Insertion of pillar[5]arene into Tröger’s base-derived porous organic polymer for promoted heterogeneous catalytic performance in Knoevenagel condensation and CO2 fixation
2024-04-06LuLiuZiyiLiuJingnnCuiGuilingNingWeitoGong
Lu Liu ,Ziyi Liu ,Jingnn Cui ,Guiling Ning,b ,Weito Gong,b,∗
a State Key Laboratory of Fine Chemicals,School of Chemical Engineering,Dalian University of Technology,Dalian 116024,China
b Engineering Laboratory of Boric and Magnesic Functional Material Preparative and Applied Technology,Dalian 116024,China
Keywords: Pillar[5]arene macrocycle Porous organic polymers Heterogeneous catalytic Knoevenagel condensation CO2 fixation
ABSTRACT Development of new metal-free heterogeneous catalysts has long been the focus of intense research interest.The integration of multifunctional monomers into the skeletons of porous organic polymers (POPs)provides an efficient pathway to achieve this goal.Herein,we rationally designed and successfully prepared a new Tröger’s base (TB)-derived POPs by insertion of pillar[5]arene macrocycle as a positively auxiliary group.Combined the both merits of pillar[5]arene macrocycle and TB moiety,the as-prepared polymer was further explored as an effective metal-free heterogeneous catalyst and exhibited promoted catalytic performance in Knoevenagel condensation and CO2 conversion.This work provides a new strategy to fabricate metal-free heterogeneous catalysts based on macrocyclic POPs.
Heterogeneous catalysts based on various porous materials,including zeolites,silicas,activated carbon,and metal-organic frameworks (MOFs) [1–3],offer a promising recyclable platform for exploring abundant new organic transformations.Specifically,metalfree heterogeneous catalysts have garnered considerable attention from both the academic and the industrial communities due to their elemental sustainability and lost cost compared with their metal-containing counterparts.In recent years,porous organic polymers (POPs) have emerged as a versatile platform for the development of new metal-free heterogeneous catalysts,because of their structural diversity,tunable pore size,designability,and excellent thermal and chemical stability,etc.[4–10].Catalytic-active POPs can be fabricatedviaboth pre-synthetic and post-synthetic approaches by cooperative insertion of different functional groups into the skeletons of POPs,and the resultant catalytic performance is largely influenced by those adjustable chemical functionalities[11–15].Accordingly,the development of diverse combination of functional groups in POPs for enhanced catalytic performance is highly desired and continuously attracting growing research interests.
Tröger’s base (TB) and its analogues have long been the focus of intense research interest in broad applications,including molecular recognition,bioorganic chemistry,functional materials,and specifically metal-free catalysis [16–21].Featuring the unique structural properties of rigidity,V-shaped geometry,chiral C2symmetry,and multiple alkaline nitrogen atoms,the incorporation of TB group into POPs skeletons affords an attractive pathway to construct new metal-free heterogeneous catalyst.In particular,combining various other chemical functionalities with TB-derived POPs delivers further win-win merits and enhanced the corresponding performance.For example,Yu and Dai developed two porous TB polymers with tetraphenylethene and triazine units,respectively.Both of them showed enhanced CO2adsorption,heterogeneous catalysis,and sensing properties,demonstrating the vital role of cooperative chemical struts in TB-derived POPs [22,23].
More recently,macrocyclic cavitands,such as cyclodextrins,calix[n]arenes and pillar[n]arenes,with intrinsic cavity structure and excellent host-guest abilities,have been widely explored as appealing functional monomers to construct a new generation of macrocyclic POPs [24–28].Those macrocycle-derived POPs have shown promising applications in broad fields of environmental remediation,gas adsorption,heterogeneous catalysis,fluorescence sensing and ionic conduction.Among all these macrocyclic cavitands investigated,pillar[n]arenes exhibit privileged advantages over others due to their rigid cylinder architectures and diverse modifiable rims,making them attractive building blocks to be equipped into POPs for promoted performance [29–33].For instance,Yang fabricated several kinds of pillar[n]arene-inserted conjugated macrocycle polymers and all of them exhibited impressive catalytic,adsorptive,and sensing properties [34–38].In our previous work [39],we successfully constructed pillar[5]arenebased COF for the first time and it showed enhanced host-guest interactions in solid state.Despite increasing attention that has been paid to developing new pillar[n]arene-based POPs with multifunctionalities,studies on their metal-free heterogeneous catalysis are still largely unexplored.
