A H4SiW12O40-catalyzed three-component tandem reaction for the synthesis of 3,3-disubstituted isoindolinones
2024-04-06YufengLiuGuodongZengYutoChengLeiChenYunhiLiuYonggeWeiGuopingYng
Yufeng Liu ,Guodong Zeng ,Yuto Cheng ,Lei Chen ,Yunhi Liu,∗ ,Yongge Wei ,Guoping Yng,b,∗
a School of Chemistry,Biology and Material Science,Jiangxi Province Key Laboratory of Synthetic Chemistry,Jiangxi Key Laboratory for Mass Spectrometry and Instrumentation,East China University of Technology,Nanchang 330013,China
b Key Lab of Organic Optoelectronics &Molecular Engineering of Ministry of Education,Department of Chemistry,Tsinghua University,Beijing 100084,China
Keywords: Polyoxometalates Dehydrative coupling Three-component tandem reaction 3,3-Disubstituted isoindolinones Organic carbonate
ABSTRACT A H4SiW12O40-catalyzed three-component tandem reaction of 2-acylbenzoic acids,primary amines and phosphine oxides to form 3,3-disubstituted isoindolinones was developed.By employing H4SiW12O40 as the catalyst and dimethyl carbonate (DMC) as the solvent,a diverse range of 2-acylbenzoic acid derivatives and primary amines worked well to give the C3-phosphinoyl-functionalized 3,3-disubstituted isoindolinones with the yield range of 61%-87%.Advantages of this transformation include green catalyst and solvent,available starting materials,broad substrate scope,high efficiency and operational simplicity with water as the sole by-product.The strategy achieved an efficient and green molecular fragment assembly to access isoindolinones,which would provide opportunities for the synthesis of potential biologically active molecules in a green manner.
The 3,3-disubstituted isoindolinone compounds featuring quaternary carbon centers at the C3-position are ubiquitous heterocyclic motifs in many synthetic and naturally occurring molecules[1,2].The skeleton of 3,3-disubstituted isoindolinones is the core of numerous bioactive and pharmaceutically active compounds[3,4].For instance,Rhodamine spirolactam (A) exhibits great superiority for subcellular imaging [5,6].DrugBis used to treat cardiac arrhythmias [7].Furthermore,the 3,3-disubstituted isoindolinones with a heteroatom-functionality at the C3-position also show unique biological activities such as compoundCacting as an MDM2-p53 inhibitor (Scheme 1a) [8–10].On the other hand,organophosphorus compounds are widely used in organic synthesis,medicinal chemistry,agricultural chemistry,and materials science [11–16].Notably,phosphorus substituents can modulate important biological functions by mimicking the carboxylic acid functionality [17].Phosphorus-substituted isoindolinones that combine the advantage of both phosphorus moi- ety and isoindolinone scaffold,represent an important class of organophosphorus compounds [18].Therefore,the development of compound libraries based on phosphorus-substituted isoindolinone motifs is of great value for drug discovery [19,20].However,only a few methods have been reported for the preparation of 3,3-disubstituted isoindolinones with a phosphorusfunctionality at the C3-position.Singh’s group developed a chiral phosphoric acidPA5catalyzed asymmetric synthesis of C3-phosphorylated 3,3-disubstituted isoindolinones using 3–hydroxy-3-phenylisoindolinones as raw material (Scheme 1b) [21].Recently,Xu and co-works reported FeCl3-catalyzed phosphorylation of 3-methyleneisoindolinones for the construction of phosphorusfunctionalized 3,3-disubstituted isoindolinones (Scheme 1c) [22].Both approaches applied the strategy of direct functionalization of parent isoindolinones,which is not conducive to the diversity and complexity of the constructed products.It is still challenging to develop efficient and practical methods to construct C3-phosphinoylfunctionalized 3,3-disubstituted isoindolinones from simple starting materials,especially in a green and sustainable manner.
