Ultrafast photoexcitation dynamics behavior of hydrogen-bonded polyfluorenol
2024-04-06MnXuChunxinWeiYunlongZhngHoLiJingyoJinyiLinShengjieWngWeiXueQiWeiLinghiXieWeiHung
Mn Xu ,Chunxin Wei ,Yunlong Zhng ,Ho Li ,Jingyo M ,Jinyi Lin ,Shengjie Wng,Wei Xue,Qi Wei,Linghi Xie,Wei Hung,,
a State Key Laboratory of Organic Electronics and Information Displays &School of Chemistry and Life Sciences &Institute of Advanced Materials (IAM),Nanjing University of Posts &Telecommunications,Nanjing 210023,China
b Key Laboratory of Flexible Electronics (KLOFE) &Institute of Advanced Materials (IAM),Nanjing Tech University (NanjingTech),Nanjing 211816,China
c Frontiers Science Center for Flexible Electronics (FSCFE),Shaanxi Institute of Flexible Electronics (SIFE) &Shaanxi Institute of Biomedical Materials and Engineering (SIBME),Northwestern Polytechnical University,Xi’an 710072,China
Keywords: Photoexcitation dynamics Polyfluorenol Aggregation behavior Energy transfer Exciton migration
ABSTRACT Exciton behavior is crucial to the exploitation of light-emitting conjugated polymer (LCPs) for optoelectronic devices.Singlet excitons are easily trapped by the intrinsically defect structures.Herein,we set a polyfluorenol (PPFOH) as an example to systematically investigate its photophysical behavior to check the role of defect structures in LCPs.According to time-resolved photoluminescence analysis,the feature emission peaks from individual chain of PPFOH in diluted DMF solution is effectively avoided the influence of fluorenone formation,but the residual green-band emission at 550 nm is easily observed in the PL spectra of PPFOH dilute toluene solution obtained delay 1.5 ns,confirmed the formation of “guest”physical aggregation-induced defect structure.Remarkably,efficient and ultrafast energy transfer from individual chain to defect structure is observed in PPFOH films.Interestingly,the efficient energy transfer happened for the film obtained from DMF solution (200 ps) than those of toluene ones (600 ps).Meanwhile,compared to relatively stable green-band emission in PPFOH film from toluene solution,red-shifted emission peak (11 nm) of PPFOH film from DMF solutions exposed to saturated DNT vapor also confirmed their different aggregation-induced green-band emission.Therefore,this aggregation defect structures are influenced on the photophysical property of LCPs in solid states.
Since first generation semiconducting polymer originally discovered by three pioneers,Chianget al.[1,2],this type of material has attracted more attentions in fundamental research and industrial area,attributed to their greatly potential applications in light-emitting diodes [3–5],solar cell [6–9] and field-effect transistors [10–12].Alternated single and double-bonded backbones enable mainchains to present aπ-conjugated backbone structure,which is fundamental topological structure to obtain conducting and emissive property for the realization of optoelectronic application [13–15].The origin of the optoelectronic behavior of conjugated polymers in condensed structure is the one-dimensional intramolecularπ-electron delocalization (π-conjugated channel) and interchainπ-electron coupling (π-stacked channel),to obtain theπ-conjugated andπ-stacked channel for electron and hole transport [10,16–20].Former ones always induced one-dimensional in- trachain charge transporting [10,16,21],relatively narrow band-gap to obtain visible light emission [13,22],and latter will allow for interchain charge hoping/jumping to ensure excellent conductivity and charge mobility in the solid states [10,16,23].Due to interchainπ-stacking and electrostatic interaction,ubiquitous self-doped domains (amorphous or ordered aggregates) are existed in the thinfilm,which can induceπ-electron coupling and the formation of multi-chain excited states [18,24–29].Therefore,for light-emitting conjugated polymers (LCP),this interchainπ-electron coupling may cause the formation of multi-chains excited states in the random aggregate,compared to the relatively wide band-gap individual chain [5,24,25,30].This self-doped aggregate with a relatively narrow band-gap will act as a physical defect center to trap exciton and energy from “host” individual chain,result into undesirable energy transfer and low color purity [5,25,31,32].In this word,it is emergent to systematically investigate the ultrafast photoexcitation dynamics behavior of wide bandgap LCP to check the role of these defect structures,which is essential to improve the emission efficiency and stability of wide band-gap LCPs for optoelectronic applications.