Herewith,we rationally integrated pillar[5]arene macrocycle and TB moiety for the first time into one system to fabricate a new functional macrocyclic POPs (denoted as P5-TB) as the metal-free heterogeneous catalyst and explored its catalytic properties in two classic organic transformations,including Knoevenagel condensation between benzaldehyde derivatives with malonic acid and the cycloaddition of CO2with various epoxides.To clarify the positive role of the inserted pillar[5]arene macrocycle,a control TBbased polymer without pillar[5]arene,named as DA-TB,was also prepared.Combined both merits of pillar[5]arene macrocycle and TB moiety,as expected,P5-TB exhibited promoted catalytic performance for these two conversions compared with DA-TB.Especially,the apparent host-guest interactions between pillar[5]arene macrocycle and malonic acid was observed,which might be a favorable factor to promote the catalytic performance.We believe this work would provide a new insight for developing new metal-free heterogeneous catalysts from macrocyclic POPs.
The synthetic routes towards TB-based polymers P5-TB and DATB were shown in Scheme 1.A trifluoroacetic acid-catalyzed onepot polymerization reaction was performed between APP5/DA and dimethoxymethane at room temperature under an N2atmosphere.The infrared analysis confirmed the bonding and structural features of P5-TB and DA-TB.The absorption bands for amine at 3360 cm-1and 3450 cm-1disappeared,whereas the peak appearing around 1128 cm-1was attributed to the -C-N-stretching vibrations of the P5-TB and DA-TB (Fig.1a and Fig.S3 in Supporting information).Meanwhile,13CP-MAS NMR spectra displayed broad signals between 114 and 152 ppm,which were attributed to the carbon atom of the phenyl groups.The peak at 30 ppm was assigned to the methoxy carbon and benzyl carbon of pillar[5]arene.The signals that appeared at 67 and 55.8 ppm corresponded to the methylene carbon atoms of TB,clearly indicating the formation of TB linkages (Fig.1b and Fig.S4 in Supporting information)[18,23,40].
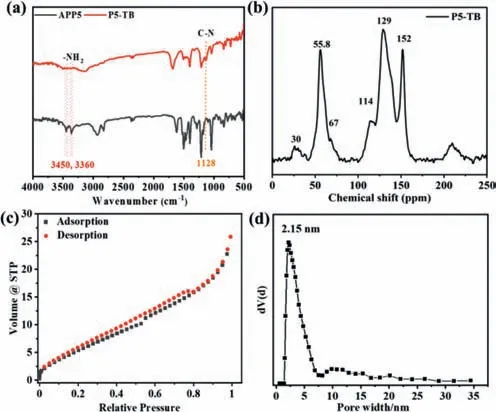
Fig.1.(a) FT-IR spectra of P5-TB.(b) 13CP-MAS NMR spectra of P5-TB.(c) Isotherm and (d) pore size distribution of P5-TB.

Scheme 1.Synthetic route to porous TB-derived polymers P5-TB and DA-TB.
TGA results under the nitrogen atmosphere indicated that the TB polymers possessed relatively high thermal stability with decomposition temperatures of approximately 350°C for P5-TB and 390°C for DA-TB,respectively (Figs.S5a and b in Supporting information).The analysis of the powder XRD pattern of P5-TB and DA-TB at room temperature showed the existence of a broad peak in the region from 16° to 26° (2θ) suggesting that the two polymers are amorphous (Figs.S5c and d in Supporting information).Further,SEM images revealed that both P5-TB and DA-TB consist of submicrometer-sized flakes (Fig.S6 in Supporting information).The porosity of P5-TB and DA-TB were evaluated by a nitrogen adsorption isotherm measured at 77 K.As shown in Fig.1c and Fig.S7a (Supporting information),the adsorption isotherm of P5-TB and DA-TB exhibited a pattern closely related to a Type IV isotherm according to the IUPAC classification.From the BET isotherms of DA-TB,the surface area was found to be 66 m2/g.However,the surface area of P5-TB was found to be 26 m2/g due to the extra space occupied by the pillar[5]arene (Fig.S8 in Supporting information).The pore size distribution plot of P5-TB and DA-TB displayed a mean pore width of 2.15 nm and 16.5 nm,respectively,further validating the mesopore nature of P5-TB and DA-TB (Fig.1d and Fig.S7b in Supporting information).Meanwhile,the pore volume of P5-TB and DA-TB was determined to be 0.040 cm3/g and 0.085 cm3/g,respectively,which is consistent with the fact that pillar[5]arene occupies a specific space (Table S1 in Supporting information).