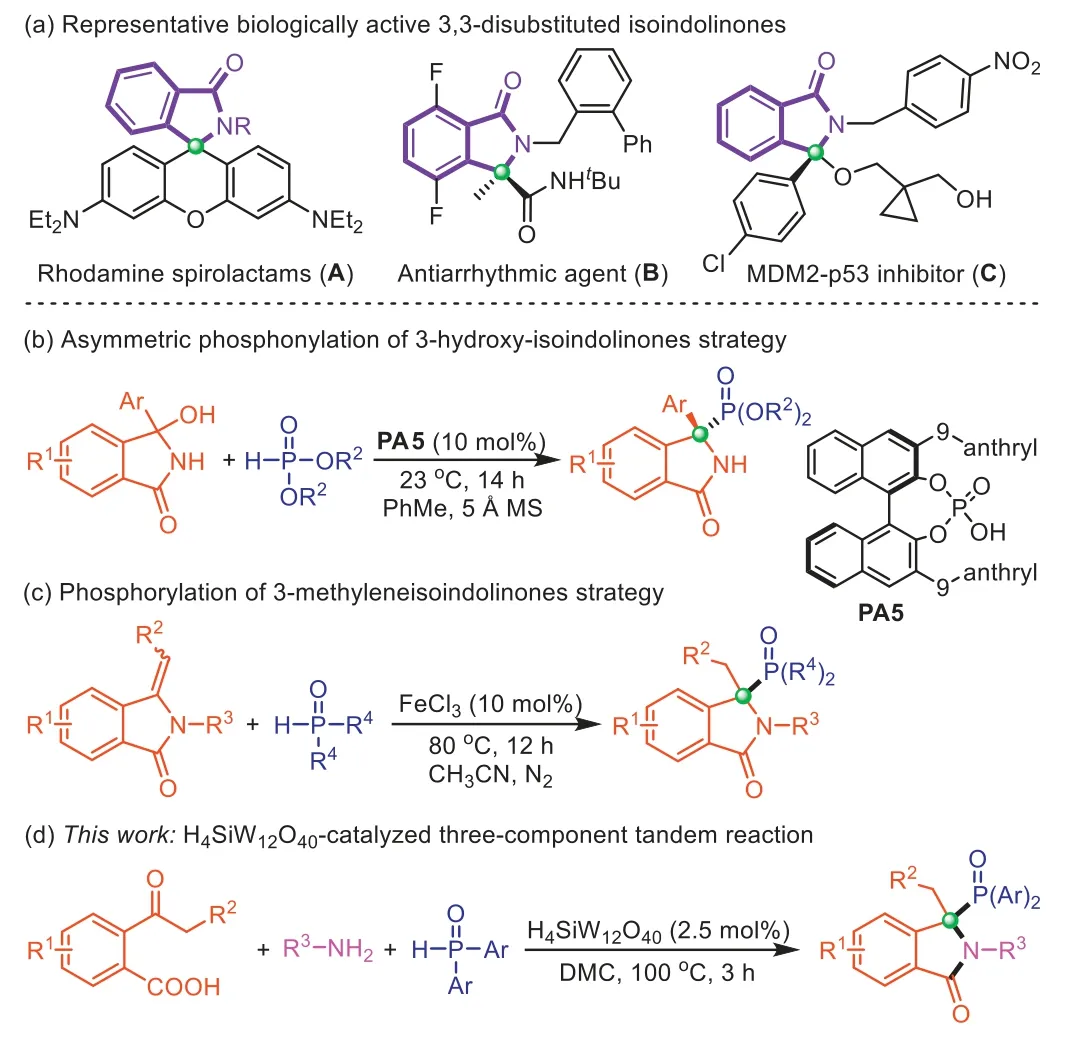
Scheme 1.Background of 3,3-disubstituted isoindolinones synthesis and our strategy.
Multicomponent reactions (MCRs) consist of a process involving the condensation of three or more reactants.The obtained products are formed in a single step from simple starting materials in high atom efficient reactions.The possibility of applying diverse reagents makes them ideal for creating new molecular libraries[23–27].In this regard,MCRs is one of the most useful and ideal tools for the synthesis of versatile 3,3-disubstituted isoindolinones.In addition,polyoxometalates (POMs) are a well-known class of anionic metaloxo clusters with diverse structures and excellent properties [28–35].Given their tunable acidity and redox properties,good thermal stability and environmental friendliness,POMs were widely used as a kind of green catalyst in organic synthesis [36–42].Particularly,our previous works demonstrated that POMs are practical and excellent catalysts for the construction of heterocyclic compounds [43–47].In this regard,POMs would be a promising catalyst for the synthesis of 3,3-disubstituted isoindolinones.
