Boosting the proton conduction in a magnetic dysprosium-organic framework by introducing conjugate NH4+-NH3 pairs
2024-04-06YiPingQuQianZouSongSongBaoLiMinZheng
Yi-Ping Qu ,Qian Zou ,Song-Song Bao,Li-Min Zheng
State Key Laboratory of Coordination Chemistry,School of Chemistry and Chemical Engineering,Collaborative Innovation Center of Advanced Microstructures,Nanjing University,Nanjing 210023,China
Keywords: Metal-organic framework Proton conduction Ammonia adsorption Metal phosphonate Single molecule magnet
ABSTRACT Metal-organic frameworks (MOFs) with inherent porosity and suspended acidic groups are promising proton conducting materials in water or aqua-ammonia media.Herein we report a new lanthanide phosphonate,namely,Dy2(amp2H2)2(mal)(H2O)2·5H2O (MDAF-6).It possesses a 3D open-framework structure,and shows a high NH3 adsorption capacity of 142.4 cm3/g at P/P0=0.98 at 298 K due to acid-base interaction.Interestingly,the proton conductivity of MDAF-6-NH3 is enhanced by five orders of magnitude compared to MDAF-6 after 8.5 h exposure in saturated NH3-H2O vapor,indicating the importance of coexistent conjugate acid-base pairs of H3O+-H2O and NH4+-NH3 in promoting proton conduction.Magnetic studies of MDAF-6 revealed slow magnetization relaxation under zero dc field,characteristic of singlemolecule magnet behavior.This work provides not only a new multifunctional MOF material,but also a new strategy to improve proton conduction in aqua-ammonia medium.
Metal-organic frameworks (MOFs) with intrinsic porosity and tailorable surface chemistry are an emerging class of protonconducting materials for clean-energy related applications [1,2].In order to improve proton conductivity of the material,the proton carrier concentration and mobility must be increased.To this end,great efforts have been made in developing water-mediated proton conducting MOFs because water can offer conjugated acid-base pair of H3O+-H2O and contributes to the construction of efficient proton-conducting pathway [3–7].While the concentration of proton carriers can be increased by introducing acidic groups in the framework and/or acidic counterions and guests [8–12].Besides water,NH3is also considered as a promising medium for proton conduction because of its similarity to water [13].Recent reports have shown that exposing the material in NH3-H2O vapor can significantly increase the proton conductivity by 1–2 orders of magnitude [14–19].However,it is unclear whether the coexistence of conjugate NH4+-NH3and H3O+-H2O pairs can further enhance the proton conductivity.To answer this question,MOFs with good ability to adsorb ammonia and possessing structural stability in basic media are highly desired.
Metal phosphonates are known to exhibit high thermal and chemical stability because each phosphonate group can afford three oxygen atoms to bind metal ions [20].The water stability of trivalent and tetravalent metal phosphonates is higher compared to monovalent and divalent metal compounds [21].By selecting suitable metal ions and phosphonate ligands,porous metal phosphonates containing P-OH acidic groups can be designed and synthesized for ammonia adsorption and proton conduction [22–26].Herein,we report a mixed-ligand dysprosium framework Dy2(amp2H2)2(mal)(H2O)2·5H2O (MDAF-6),where amp2H4is a pre-photodimerized 9-anthrylmethylphosphonic acid and malH2is malonic acid.The amp2H4was chosen because it contains a large and flexible dianthracene group,which facilitates the formation of a 3D framework structure [27–29].DyIIIion was chosen because its trivalent state can improve the framework stability and its unique magnetic properties lead to multifunctional materials[30–36].Indeed,MDAF-6shows a 3D open framework structure in which the uncoordinated P-OH group dangles over the channel surface.It exhibits excellent NH3adsorption capacity due to the acid-base reaction between NH3and P-OH groups.The resulted material,MDAF-6-NH3,shows five orders of magnitude enhancement in proton conductivity compared toMDAF-6in saturated NH3-H2O vapor,indicating the importance of the coexistence of NH4+-NH3and H3O+-H2O conjugate acid-base pairs for proton conduction (Scheme 1).The magnetic properties ofMDAF-6were also studied.