Considering the presence of TB within both POPs,these two polymers might be used as heterogeneous catalysts for basecatalyzed reactions.In this respect,the base-catalyzed Knoevenagel condensation between benzaldehyde and malonic acid was initially chosen as the model reaction to evaluate the catalytic activity of both POPs.As shown in Fig.S9a (Supporting information),the different catalyst amounts have been tested to verify the effect of loading.It is noteworthy that 1.5 mol% catalyst loading was found to be the optimum.In addition,the reaction time is another parameter that needs to be optimized.Fig.S9b (Supporting information) shows that the conversion of benzaldehyde was maximum at 8 h.
Furthermore,we explored the reactions of malonic acid with various aromatic aldehydes under this standard condition.The yields of the substrates in the Knoevenagel condensation reaction were summarized in Table 1.It was clear that the reaction exhibited a certain degree of substituent effect and the aldehydes with electron-withdrawing groups gave higher yields compared with those counterparts with electron-rich groups (entries 2–8).However,when 9-anthraldehyde was selected as the substrate,no reaction was observed,which might be due to the steric effect (Table 1,entry 9).This result indicated the presence of pillar[5]arene macro-cycle in P5-TB could endow the catalyst potential shape selectivity.Importantly,compared to DA-TB,P5-TB was found to improve the conversion yield significantly (Table 1,entry 1b),which further implied the essential role of pillar[5]arene macrocycle played in the reaction.
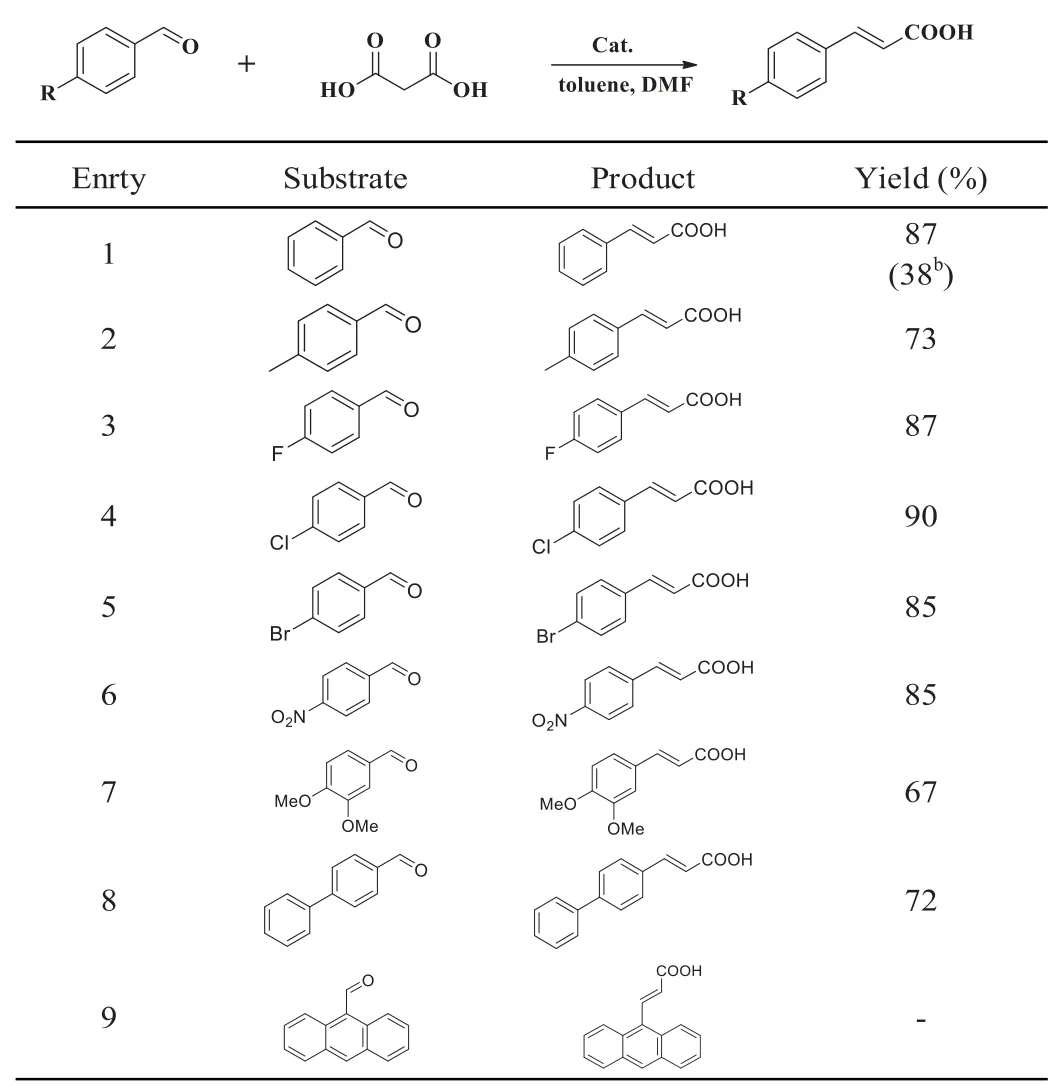
Table 1 Catalytic activity of the polymer for the heterogeneous Knoevenagel reaction.a
To verify the role of pillar[5]arene in this reaction,the hostguest properties of pillar[5]arene with malonic acid,and benzaldehyde were tested.The1H NMR experiment of host P5 in the absence and presence of guest malonic acid in DMSO–d6were explored and the spectra are shown in Fig.S10 (Supporting information).The carboxylate proton of malonic acid (H1) showed a substantial downfield shift (Δδ=0.40) and broadening effects in the presence of P5.Meanwhile,the aromatic (Ha) and methoxy proton(Hb) signals of P5 displayed upfield displacement.This result indicated that proton H1 resides in the cavity of P5,resulting from an inclusion