Based the above considerations,we described the first MCRs for the preparation of C3-phosphorylated 3,3-disubstituted isoindolinonesviathe condensation of 2-acylbenzoic acids,primary amines and phosphine oxides using H4SiW12O40as an environmentally benign and inexpensive catalyst,and DMC as the green solvent (Scheme 1d).The simple starting materials,green catalyst and solvent,and water as the sole by-product make this method suitable for the 3,3-disubstituted isoindolinones library synthesis.
Initially,2-acetylbenzoic acid (1a),hexylamine (2a) and diphenylphosphine oxide (3a) were employed as the model substrates to optimize the reaction conditions (Table 1,Tables S1–S5 in Supporting information for more details).A variety of heteropolyacids (HPAs) (2 mol%) as Brønsted acid catalysts were screened in PhCl (1 mL) in a sealed tube at 100 °C for 2 h at first.It was revealed that H4SiW12O40performed better than other HPAs such as H3PW12O40and H3PMo12O40,affording product4ain 61% yield(entries 1–3).Other Brønsted acids likep-TSA andPA5did not improve the yields compared to HPAs (entries 4 and 5).In addition,Lewis acids such as CuCl2,CoCl2and FeCl3were also tested as catalysts,but none of them could catalyze this transformation with higher yield (entries 6–8).Control experiments indicated that H4SiW12O40played a crucial and essential role in this threecomponent reaction.Subsequently,the solvents screening showed the transformation was strongly influenced by the solvents,and DMC turned out to be the