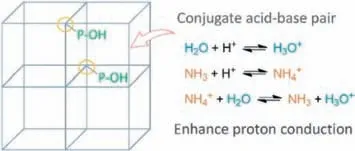
Scheme 1.The framework of MDAF-6 containing P-OH for NH3 and H2O adsorption.
CompoundMDAF-6was obtained as pale-yellow rhombus crystals after solvothermal reaction of DyCl3·6H2O,amp2H4and malonic acid in CH3OH/H2O at 90°C for 48 h,and was characterized by PXRD,TG and IR measurements (Figs.S1–S3 in Supporting information).Single crystal structural analysis revealed that it crystallizes in the orthorhombic system,polar space groupPmn21(No.31) (Table S1 in Supporting information).The asymmetric unit contains two kinds of Dy atoms (each with half occupancy),one amp2H22-ligand,half an occupied malonate,one coordinated and 2.5 lattice water molecules (Fig.S4 in Supporting information).The lattice water molecules were treated with PLATON/SQUEEZE program because of heavy disorder,and the number was determined by elemental and thermal analyses (Fig.S2 in Supporting information).Both Dy atoms reside on the mirror plane,and each is seven-coordinated by four O atoms from four amp2H22-ligands(O1,O1A,O5B and O5C for Dy1;O2,O2A,O4D and O4E for Dy2),two O atoms from the chelated malonate anion (O7 and O9 for Dy1;O7 and O8 for Dy2),and one from water molecule (O1W for Dy1;O2W for Dy2) (Fig.1a).The Dy-O bond lengths and ODy-O angles are 2.218(9)–2.465(16) ˚A and 69.2(2)°–156.4(4)° for Dy1,and 2.232(9)–2.749(9) ˚A and 50.5(3)°–167.0(4)° for Dy2 (Table S2 in Supporting information).The {DyO7} core has a distorted capped trigonal prism geometry (CShM=0.755 for Dy1 and 1.434 for Dy2,Table S3 in Supporting information) [37].The adjacent Dy1 and Dy2 atoms are connected alternatively by triple bridges of two O-P-O and oneμ-O and double bridges of O-P-O units (Dy1…Dy2 distance: 4.558,5.389 ˚A),forming an infinite polar chain running along theb-axis,where the disordered malonate ligands locate on the same side (Fig.1b).Each amp2H22-serves as a tetradentate ligand binding to four Dy atoms,whereas each mal2-also acts as a tetradentate ligand chelating and bridging the Dy1 and Dy2 atoms (Fig.S5 in Supporting information).The neighboring chains are cross-linked by the amp2H22-ligands forming a 3D framework structure (Fig.1c).A 1D channel is generated along theb-axis with the window dimension ofca.7.0×6.2 ˚A2(van der Waals radii not accounted).It is noted that one of the two protonated phosphonate oxygen atom (O6) forms intrachain H-bond with the malonate oxygen atom O8,while the other (O3) points towards the channel center,and should form H-bond with lattice water molecule.Thus,the channel wall is hydrophobic on the dianthracene side,but hydrophilic on the chain side with pendent P-OH,malonate oxygen atom,and coordination water molecules.