As a wide investigated deep-blue LCP,polyfluorenes are attracted more attentions for their easily structural modification,high emission efficiency and robust deep-blue emission [5,25,33–35].Compared to the red and green LCPs,the physical defect structure involved above with a distributed narrow bandgap is easily formed in this wide bandgap deep-blue LCPs,associated with their easily self-organization,high energy absorption and active excited states [25,26,31,32,36–38].Therefore,both chemical and physical defects of polyfluorenes,included fluorenone and excimer,caused a severe green-band emission [25,29,34].It is obvious that the formation of chemical defect,such as fluorenone in pofluorene,resulted into serious low-band emission at 500–560 nm [39–42].Beyond fluorenone mechanism,random aggregation-induced physical defect,included excimer and distorted conformation,can also cause serious broaden defect green emission at 500–620 nm(Scheme 1) [43–48].Compared to the widely reported and accepted fluorenone mechanism,to establish the convinced aggregation mechanism will re-inspire researcher to believe the potential application of polyfluorene in light-emitting applications.One major challenge is impossible to avoid the effect of trace and residual fluorenone on the emission behavior in the solid states.And there is rarely material platform to explore the role of defect structure in modulating photophysical behavior of deep-blue LCPs.In the last several years,platform of polyfluorenols is designed and prepared to deeply explore the origin of aggregation-induced green emission in the various states [49–51].It is believed that the greenband emission ranges from 500 nm to 600 nm are easily observed in polyfluorenols,associated with their strong hydrogen-bondedassisted interchain and intrachain aggregation [49–51].As we discussed in our previous works,there are some serious problems need to further explain such as: (1) How is the energy transfer and exciton migration from individual chains to the aggregationinduced defect structure;(2) It is any different excited behavior in the two type film states such as gelation film (type II) and nongelation film (type I);(3) How to check the effect of fluorenone formation on the emission behavior.The establishment of the molecular mechanism of aggregation-induced green emission is the preconditions to realize the practical application of polyfluorene in optoelectronic devices.Meanwhile,this physical defect structure is not only existed in the polyfluorene but also appeared in the other LCPs,which will provide a guidance to improve the efficiency and stability of LCPs.Therefore,herein,we systematically explored the ultrafast photoexcitation dynamics behavior of polyfluorenes in various states to systematically confirm the mechanism of aggregation defect structures in green-band emission.
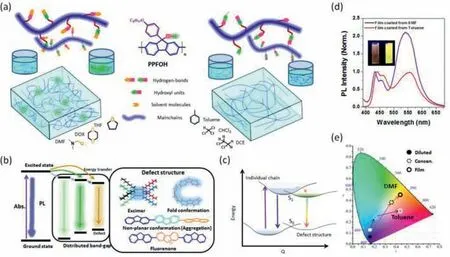
Scheme 1.Schematic illustration of the aggregation behavior of light-emitting conjugated polymers in various states.(a) Schematic illustration of PPFOH chain in two type solvents: protic solvent (Type I: DMF,THF and DOX) and non-protic solvents (Type II: Toluene,CHCl3 and DCE).The hydrogen-bonds between PPFOH chain and solvent molecules are observed in the Type I solution,but interchain hydrogen-bonds formed in the Type II solution,similar to previous works.(b) Formation of defect structure with a relatively narrow bandgap in the wide band-gap LCPs.Defect structures in polyfluorenes can be divided into two types,chemical defect (such as fluorenone) and physical ones (such as excimer),which can be act as the emission center various states.(c) The Jablonski diagram showing the mechanism of absorption and fluorescence from the individual chain to the defect structures.(d) Emission spectra of PPFOH two type films spin-coated from DMF and toluene solutions (10 mg/mL).Inset showed the photographs of the spin-coated films under UV irradiation (365 nm).(e) CIE chromaticity coordinates of PPFOH in toluene and DMF diluted and concentrated (concen.)solutions,corresponding spin-coated film.