best choice resulting in a 73% yield (entries 9–13).The yield of4acould not be increased by changing the reaction temperature (entries 14 and 15).Applying a longer reaction time (3 h) and more catalyst loading (2.5 mol%),led to the desired product4awith 80% and 89% yield,respectively (entries16 and 17).Finally,the optimum results were obtained when 2-acetylbenzoic acid (1a,0.2 mmol),hexylamine (2a,0.2 mmol) and diphenylphosphine oxide (3a,0.2 mmol) were treated with 2.5 mol% of H4SiW12O40in DMC (1 mL) in a sealed tube at 100 °C for 3 h.
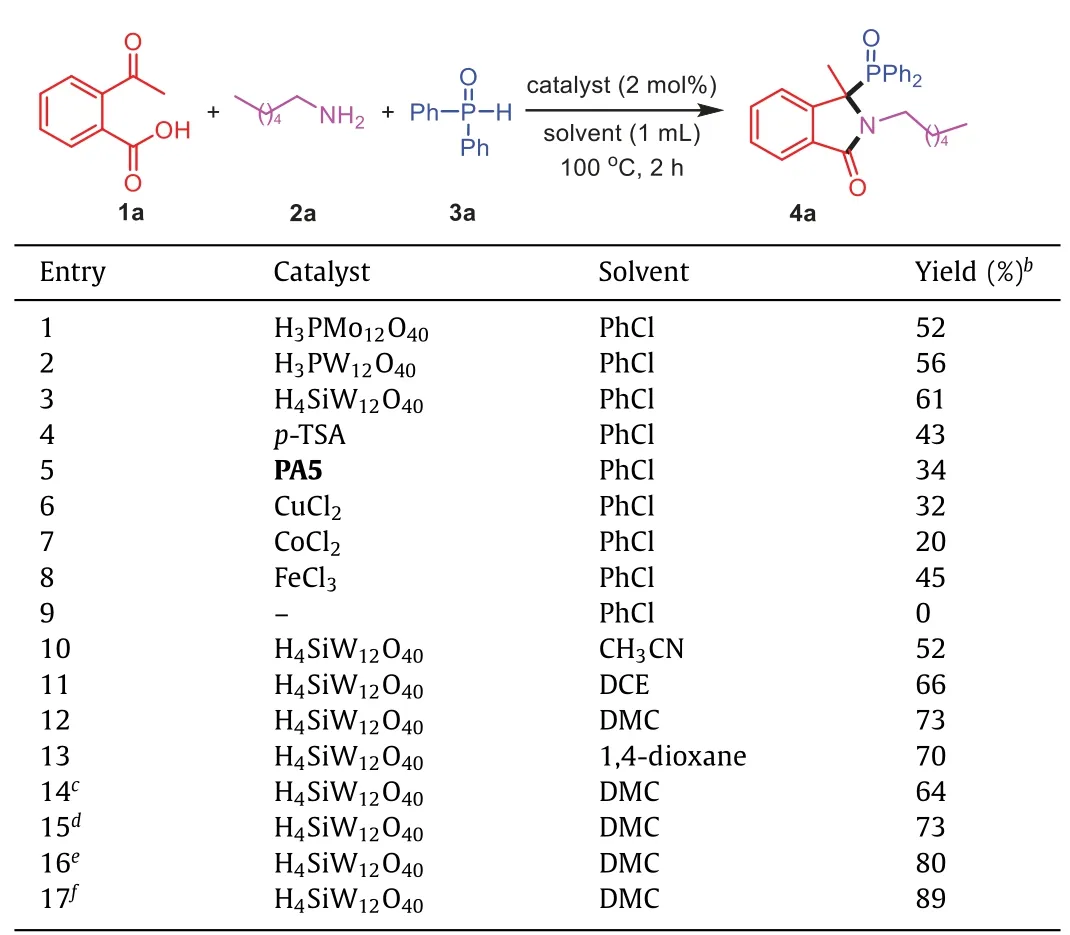
Table 1 Optimization of reaction conditions.a
Next,the substrate scope of this three-component reaction process was investigated under the optimized conditions.We first explored a range of 2-acetylbenzoic acids (1) with hexylamine (2a) and diphenylphosphine oxide (3a) as partners.As shown in Scheme 2,2-acetylbenzoic acids bearing either electronwithdrawing or electron-donating groups are efficient substrates,giving the desired 3,3-disubstituted isoindolinones4a-4hin good yields.Furthermore,2-propionylbenzoic acid was also tolerated well,delivering the corresponding product4iin 84% yield.
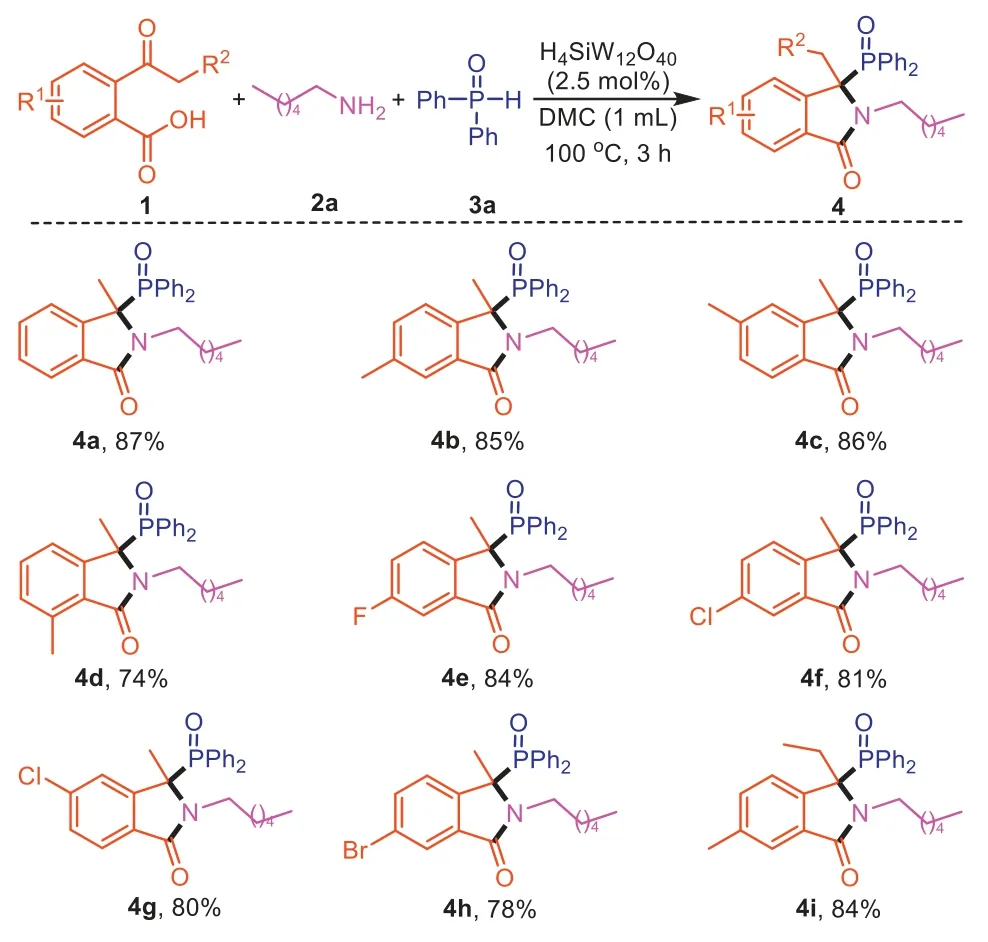
Scheme 2.Scope of 2-acylbenzoic acids for the synthesis of 3,3-disubstituted isoindolinones.Reaction conditions: 2-acylbenzoic acid (1,0.2 mmol),hexylamine (2a,0.2 mmol),diphenylphosphine oxide (3a,0.2 mmol),DMC (1.0 mL),H4SiW12O40 (2.5 mol%) for 3 h.