complex.However,the1H NMR signals of benzaldehyde did not show a similar shift in the presence of P5 in DMSO–d6(Figs.S11–S13 in Supporting information).Therefore,we speculated that a plurality of weakly interacting sites provided by the cavity of P5,is preferable to facilitate anchoring of the malonic acid after entering the cavity,to reduce the molecular motion,and generate the confinement effect to activate the substrates.
On the other hand,there is an increasing research interest for the development of efficient catalyst to convert CO2into valuable products.In previous work,pillar[5]arene macrocycle exhibited high-adsorption affinity towards CO2enabled by its proper cavity size [41,42];however,the pillar[5]arene-based catalyst for CO2conversion has not yet to be explored.
With the aim to investigate the catalytic performance of P5-TB for CO2conversion,the isosteric heat of adsorption (QSt) plot of CO2was first calculated from isotherms recorded at 273 K and 298 K using the Clausius-Clapeyron equation for above TB-based polymers (Figs.S14c and d in Supporting information).As expected,P5-TB showed higher isotherms than DA-TB,indicating its stronger interaction affinity with CO2.Then,the catalytic activity of P5-TB and DA-TB was examined in which propylene oxide waschosen to react with CO2.As shown in Table S2 (Supporting information),the optimal reaction conditions were determined to be as follows: Catalyst amount of 2.0 mol% (20 mg),reaction time of 48 h,reaction temperature of 80°C,and pressure of 1 MPa.We further investigated the catalytic performance of P5-TB towards different epoxides under the optimum reaction conditions.As shown in Table 2,the small-sized linear epoxides (entries 1–4,propylene oxide,epichlorohydrin,1,2-epoxybutane,and allyl glycidyl ether)could be smoothly converted to corresponding cyclic carbonates in high yields.However,for large-sized cyclic substrates,such as cyclohexene oxide,only a 51.3% yield could be achieved (Table 2,entry 5).This might be ascribed to the steric effect of cyclohexene substituent which limited the ring-opening reaction of the epoxy substrate.Furthermore,DA-TB exhibited relatively lower yield in CO2conversion compared to that of P5-TB (Table 2,entry 1b),indicating the positive role of pillar[5]arene macrocycle played in this reaction through the enrichment of CO2owing to the matched pore size [25].