According to traditional polymer physic,the physic-chemical property of solvent is a key factor to influence on the interchain aggregation behavior and arrangement in solution states [25,31].Due to the unique planeπ-conjugated structure,precisely controlling of diverse secondary interactions,such asπ-πinteraction [50],is a promising tool to tune the self-assembly behavior of conjugated polymer [21,25,31].In this regard,the competition between the interchain interaction (interchain packing) and chain-solvent interaction (solvation effect) may modulate the interchain aggregation behavior in the solution states [21,25,31].In solution states,polymer chains usually do not have a stable and ordered microstructure (aggregate),caused by the freedomσ-band motion and interchain supramolecular interactions [21].Besides,polymorphic behaviors of LCP in the film states are strongly depended on film fabrication procedures such as ink formulations,film-processing methods and rheological kinetics [52,53].Therefore,to explore the aggregate structure in the amorphous film and their optoelectrical property is a challenging task.
In this work,we systematically explored the role of aggregation-induced defect structure in the photophysic property of polyfluorenes.Hydroxyl units at 9-position of fluorene in PPFOH enable chain to present a diverse hydrogen-bonding andπ-πinteraction in the solution and film states [31,49,51].According to IR spectra,there is no obvious fluorenone formation in PPFOH (Fig.S1 in Supporting information).As shown in Scheme 1a,it is believably confirmed that strong hydrogen-bonding interactions between PPFOH chain and protic solvent molecules(Type I,such as dimethylformamide (DMF) and tetrahydrofuran(THF)) can be observed in the solution state,associated the alone electron pair of solvent molecules.However,it tends to form interchain hydrogen-bonding interaction in the non-protic solvents,such as toluene,chloromethane (CHCl3) and dichloroethane (DCE)(Type II).Therefore,PPFOH show excellent solubility in the protic solvents,such as a saturated concentration of>50 mg/mL in DMF solution.Meanwhile,this interchain hydrogen-bonding interaction in Type II solvent also induced the gelation processing,also confirmed the formation of cross-linked interchain framework.
Subsequently,it is believed that PPFOH present individual chain in diluted DMF solution but inter-and intrachain complex for dilute toluene solution instead.More interestingly,PPFOH exhibited a deep-blue and sky blue emission in DMF and toluene concentrated solution,respectively,also revealed the different aggregation behavior.These aggregate in precursor solution will dominate the interchain arrangement and film morphology,which are the key factors to determine photophysical behavior.Compared to “parent”individual chain,these microstructures in the film present a distributed narrow bandgap,which can act as “defect” emissive center to trap energy from high band individual chain (Schemes 1b and c).Therefore,these interchain aggregations in the film state resulted into a strong long-wavelength emission.In general,fluorenone in the mainchain also act as the chemical defect structure in the film states (Scheme 1b).Similarly,aggregation-induced interchain excimers also act as a similar physical defect structure to trap energy transfer (or exciton) from “parent” individual chain(Scheme 1c).Therefore,both residual chemical and physical defect structure will act as “guest” emissive center to dominate the photophysical property of polyfluorene (Scheme 1d).Significantly different to deep-blue emission property of traditional polyfluorene in solid films,there are strong green-band emission observed in PPFOH films (Scheme 1d).Therefore,the emission colours of PPFOH in dilute and concentrated solution can be widely tuned from deep-blue to yellow for toluene and DMF solutions,respectively(Scheme 1e,Figs.S2 and S3 in Supporting information).In this regard,hydrogen-bonded-assisted induced aggregation may possible to cause the strong green-band emission in polyfluorenes.
