Photoinduced palladium-catalyzed 1,3-diene-selective fluoroalkylamination compounds as potential bactericidal agent against Xanthomonas oryzae pv. oryzae
2024-04-06YuShiZhoShengZhngJingShoChenFuLnTuXiongZhoDongLiZiNingCui
Yu Shi ,Zho-Sheng Zhng ,Jing Sho ,Chen Fu ,Ln-Tu Xiong ,Zho-Dong Li ,Zi-Ning Cui,∗
a National Key Laboratory of Green Pesticide,Integrative Microbiology Research Centre,Guangdong Province Key Laboratory of Microbial Signals and Disease Control,College of Plant Protection,South China Agricultural University,Guangzhou 510642,China
b Henry Fok School of Biology and Agriculture,Shaoguan University,Shaoguan 512005,China
c College of Materials and Energy,South China Agricultural University,Guangzhou 510642,China
Keywords: 1,3-Diene-selective fluoroalkylamination derivatives Xanthomonas oryzae pv. oryzae Antibacterial activity Electron microscopy Reactive oxygen Flow cytometry
ABSTRACT A series of photoinduced palladium-catalyzed 1,3-diene-selective fluoroalkylamination derivatives was rationally synthesized based on diversity-oriented synthesis via cross coupling of 1,3-dienes,amines and fluoroalkyl iodides.The reaction featured good function group tolerance and a broad substrate scope,which could be extended to the late-stage modification of bioactive molecules.Bactericidal activity of all the compounds against Xanthomonas oryzae pv. oryzae (Xoo) was evaluated.Among them,compound E14 showed significant activity against Xanthomonas oryzae pv. oryzae (Xoo) with half maximal effective concentration (EC50) value of 6.61 μmol/mL.In pot experiments,the results showed that E14 could control rice bacterial blight with protective and curative efficiencies of 37.5% and 63.2% at 200 μg/mL,respectively.Additionally,a plausible mechanism for antibacterial behavior of E14 was proposed by electron microscopy,flow cytometry,reactive oxygen species detection,and biofilm assay.In current work,it can promote the development of photoinduced palladium-catalyzed 1,3-diene-selective fluoroalkyl amination compounds as prospective antibacterial agent bearing an intriguing mode of action.
Bacterial leaf blight (BLB) is a devastating worldwide rice disease caused by the pathogenXanthomonas oryzaepv.oryzae(Xoo),and is a Gram-negative bacterium that invades a broad range of host plants [1–3].Infection withXooresulted in the development of several symptomatic white leaf blight diseases,such as abnormal growth,leaf blight,and necrosis,which can reduce rice yields by 80% and pose a serious threat to food security [3,4].
Few agrichemicals are effective in preventing and eliminating diseases caused byXoo.The commonly used fungicides are bismerthiazol (BT) and thiodiazole (TC),however,the prolonged use of these fungicides has led to the production of resistant strains of BT and TC [5].Hence,the quest for innovative antibacterial agents remains a significant challenge,and there is a severe requirement for such chemical or biological agents to control the damage caused by the disease [6–9].However,the underlying mechanism of action of compounds against pathogens in most studies remained unexplored,resulting in a scarcity of targets that can be utilized for target-based molecular design [10].This aroused our interest to further explore novel bactericidal agents and to investigate the molecular mechanism of their inhibition of bacterial growth [11–13].Meanwhile,we hope to develop inhibitors for novel targets,which will provide possibilities for developing promising new agrochemicals.
Due to the unique fluorine effects on the lipophilicity,basicity,membrane permeability,and bioavailability of the parent molecules,fluoroalkyl motifs are very beneficial when included into organic frameworks [14].Fluoroalkyl groups can significantly enhance the lipophilicity,solubility,basicity,membrane permeability,and bioavailability of organic molecules,modulating the physicochemical and pharmacokinetic properties of compounds.Due to their significant potential for advancement in the field of pharmaceutical and agrochemical compounds,a large number of scientists are currently researching new,safe,and efficient fluorinated compounds [15].Fluoroalkylamines (perfluoroalkyl,trifluoromethyl,or difluoromethyl) are crucial building blocks for advanced intermediates in the pharmaceutical and pesticide industries because they can alter the chemical and biological properties of the target product [16].1,3-Diene is a versatile chemical raw material used in a variety of applications,including organic transformation,materials science,and the development of novel drugs.Complex compounds can be quickly and easily synthesized in one step by difunctionalizing 1,3-dienes [17].Despite significant progress in the bifunctionalization of 1,3-dienes,the selective fluoroalkyl amination of 1,3-dienes remains a challenging research topic [18].
Palladium catalysis induced by visible light is an emerging field of catalysis [19–21].In this study,we extended our previous work to synthesize a series of photoinduced palladium-catalyzed 1,3-diene-selective fluoroalkylamination derivatives.Subsequently,all title compounds were well-evaluated for potential bactericide againstXooby thein vitroandin vivoantibacterial abilities.Furthermore,a plausible mechanism for the antibacterial behavior of the target compound was proposed by electron microscope,flow cytometry,reactive oxygen species (ROS) assay,and biofilm assay.