After having exhibited a broad scope for substituted 2-acetylbenzoic acids,a variety of primary amines (2) were next investigated to react with1aand3aunder the same optimized conditions and the results are summarized in Scheme 3.A wide variety of structurally and electronically diverse primary alkyl amines can be successfully subjected to this transformation.Thenbutylamine,i-butylamine,and cyclopropanemethylamine are competent coupling partners,furnishing the desired products in good isolated yields (4j-4l).Interestingly,unprotected N–H isoindolinone(4m) can be prepared by using ammonium acetate as substrate.For the substrate of benzylamines,a slightly increased amount of2and3awas necessary to reach full conversion.Benzylamines with electron-donating or -withdrawing groups reacted smoothly to afford the desired products with moderate yields (4n-4u).The structure of product4nwas characterized by single-crystal X-ray diffraction analysis.Substituents such as -CH3,-OCH3,-F,-Cl,-Br on the benzene ring of2were well tolerated,the electronic property of which slightly affected the reactivity of amine and electron-rich amine afforded better yields.In the case of phenethyl amines,the corresponding product4vand4wwere obtained in the yield of 80% and 61%,respectively.
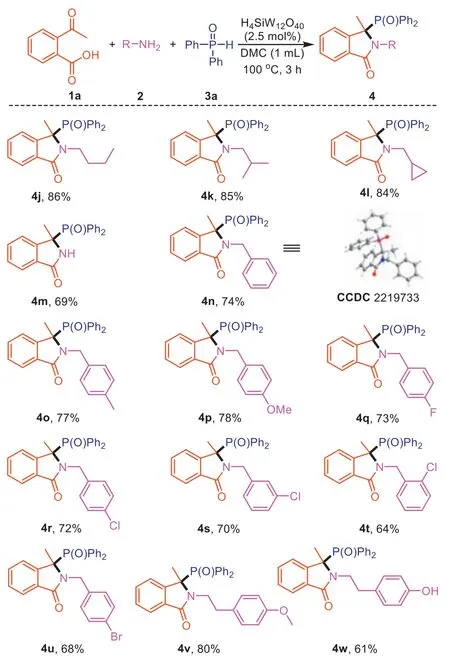
Scheme 3.Scope of primary amines for the synthesis of 3,3-disubstituted isoindolinones.Reaction conditions: 2-acetylbenzoic acid (1a,0.2 mmol),primary amines(2,0.3 mmol),diphenylphosphine oxide (3a,0.3 mmol),DMC (1.0 mL),H4SiW12O40(2.5 mol%) for 3 h.