Interchain interactions in LCP play a fundamental role in a variety of photophysical processing [37,38,54,55].Subsequently,transient absorption (TA) measurement are explored here to further probe excitonic behavior of PPFOH in various states (Fig.1)[19,56,57].Compared to concentrated solution and film,broad stimulate emission (SE) spectral profile is observed for PPFOH dilute solution,associated with singlet manifold and lack of sub-ps relaxation,suggested the weak exciton-exciton annihilation.Similar to the PL spectral profile,there is a strong SE band found in both PPFOH in DMF and toluene dilute solution (10-5mg/mL),which consisted of two feature peaks at 420 nm and 460 nm(Scheme 1d,Figs.1a and d),revealed a strong singlet manifold.However,with the enhancement of interchain interaction in concentrated solutions (0.5 mg/mL),weak SE band at 420 and 460 nm are found,and broad photoinduced absorption (PA) band from 550 nm to 750 nm,clarified as quite different PAs (singlet excitons) and PAp (polaron pairs) (Figs.1b and e),due to the dynamic of long-lived polaron pairs.This may be closely associated with the exciton-exciton annihilation,which may result into a low emission efficiency and poor color purity.Obviously,strong interchain aggregation can reasonably explain the strong polaron formation.As we expected,the intensity of SE band are further decreased,and excited state are quenching quickly in the PPFOH spin-coated films (Figs.1c and f).In this regard,these hydrogenbonded-assisted aggregations are negative to emission stability and efficiency.More interestingly,although there is a similar SE and PA band in the dilute solution,PPFOH DMF concentrated solution present a stronger SE band and PA band,than those of PPFOH toluene concentrated solution,associated with their weaker interchain aggregation.However,strong interchain interaction in the films sates also induced a weak SE band (Figs.1c and f),attributed to the formation of polaron pair under extremely concentrated states.In fact,PPFOH in DMF diluted and concentrated solution,show a strong single-molecular excitonic emission with weaker exciton-exciton annihilation than those of corresponding PPFOH in toluene solution.This is consistent with the optical results in our previous works.More interestingly,almost perfect symmetry of SE and PA band observed for PPFOH DMF and toluene diluted solution,effectively confirmed the pure singlet exciton,and indicated that there is no obvious polaron pair’s formation.However,in fact,PA band at long wavelength of 600–650 nm,clearly showed a longer-lived component than those of SE band in the PPFOH toluene solution,indicated the formation of polaron pairs,and relatively lower emission efficiency than those of PPFOH concentrated DMF solutions.More interestingly,SE band at 400–450 nm also exhibited a longer-lived component than those of PA band in the PPFOH DMF solution,compared to PPFOH toluene solution,attributed to their robust emission.Finally,PPFOH DMF solution and coated films had an efficient emission property than those of toluene ones.
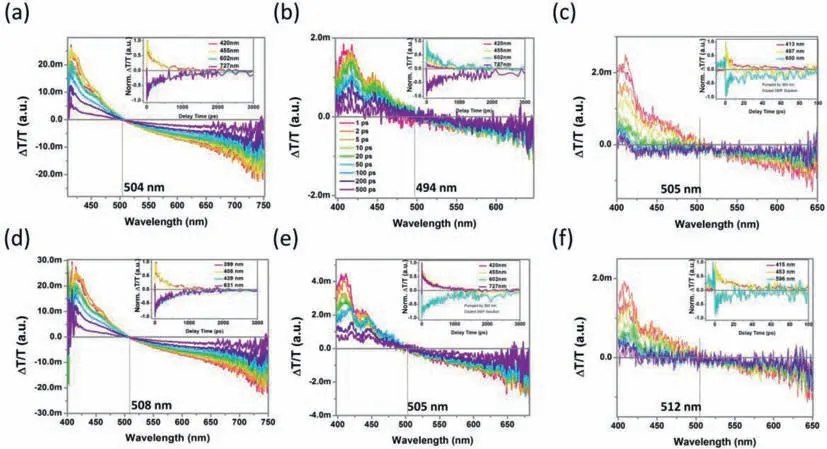
Fig.1.TA analysis of PPFOH in various states.The transient absorption spectra of PPFOH in (a,d) toluene and (b,e) DMF diluted and concentrated solution,and (c,f)their spin-coated film,respectively,which collected at different delay times after excitation.Inset showed the corresponding normalized pump-probe kinetics at selected wavelength.The excitation wavelength is 360 nm,beam diameter is 5 mm,and pump power is 30 μW.