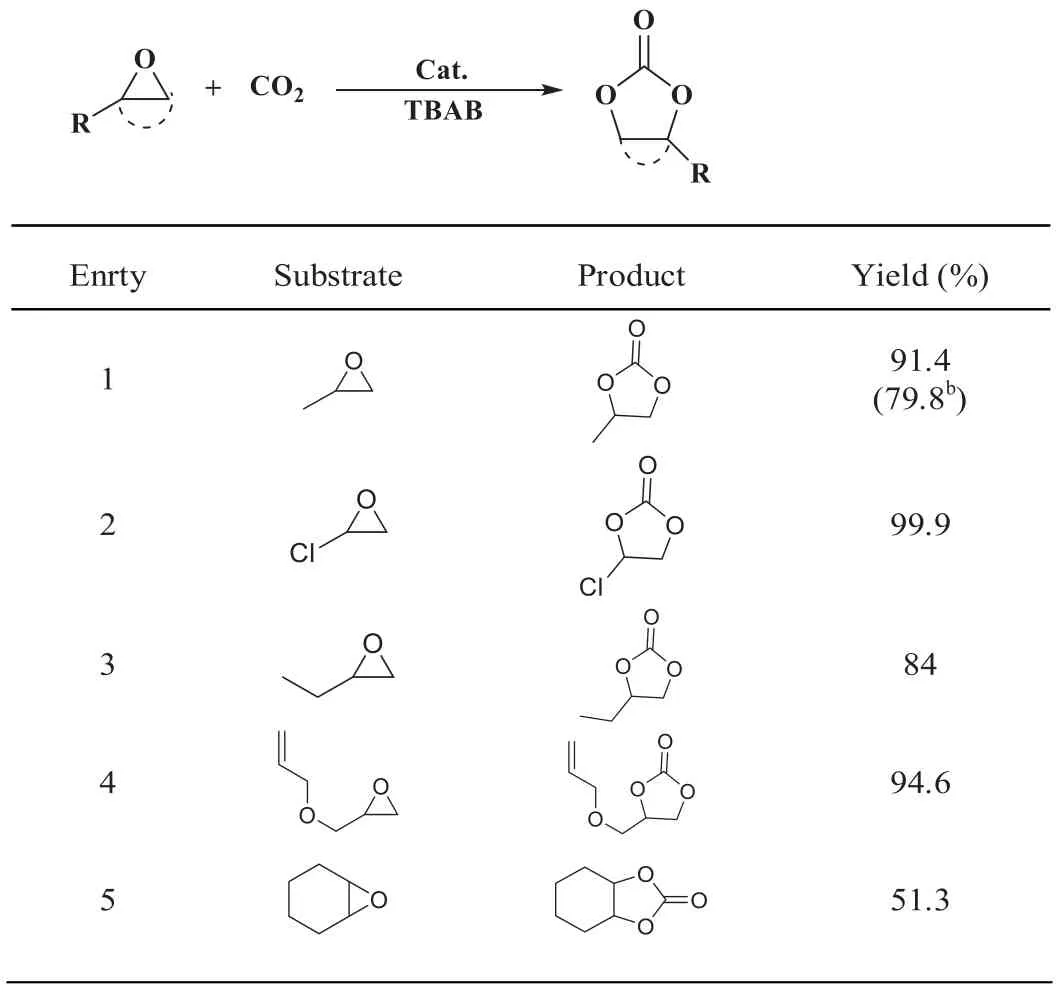
Table 2 Substrate scope of the cycloaddition of CO2 catalyzed by P5-TB.a
To evaluate the reusability of P5-TB,the used photocatalyst was collected and washed with DMF,EtOH and dried in vacuum for 24 h before the subsequent cycle.As presented in Fig.S15 (Supporting information),the Knoevenagel condensation and the CO2conversion can still reach a yield of 85% and 90%,respectively.This result suggested that P5-TB has the potential as a robust sustainable catalyst in some heterogeneous transformations.
In summary,two TB-derived POPs with and without pillar[5]arene macrocycle,named P5-TB and DA-TB,respectively,were successfully fabricated by one-pot reaction from APP5/DA and dimethoxymethane in TFA solution at room temperature.The catalytic performance of P5-TB and DA-TB in Knoevenagel condensation and CO2conversion were explored.Combining both merits of pillar[5]arene macrocycle and TB moiety,as expected,P5-TB exhibited promoted catalytic performance for these two conversions compared with DA-TB.In particular,the apparent host-guest interactions between pillar[5]arene macrocycle and malonic acid were observed,which might be a favorable factor in promoting the catalytic performance.This work would provide an insight into developing new metal-free heterogeneous catalysts from macrocyclic POPs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful for financial support from the National Natural Science Foundation of China (No.U1808210) and the Natural Science Foundation of Liaoning province (No.2019-MS-046).The authors also acknowledge the assistance of the DUT Instrumental Analysis Center.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108422.
杂志排行
Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