First,we synthesizedγ-difluromethylated allylic amines product4a–4wby varying the different 1,3-dienes1with the best yield of 20%–87%.As show in Scheme 1,the desired product4a–4wcould be isolated in the following condition: dichloromethane(DCM) (c=0.05 mol/L) as the solvent,Pd(TFA)2(10 mol%) as the catalyst,Xantphos (20 mol%) as the ligand,NaNTf2(20 mol%)as the additive,and Cs2CO3(1.5 equiv.) as the base,under the blue light for 36 h.Compounds4a–4wwere subjected to primary screening for bactericidal activity by the inhibition circle method,and those with inhibitory effect were selected for further determination of their minimum inhibitory concentration (MIC).Unfortunately,none of the compounds exhibited inhibitory activity againstXoo(MIC>400 μmol/L).
Scheme 1.Synthetic route of target compounds 4a–4w.
Then,we replaced different amines2to further investigate their inhibitory activity.It can be seen in Scheme 2 that various amines can be reacted smoothly and the corresponding target productsγdifluoromethylated allylamine (5a–5p) were obtained in moderate to excellent yields.Among them,compound5oshowed good inhibitory activity againstXoo(MIC=62.5 μmol/mL).
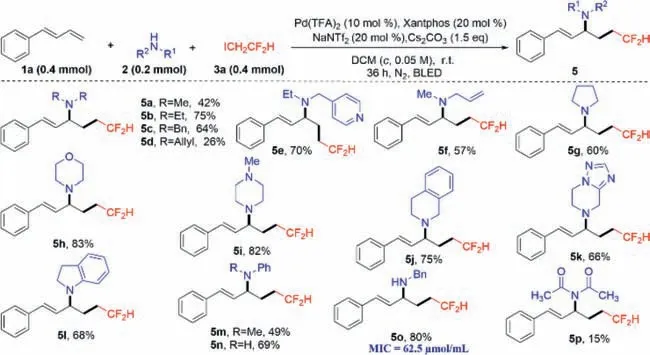
Scheme 2.Synthetic routes of target compounds 5a–5p.
After investigating the generality of 1,3-dienes and amines,the scope of fluoroalkyl iodides3was fully discussed (Scheme 3).Unfortunately,these products containing different fluoroiododecanes(6a–6h) showed poor inhibitory activity (MIC>400 μmol/mL).
Scheme 3.Synthetic route of target compounds 6a–6h.
Based on5o,we synthesized the title compoundsE1–E18(Scheme 4),whose antibacterial activity againstXoowas examined by the turbidimetric method,and this MIC value (blue letters) has been tagged in Scheme 4.All the title compounds were characterized and confirmed by1H nuclear magnetic resonance (NMR),13C NMR,and high-resolution mass spectra (HRMS) (Supporting information).
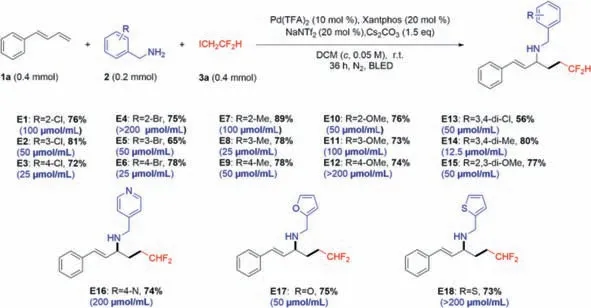
Scheme 4.Synthetic routes of target compounds E1–E18.
As listed in Table 1,five target compoundsE3,E6,E8,E13andE14demonstrated excellent antibacterial activity againstXoo.Among them,compoundE14demonstrated the best bactericidal activity againstXoo(MIC=12.5 μmol/mL).Subsequently,the results of the half maximal effective concentration (EC50) assay showed that compoundE14(EC50=6.61±0.36 μmol/mL) had the best bactericidal activity againstXoo,which was better than that of positive control bismerthiazol (EC50=15.71±0.27 μmol/mL)(Table 1).Therefore,E14was used in further study.

Table 1 Antibacterial activity of compounds E1–E18 against Xoo in vitro.
As shown in Fig.1 and Table 2,thein vivocurative activity and protective activity ofE14against bacterial leaf blight were evaluated.The results showed thatE14(dissolved with dimethyl sulfoxide (DMSO)) gave curative activity and protective activity of 63.62%and 37.5%,respectively,where the curative activity was higher than that of positive control BT (61.2%).Interestingly,the addition of 0.1% (v/v) organic sicilon or orange peel essential oil adjuvants could significantly enhance the surface wettability of compoundE14toward rice leaves,thus leading to improved control effectiveness (curative activity and protective activity can reach 67.49% and 43.06%,respectively).It also indicated thatE14(63.62%) had morepotential to be a therapeutic agent than that of the positive control BT (61.20%).