Furthermore,we examined the scope of H-phosphine oxides3using1aand2aas coupling partners (Scheme 4).Generally,H-phosphine oxides substituted with electron-donating (-Me,-OMe) or electron-withdrawing group (-Cl) were both suitable reaction partners,and the corresponding products were obtained in 72%–85% yields (4x-4aa).
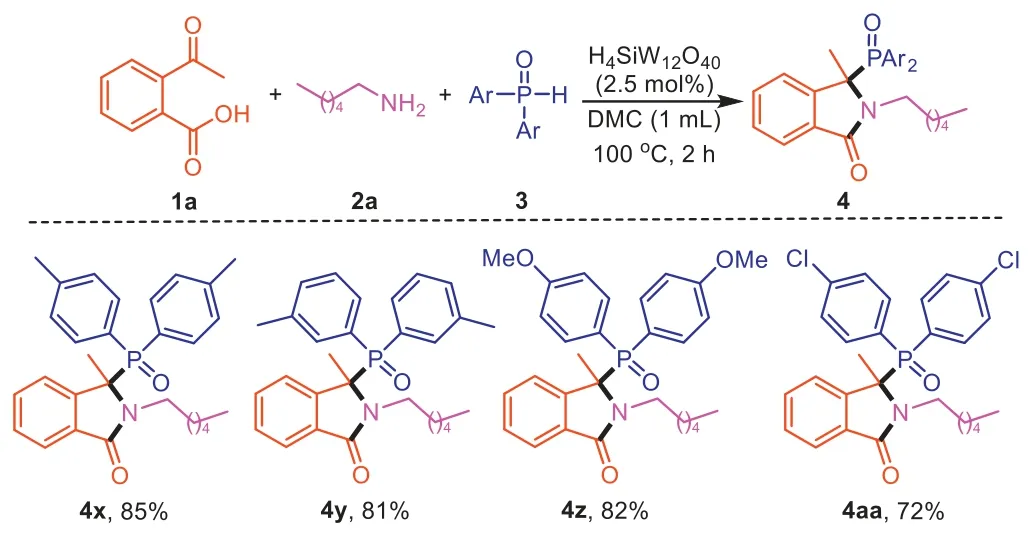
Scheme 4.Scope of diarylphosphine oxides for the synthesis of 3,3-disubstituted isoindolinones.Reaction conditions: 2-acetylbenzoic acid (1a,0.2 mmol),hexylamine (2a,0.2 mmol),diphenylphosphine oxide (3,0.2 mmol),DMC (1.0 mL),H4SiW12O40 (2.5 mol%) for 3 h.
To determine the practicability of this method,a gram-scale reaction was performed with1a(5 mol),2a(5 mol) and3a(5 mol)to synthesize4aand the reaction proceeded smoothly to give the product in good yield (4a,80%,1.73 g).The result demonstrated the potential application of this protocol in large-scale production(Scheme 5).
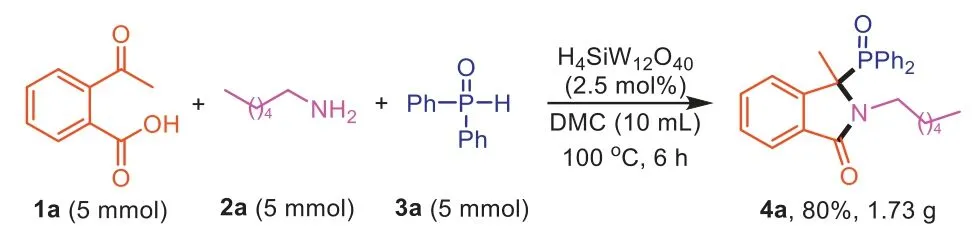
Scheme 5.Gram-scale reaction.
In order to have a deeper understanding on how the three compounds combine together,and get some insight into the mechanism,several control experiments were conducted (Scheme 6).In the presence of radical inhibitor: BHT,4awas still obtained in 83% yield,indicating that a radical pathway should be excluded(Scheme 6a).The reaction of1aand2aled to the formation of the compoundIV(Scheme 6b).On the other hand,the reaction of1aand3ain the absence of2awas performed,no reaction was observed (Scheme 6c).Furthermore,when3awas reacted with compoundIVunder standard conditions,the desired product4aobtained in 86% yield (Scheme 6d).These results proved thatIVwould be the key intermediate for this transformation.Without H4SiW12O40,1acould not be converted toIV,andIVcould also not be converted to4a,which means H4SiW12O40plays an essential role in the whole reaction process.