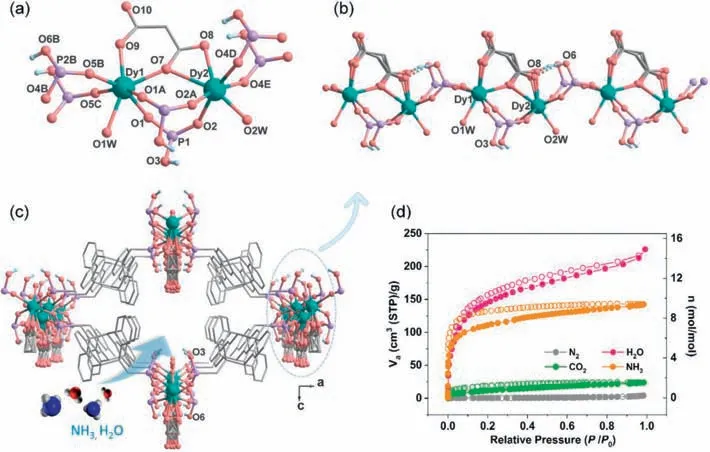
Fig.1.(a) Building unit of MDAF-6.Part of the disordered malonate and the organic group of amp2H22- ligand are omitted for clarity.(b) The infinite chain in MDAF-6 running along the b-axis.(c) The open framework structure of MDAF-6 viewed along the b-axis.All H atoms except for those attached to phosphonate oxygen atoms are omitted for clarity.(d) The adsorption (filled) and desorption (open) isotherms for solvent-free MDAF-6: N2 (77 K),CO2 (298 K),NH3 (298 K) and water vapor (298 K).
To examine the stability ofMDAF-6,the crystalline sample was immersed in water with different pH (1–14) for 24 h at room temperature.The PXRD measurements confirmed the stability of the material at pH 2–12 (Fig.S6 in Supporting information).Thermal analysis revealed thatMDAF-6lost seven water molecules below 170°C (obs.7.2%,calcd.7.7%).The weight loss above 170°C is due to the decomposition of organic components and the collapse of the framework structure (Fig.S2).
We next investigated the adsorption/desorption performance ofMDAF-6,activated under vacuum at 100°C for 4 h (weight loss: obs.7.8%,calcd.7.7%),toward N2,CO2,NH3and H2O gasses(Fig.1d).The N2adsorption/desorption isotherm at 77 K is a Type II isotherm with a small loading of 4.1 cm3/g atP/P0=0.98,indicating a very weak interaction between nitrogen and pore walls.The adsorption was increased for CO2at 298 K with a maximum uptake of 23.6 cm3/g (1.6 mol/mol) atP/P0=0.98.A quick and dramatically enhanced adsorption was found for NH3with uptakes of 92.0 cm3/g (6.1 mol/mol) atP/P0=0.04 and 142.4 cm3/g (9.4 mol/mol,6.35 mmol/g) atP/P0=0.98 at 298 K,attributed to the favourable acid-base interaction between P-OH and NH3.The capacity is higher than MIL-53 (4.40 mmol/g) but lower than NH2-MIL-53 and MIL-100 (8.00 mmol/g) [38].After desorption,there remained 75.0 cm3/g (4.9 mol/mol) of ammonia atP/P0=0.001,which is higher than the expected value of 4.0 mol-1when all four P–OH groups per molecular formula interacted with NH3to form non-volatile PO32--NH4+pairs.This result suggests that NH3may also occupy the vacancy left by the Dy atom after removing the coordination water.The formation of PO32--NH4+pair breaks the intrachain H-bond between P-OH and malonate oxygen and weakens the O(P)-H bond,and thus should increase the proton concentration for conduction in the framework channel.The sample after adsorption/desorption of NH3at 298 K is named asMDAF-6-NH3.Interestingly,the re-activated sample,after heatingMDAF-6-NH3at 150°C under vacuum for 10 min (weight loss: obs.5.8%,calcd.5.6%),showed similar capacity of NH3adsorption atP/P0=1.0 (Fig.S7 in Supporting information).
