sample contain pyrene with high purity (>98%,Fig.S23 in Supporting information).The high purity of recovered pyrene was further confirmed by high performance liquid chromatography (HPLC),and a purity of 96.1% was determined (Fig.S24 in Supporting information).The separation experiment was performed when pyrene was present in an equal amount in the mixture of PAHs.The results showed that MOC1was able to separate pyrene with>90.9% purity (Fig.S25 in Supporting information).In addition,ethyl acetate as a guest molecule bound to the cage does not affect the separation performance.Similar results can be obtained when other poor solvents (ether,toluene,etc.) were used for separation experiments.Moreover,the recovered1can be further used to separate the pyrene from the mixture.Cage1was stable after repeating the separation three times (Fig.S22d in Supporting information),suggesting that1was a robust material for the selective separation of pyrene from the PAHs mixture.

Fig.6.Selective separation and extraction of pyrene from PAHs mixtures: (a) 1 was dissolved in CH3CN and (b) selectively encapsulated pyrene to form pyrene⊂1.(c)Pyrene⊂1 was precipitated,and the free PAHs were dissolved in a mixed solvent of ether and CH3CN.(d) The powder of pyrene⊂1 was obtained by filter.(e) The bound pyrene was released by solid-liquid extraction with ethyl acetate to obtain empty 1 and pure pyrene.
In summary,we have designed and synthesized a low symmetric hexahedral metal-organic cage with a cavity volume of 520 ˚A by subcomponent self-assembly.In MOC1,zinc centers displayΛΛ/ΔΔΔΔΔΔandΛΛΛΛΛΛ/ΔΔconfigurations in its crystal structure,which is discovered in hexahedral MOCs for the first time.The molecular volume of pyrene matches well with the cavity volume of cage1(55.2% packing coefficient).Cage1has a high affinity to pyrene and can selectively separate pyrene from a mixture of PAHs with high purity.X-ray structure of pyrene⊂1showed there exist multi CH∙∙∙πinteractions between them,which stabilize the host-guest complex.Moreover,cage1is robust and recyclable,providing an advanced material for the selective separation of pyrene from the PAHs mixture.This study provides a new strategy for designing materials based on metal-organic cages for the separation of PAHs,which will probably pave the way for developing new technologies in separation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.22171106,21731002,21975104,21871172 and 22201101),the Guangdong Major Project of Basic and Applied Research (No.2019B030302009),Guangdong Natural Science Foundation (No.2022A1515011937),the Guangzhou Science and Technology Program (No.202002030411),the Fundamental Research Funds for the Central Universities (No.21622103),the China Postdoctoral Science Foundation (No.2022M711327),and Jinan University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108326.
杂志排行
Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