In fact,steady optical analysis is not enough to comprehensively evaluate the photophysics of neutral excited species,especial the residual defect emission [29,57–59].Therefore,time-resolved photoluminescence analyses of PPFOH in various states are explored here to check the efficiency of the energy transfer from individual chain to defect structures (Fig.2 and Figs.S4–S7 in Supporting information) [54].In fact,the deep-blue emissions (high energy band) from individual chain of polyfluorenes always present a shorter life-time about ∼500 ps (Figs.S4 and S5) [55,60].However,the green-band emission from the defect structures,both fluorenone and excimers,had a long lifetime of>1 ns (Figs.S4 and S5)[31,45,49].First of all,fluorenone segments in the backbone structure of polyfluorenes will believably cause a strong green-band emission at long wavelength,even at individual chain in extremely dilute solution.Fig.2,Figs.S6 and S7 showed the extracted spectra with time decay from 0 ps to 9.1 ns.As we expected,the initial emission spectrum (obtained at 0 ps) of PPFOH DMF diluted solution consisted of well-resolved peaks at 420,440 and 465 nm,consistent with the steady PL spectrum.More interestingly,PL spectra of PPFOH in DMF diluted solution recorded at delay time from 0.0 ns to 9.1 ns are identically similar profiles,strongly confirmed the individual chain without any fluorenone segment in the mainchain backbone structures.More interestingly,there are similar PL spectral profiles of PPFOH in toluene solution obtained from 0.0 ns to 1.6 ns.However,residual green-band emission at 550–600 nm are appeared in the PL spectra of PPFOH dilute toluene solution recorded from 1.6 ns to 9.1 ns,different to those in dilute DMF solution.Combination of the results above,interchain or intrachain complex in the toluene solution may result into these weaker green-band emissions,caused by the strong hydrogen-bonding interaction,similar to the previous works [42,46,47].With increasing the solution concentration from 10-5mg/mL to 0.5 mg/mL,the emission region at 420 nm was disappeared in both PL spectra of PPFOH in diluted DMF and toluene solutions.However,it presents a strong green-band emission at 520–600 nm in the PL spectra of PPFOH DMF concentrated solution.Compared to PPFOH DMF solution,interchain hydrogen-bonding interactions in toluene solution are easily induced defect green-band emissions.Therefore,all experimental results effectively confirmed the aggregation mechanism in the green-band emission of polyfluorene.
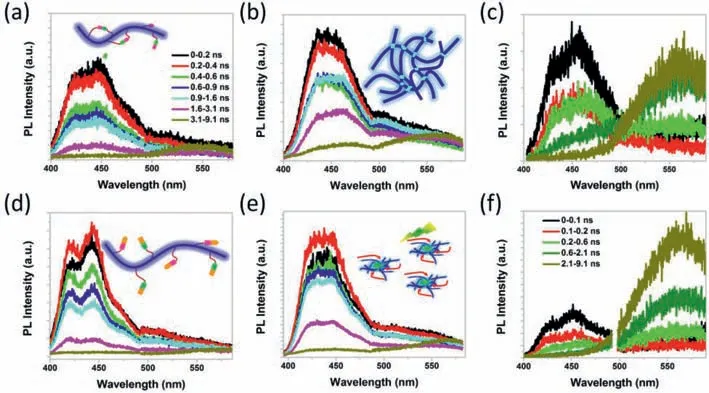
Fig.2.Pseudocolor time-resolved photoluminescence (TRPL) spectra of PPFOH in various states.Time-resolved PL spectra of PPFOH in (a,b) toluene and (d,e) DMF diluted and concentrated solution,and (c,f) corresponding spin-coated films obtained at different delay time range from 0.0 ps-9.1 ns.All PL spectra are obtained under excited by 360 nm laser source.
Compared to dilute solution states,it shows a relatively complicated Förster energy transfer in the solid states (Figs.2c and f,Fig.S3).Both PL spectra of two-type PPFOH films recorded at delay time of 0.0 ns had two emission bands at 425 and 460 nm,respectively (Fig.S3).However,blue emission band at 400–500 nm are weakened but a strong emission peak at 500–600 nm appeared in the PL spectra recorded after delay about 0.6 ns,revealed that the simple Förster energy transfer to induce exciton migrate from individual chain to aggregation defect structures.Obvious greenband emission at 500–600 nm is easily observed in the PPFOH spin-coated from DMF solution after delay time about<0.2 ns,supported the more efficient energy transfer than those obtained from toluene solution.According to previous work,the “amphiphilic”property of PPFOH chain in the polar DMF solution may cause the strong interchainπ-electron coupling of conjugated backbone structure,and result into a strong interchain aggregation.Meanwhile,freedom hydroxyl units are existed and distributed on the surface of film,different to the cross-linked framework in the PPFOH film spin-coated from toluene solution [49].These hydrogenbonded-assisted interchain aggregation of PPFOH plays a fundamental role to induce efficient energy transfer from individual chain to aggregation defect structure in PPFOH spin-coated from DMF solution than those from toluene ones.