The water adsorption isotherm of the fully dehydrated sample ofMDAF-6showed a quick uptake of water vapor,and the value reached 118.7 cm3/g (7.8 mol/mol) atP/P0=0.08 and 226 cm3/g(15 mol/mol) atP/P0=0.99 at 298 K (Fig.S8 in Supporting information).When desorbed,there were still about 100.3 cm3/g(7 mol/mol) H2O in the structure atP/P0=0.03,in agreement with the presence of two coordinated and five lattice water molecules.We also measured the water adsorption capacity ofMDAF-6-NH3at 298 K and observed an uptake of 78.4 cm3/g (5.3 mol/mol)atP/P0=0.96,which is much lower than that forMDAF-6(14.5 mol/mol).This is reasonable because the channel space inMDAF-6-NH3is partially filled with the loaded NH3molecules.In addition,the hysteresis of water adsorption/desorption isotherm is more significant forMDAF-6-NH3than forMDAF-6,indicating a strong interaction between water and NH4+/NH3.Notably,the water adsorption capacity ofMDAF-6andMDAF-6-NH3drops significantly upon slight increase of temperature.At 308 K,the capacities became 179.8 cm3/g (11.8 mol/mol) forMDAF-6atP/P0=0.98 and 67.1 cm3/g (4.4 mol/mol) forMDAF-6-NH3atP/P0=1.00,respectively (Fig.S9 in Supporting information).
The proton conductivity (σ) ofMDAF-6andMDAF-6-NH3was evaluated by impedance spectroscopy measurements using a pellet sample placed in a temperature and humidity-controlled chamber for 12 h.ForMDAF-6,the conductivity at 298 K was 1.8×10-12S/cm at 40% RH and increased to 1.5×10-9S/cm at 95% RH (Fig.2a and Fig.S10 in Supporting information).The values are very low compared to other proton conductive metal phosphonates with protonated phosphonate groups [22].From the structure ofMDAF-6,we can see that hydrophobic dianthracene moieties are present in the channel which is unfavorable for the formation of continuous hydrogen bond network.Besides,half of the protonated P-OH groups participate in strong intrachain hydrogen bonds,which may‘freeze’the protons,prevent them from migration,and thus affect their conduction.The adsorption of ammonia lifted the freeze of protons due to the acid-base interaction between P-OH and NH3to form NH4+inMDAF-6-NH3.Proton conductivity measurements at 298 K revealed a remarkably improved conductivity of 1.0×10-10S/cm at 40% RH and 6.8×10-6S/cm at 95% RH (Fig.2a and Fig.S11 in Supporting information).The latter is three orders of magnitude higher than that forMDAF-6.This value is comparable to some metal-triphosphonates [22,39],but lower than a few 3D metaltetraphosphonates (ca.10-2–10-4S/cm) [26,40,41].Obviously,the NH4+ions formedin-situpromote the water-mediated proton conduction.
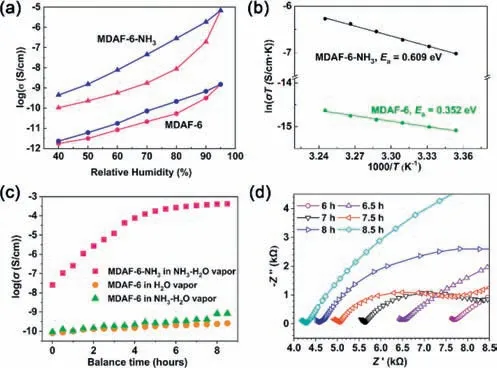
Fig.2.(a) Proton conductivities of MDAF-6 and MDAF-6-NH3 from 40% to 95% RH at 298 K (pink for increasing RH and blue for decreasing RH).(b) Arrhenius plot of the temperature dependence at 95% RH for MDAF-6 (green) and MDAF-6-NH3(black).(c) Proton conductivities of MDAF-6-NH3 in ammonia vapor, MDAF-6 in water vapor and MDAF-6 in ammonia vapor.(d) Nyquist plots of MDAF-6-NH3 in ammonia vapor for 6–8.5 h.
The temperature-dependent proton conductivities ofMDAF-6andMDAF-6-NH3were measured at 95% RH.The proton conductivities were almost unchanged for both compounds when the temperature went up to 35°C and further reduced above 35°C(Fig.S12 in Supporting information),in consistence with the partial release of water molecules at this temperature.Based on the data in the temperature decreasing process,the activation energies (Ea)were estimated to be 0.35 eV forMDAF-6and 0.61 eV forMDAF-6-NH3(Fig.2b).The largerEa(>0.6 eV) suggests that NH4+may migrate directly as a proton attached to a vehicle inMDAF-6-NH3.