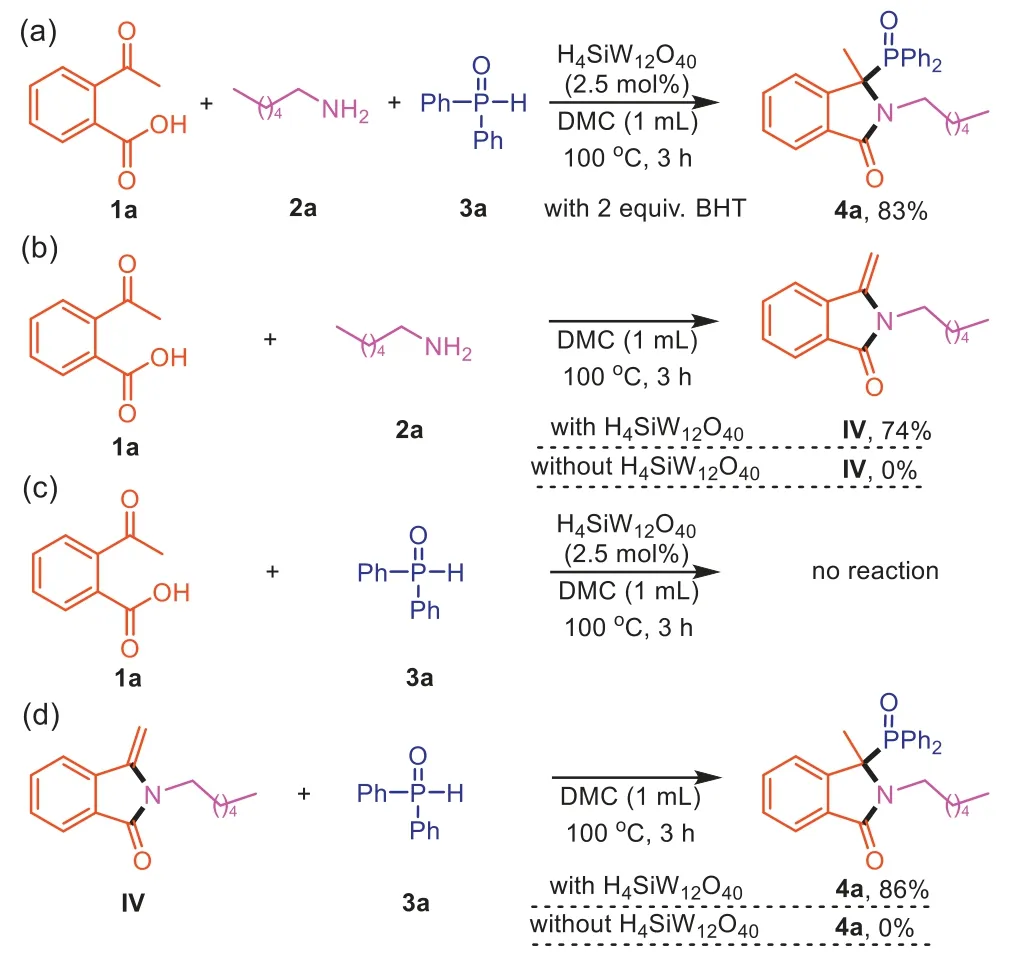
Scheme 6.Control experiments.
Based on the above experimental results and previous literature reports [22,48,49],a plausible mechanism was proposed in Scheme 7.The reaction initially goes through the formation of imineI,which was protonated by H4SiW12O40to generate a higher electrophilic intermediateII.Next,the nucleophile imine attacked carbonyl to provide the intermediateIII,followed by the elimination of H2O and regeneration of catalyst H4SiW12O40to afford the key intermediateIV.The acyliminium intermediateVis generated fromIVin the presence of Brønsted acid H4SiW12O40.Then,3aas the nucleophile reacts with the acyliminium intermediateVto form intermediateVI,which produces the product4aalong with releasing the catalyst H4SiW12O40.