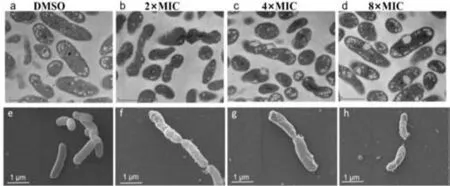
Fig.2.TEM (a-d) and SEM (e-h) images of Xoo treated with different amounts of the compound with E14.The scale bar of TEM and SEM image is 500 nm and 1 μm,respectively.

Fig.3.Accumulation of ROS in Xoo was monitored by fluorescence microscope after treatment with title compound E14 of DMSO (a),2× MIC (b),4× MIC (c) and 8× MIC (d).Scale bar: 10 μm.(e) ROS was detected by flow cytometry.DMSO is the negative control.All experiments were repeated at least three repeats.Asterisks indicate statistically significant differences.∗∗∗P < 0.001 vs.DMSO group.
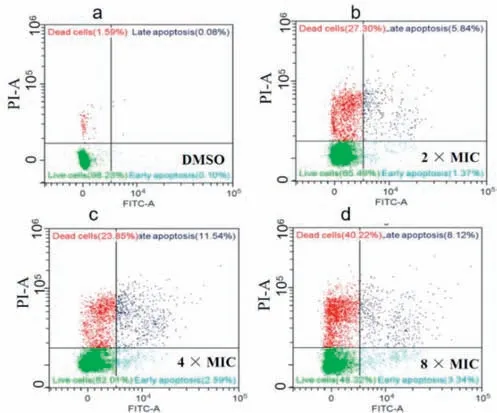
Fig.4.Apoptosis assays stained with Annexin V-fluorescein isothiocyanate (FITC)and propidium iodide (PI) using flow cytometry after treatment with compound E14 of DMSO (a),2× MIC (b),4× MIC (c) and 8× MIC (d).
Table 2 Protective and curative effects of title compound E14 on the BLB of rice.
The morphological changes and membrane integrity ofXootreated with compoundE14were further evaluated by scanning electron microscopy (SEM) and transmission electron microscopy(TEM).The scanning electron micrograph revealed that normal cells (treated with DMSO) ofXoowas typically rod-shaped with a normal,smooth,and bright surface without any apparent cellular debris,whileE14(2×,4× and 8× MIC) treatedXooshowed irregular shape with sunken surface (Figs.2a–d).TEM observations indicated that theXoocell membranes were heavily disrupted with noticeable irregular shape and morphology (Figs.2e–h).Serious structural changes were also evidenced by the presence of a large amounts of debris and distinct formation of potholes on the surface.The cell wall disruption instigated the leakage of the intracellular bacterial content.By electron microscopy,we concluded that compoundE14disrupts the membrane integrity,and causes the release of bacterial content,which eventually leads to the inhibition ofXoogrowth.
To further explore the possible antibacterial mechanism,the antibacterial behavior of compoundE14was investigated by fluorescence microscope,flow cytometry,apoptosis assay,ROS detection and biofilm formation.In this study,compoundE14bearing remarkable anti-Xooeffects was selected.AfterXoocells with various (2×,4× and 8× MIC) does of compoundE14,the fluorescence microscope and flow cytometry analysis results showed that apoptosis behaviors and ROS levels were also in a concentrationdependent manner (Figs.3 and 4).Subsequently,we treatedXoowith 2× MIC to examine the biofilm,and the results showed that the 2× MIC treatedXooalmost lost biofilm formation ability compared with the untreated (Fig.5).These results further indicate the promising potential ofE14as a prospective antibacterial agent forXoo.In view of the excellent control effect of the compoundE14againstXoo,the bactericidal spectrum of compoundE14was evaluated (Table S1 in Supporting information).The result showed thatE14also possessed obvious activity againstXanthomonas oryzaepv.oryzicola(Xoc) (MIC=12.5 μg/mL),Xanthomonas citrisubsp.citri(Xac) (MIC=25 μg/mL),andXanthomonas campestrispv.campestris(Xcc) (MIC=12.5 μg/mL).
Fig.5.Biofilm formation assay with the candidate compound E14 treatment.DMSO is the negative control.Asterisks indicate statistically significant differences.∗∗∗∗P < 0.0001 vs.wild type strain PXO99A (WT) group.
In summary,we synthesized a series of fluoroalkylated amines(total 47),and only compound5oshowed potential inhibitory activity againstXoowith MIC of 62.5 μmol/L.Based on5o,we synthesized a series of derivatives (E1–E18),and found thatE14demonstrated excellent antibacterial activity againstXoowith MIC(12.5 μmol/mL) and EC50(6.61±0.36 μmol/mL).E14showed better curative activity than that of the positive control in pot experiments.Additionally,a plausible mechanism for the antibacterial behavior of compoundE14was proposed by SEM assay,TEM assay,ROS detection,apoptosis assay,and biofilm assay.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China (No.32072450),the National Science Fund for Distinguished Young Scholars of Guangdong Province(No.2021B1515020107),the International Science and Technology Cooperation Program in Guangdong (Nos.2020A0505100048 and 2022A0505050060).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108794.
杂志排行
Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