The above results showed that the water-mediated proton productivity ofMDAF-6was significantly enhanced after the incorporation of NH4+.In this case,there exist two acid-base pairs,e.g.,H3O+-H2O and NH4+-H2O,which participate in the proton conduction pathway.We postulate that the presence of conjugate acid-base pair of NH4+-NH3would facilitate the proton mobility.
To confirm this,we first evaluated the proton conductivity ofMDAF-6-NH3under a dry NH3gas atmosphere at 100 kPa.The sample pellet was dried at 100°C under vacuum for activation using a hot stage with electrical probes and then filled with dry NH3gas after cooling to room temperature.After being exposed to NH3gas for about 7 h,the gas adsorption of the sample pellet reached equilibrium and exhibited a conductivity of 3.4×10-9S/cm at 304 K (Fig.S13 in Supporting information).This value is higher than that obtained at 298 K and 40% RH (1.0×10-10S/cm),but much lower than that obtained at 298 K and 95% RH.The result indicates that the filling of NH3gas is not sufficient to form a continuous hydrogen bond network for efficient proton conduction.We then exposed the same pellet ofMDAF-6-NH3to saturated NH3-H2O vapor.Interestingly,the proton conductivity drastically increased along with prolonged time and reached a maximum value ofca.4.2×10-4S/cm after 8.5 h (Figs.2c and d,Fig.S14 in Supporting information).Controlled experiments were carried out under the same conditions forMDAF-6in water or NH3-H2O vapor.As shown in Fig.2c,the proton conductivity ofMDAF-6wasca.10-9S/cm after exposure to water or NH3-H2O vapor for 8.5 h,which is 5 orders of magnitude lower than that forMDAF-6-NH3.The conductivity ofMDAF-6reached the equilibrium value of 2.1×10-8S/cm after 32 h exposure to water vapor,and 6.9×10-5S/cm after 172 h exposure to NH3-H2O vapor (Fig.S15 in Supporting information).Notably,the framework structure ofMDAF-6remained the same after thermal treatment,the NH3gas adsorption/desorption process,and exposure to saturated NH3-H2O vapor(Fig.S16 in Supporting information).
To understand the mechanism of enhanced proton conductivity ofMDAF-6-NH3in NH3-H2O vapor,we measured the solidstate IR and1H MAS NMR spectra of the samples.Fig.S3 shows the IR spectra ofMDAF-6andMDAF-6-NH3.Compared toMDAF-6(3619,3522 and 3441 cm-1),the O-H stretching vibrations ofMDAF-6-NH3are red-shifted to 3603,3499 and 3418 cm-1,indicating the weakening of the O-H stretching vibration.In addition,there appears a new broad peak at 3196 cm-1,attributed to the NH stretching vibration.The P-O stretching vibrations are very different for the two samples with additional strong peaks appearing at 1042 and 1001 cm-1forMDAF-6-NH3.These results corroborate with the fact that the P-OH acidic group reacted with NH3base forming PO32--NH4+pair inMDAF-6-NH3.
After exposure to NH3-H2O vapor for 8.5 h,bothMDAF-6andMDAF-6-NH3show broad bands at 3680–3280 cm-1,ascribed to the presence of extensive hydrogen bonding networks (Fig.S17 in Supporting information).Compared toMDAF-6,MDAF-6-NH3shows an enhanced stretching vibration atca.3435 cm-1and additional bands at 3207,1402 and 1207 cm-1.The latter three peaks are attributed to the N-H vibrations of NH4+and NH3[42,43].The broad N-H stretching peak at 3207 cm-1may be related to the formation of hydrogen bonds between NH3and NH4+[44].The observation of these N-H vibrations forMDAF-6-NH3but not forMDAF-6indicates thatMDAF-6-NH3adsorbs NH3-H2O vapor quickly and reaches equilibrium within 8.5 h.By contrast,MDAF-6adsorbs NH3-H2O vapor slowly and needs much longer time(ca.172 h) to reach equilibrium.This fact gives us an opportunity to distinguish the role of the NH4+-NH3conjugate pair in proton conduction.ForMDAF-6exposed to NH3-H2O or pure water vapor,there was negligible or no NH4+-NH3conjugate pair generated within 8.5 h,and their conductivities were extremely low (ca.10-9S/cm2).Therefore,the significantly enhanced proton conductivity ofMDAF-6-NH3in saturated NH3-H2O vapor (ca.10-4S/cm2) has to contribute to the coexistence of the NH4+-NH3and H3O+-H2O conjugate pairs.