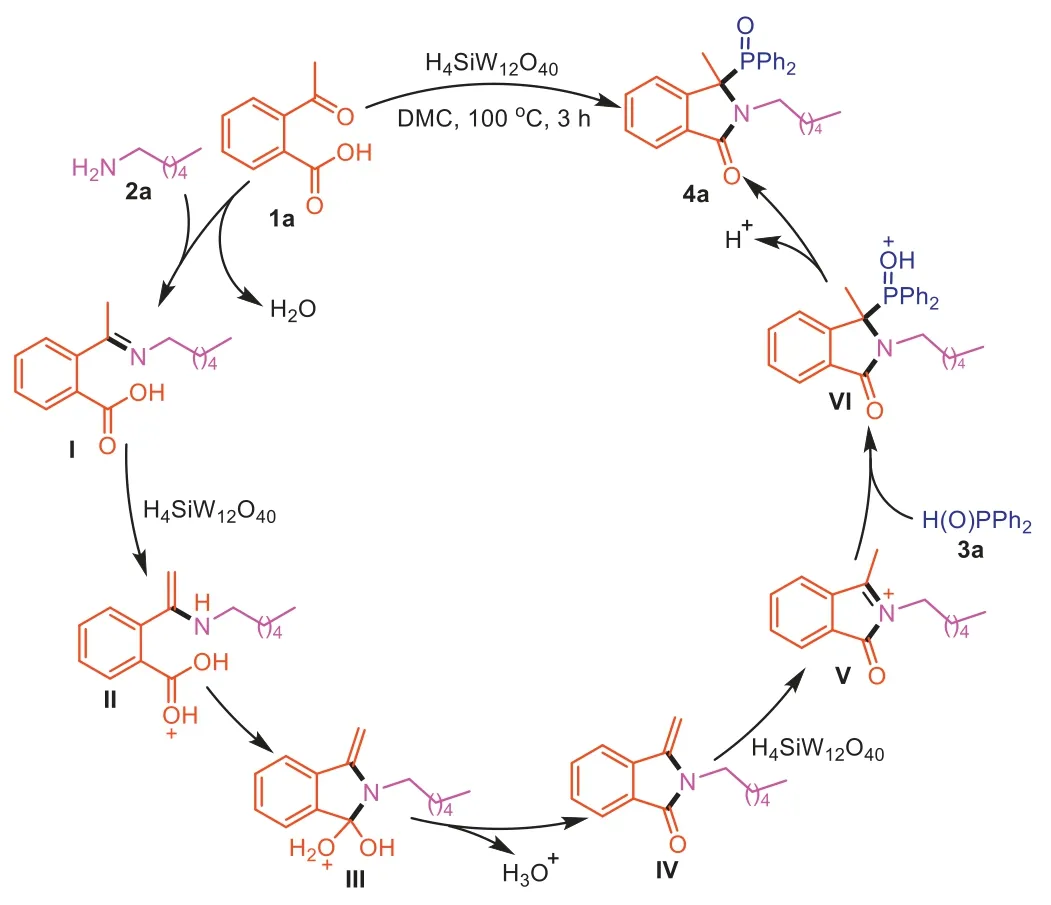
Scheme 7.Possible reaction mechanism.
In conclusion,we have developed the first facile and efficient H4SiW12O40-catalyzed three-component tandem reaction for the synthesis of 3-phosphoryl-substituted isoindolinones from readily accessible 2-acylbenzoic acids,primary amines and phosphine oxides in one pot.Importantly,the environmentally benign and inexpensive catalyst,green organic carbonate solvent DMC,available starting materials and water as the sole by-product mean that this protocol provides a green and efficient cyclization pattern for the synthesis of potential biologically active C3-phosphinoyl-functionalized 3,3-disubstituted isoindolinones,which promises potential applications in synthetic and medicinal chemistry.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported the National Natural Science Foundation of China (No.22001034),Jiangxi Provincial Natural Science Foundation (No.20212BAB213001).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108480.
杂志排行
Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