As we discussed above,two types of green-band emission are observed in the PPFOH spin-coated films from DMF and toluene solutions.Here,we set DNT as an exciton trapped molecule to check this assumption (Fig.3).As displayed in Fig.3a,PPFOH spincoated films from DMF solutions have two feature emission peaks of single polyfluorene chain at 420 and 445 nm,together with a strong emission peak at 540 nm.And PL spectra of PPFOH film coated from toluene solution also consisted of two emission peaks at 460 and 555 nm (Fig.3c and Fig.S3),similar to the optical analysis above.When PPFOH films were exposed to DNT saturated vapor,the emission intensity was severely quenched (Fig.3).Fig.3a shows that the quenching intensity of PPFOH film coated from DMF solution is about 38% after 5 min and 90% after 60 min indicated that the excitons were mostly quenched by DNT molecules.However,the PPFOH film coated from toluene solution have a relatively low quenching efficiency of about 48% after 5 min and 66%after 60 min under the same experimental addition (Fig.3c).Strong hydrogen-bonding interaction between the freedom hydroxyl units and DNT molecules may explain this relatively high quenching effi-ciency for the PPFOH spin-coated film from DMF solution (Figs.3e and f).Interestingly,compared to the blue-emission region,similar green-band emission peak at 555 nm but weak emission intensity are found in the PL spectra of PPFOH film coated from toluene solution under prolonging the explosion time in the saturated DNT vapor,revealed the simple trapped exciton energy by the DNT molecules (Fig.3d).Remarkably,green-band emission of PPFOH spin-coated from DMF solution is red-shifted from 545 nm to 556 nm,about 11 nm,with prolonging the exposure time in the saturated DNT vapor (Fig.3b).However,the mechanism for this red-shift has not yet been elucidated,but whatever process is involved,all observations are two type green-band emissions for polyfluorene,directly supports the aggregation mechanism of green-band emission.
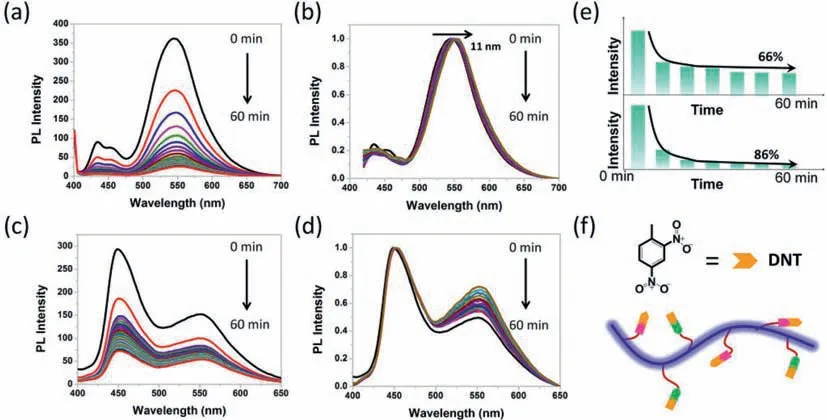
Fig.3.Time-dependent emission quenching processing of two type PPFOH films spin-coated from (a) toluene and (c) DMF solutions in DNT saturated vapor for 0–60 min(λex=390 nm),(b,d) together with their normalized PL spectra.(e) Efficiency of the time-dependent fluorescence quenching of their relative to the exposure times.(f)Detective mechanism of PPFOH chain on the DNT in films coated from DMF solutions.Hydrogen-bonding bond are formed between hydroxyl unit and DNT molecules in films coated from DMF solutions,which may reasonably explain this high quenching efficiency.
In summary,we set a supramolecular polyfluorene to systematically investigate the self-organization behavior of LCPs in various states toward confirming the aggregation defect mechanism of green-band emission in polyfluorenes.PPFOH presents an individual chain in dilute DMF solution but interchain or intrachain complex in the dilute toluene solution.More interestingly,significantly different to the feature emission spectra of single chain in DMF solution,residual green-band emission is observed in the PL spectra of PPFOH dilute toluene solution,effectively confirmed the aggregation mechanism in green-band emission.Efficient energy transfer from individual chain to defect structure is appeared in two type PPFOH films,according to the time-resolved photoluminescence analyses.Therefore,all experimental results above are effectively confirmed that interchain and intrachain aggregation may induce the green-band emission in polyfluorene.This result will inspire us to obtain high performance and stable deep-blue polyfluorene for light-emitting optoelectronic devices.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Nos.22105099,61874053),and Natural Science Foundation of Jiangsu Province (No.BK20200700),the open research fund from Anhui Province Key Laboratory of Optoelectronic Materials Science and Technology (No.OMST202101).
杂志排行
Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