We also measured the solid-state1H MAS NMR spectra of the above three samples.As shown in Fig.S18 (Supporting information),a resonance signal appears at 4.2 ppm forMDAF-6exposed to H2O and NH3-H2O vapor for 8.5 h,corresponding to physisorbed water moving freely in the pore system of the material [45].ForMDAF-6-NH3exposed to NH3-H2O vapor for 8.5 h,the signal is slightly shifted to 5 ppm,which could be related to the presence of NH4+(NH3) and H2O interactions.
Based on the above results,we propose the mechanism of proton conduction ofMDAF-6-NH3in NH3-H2O vapor as below: (1)The formation of PO32--NH4+pair breaks the intrachain H-bond between P-OH and malonate oxygen and weakens the O(P)-H interaction,thus increasing the proton concentration for conduction;(2) The NH4+ion forms H-bonds with neutral NH3and transfers proton with low energy barrier [44];(3) Saturated NH3-H2O atmosphere provides a continuous network of H-bonds for efficient proton conduction (Scheme 2).
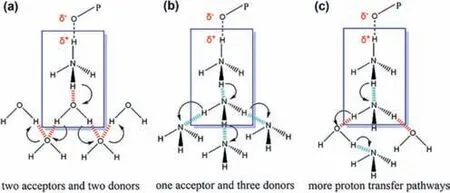
Scheme 2.Proposed proton transfer pathways of MDAF-6-NH3 in (a) water,(b) NH3 and (c) NH3-H2O atmosphere.
In order to explore the possibility of usingMDAF-6as a magnetic proton conductor [46,47],we also investigated its magnetic properties.As shown in Fig.S19 (Supporting information),theχMTvalue ofMDAF-6(28.17 cm3K/mol per Dy2) at room temperature agrees well with the spin-only value of 28.34 cm3K/mol for two isolated DyIIIions (6H15/2,S=5/2,L=5,gJ=4/3).TheχMTdecreases progressively upon cooling,attributed to the thermal depopulation of DyIIIStark sublevels and possible weak antiferromagnetic interactions.Field-dependent magnetization measured from 2 K to 10 K showed unsaturation up to 70 kOe (Fig.S19,inset),suggesting the presence of magnetic anisotropy and/or lower lying excited states.The alternating current (ac) susceptibility data ofMDAF-6revealed a frequency dependence of the out-of-phase (χ’’)signals under zero or 1 kOe dc field (Fig.S20 in Supporting information),characteristic of single-molecule magnet (SMM) behavior.But no maximum was observed,thus excluding the possibility to derive the energy barrier of the material.
In summary,we report a new porous dysprosium phosphonate framework Dy2(amp2H2)2(mal)(H2O)2·5H2O (MDAF-6) which shows a high adsorption capacity toward NH3due to the presence of P-OH acidic groups.Impressively,we observed a dramatic enhancement of proton conductivity after exposingMDAF-6-NH3in saturated NH3-H2O vapor,demonstrating the importance of coexistent conjugate acid-base pairs of H3O+-H2O and NH4+-NH3in promoting proton conduction.In addition,MDAF-6shows SMM behavior at low temperature.This work not only provides a new example of proton conductive magnetic materials,but also a new approach to boost the proton conduction of MOFs by introducing conjugated acid-base pairs of H3O+-H2O and NH4+-NH3in NH3-H2O media.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grant from the National Natural Science Foundation of China (No.21731003).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108320.
杂志排行
Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
