Cu single atoms embedded on hollow g-C3N4 nanospheres with enhanced charge transfer and separation for efficient photocatalysis
2024-04-05LinlinZhngJinjunLioYkunLiWeiSunChengjunGe
Linlin Zhng ,Jinjun Lio,* ,Ykun Li ,Wei Sun ,Chengjun Ge,*
a Key Laboratory of Agro-Forestry Environmental Processes and Ecological Regulation of Hainan Province,School of Ecology and Environment,Hainan University,Haikou 570228,China
b Shandong Ocean Chemical Industry Scientific Research Institute,Weifang 262737,China
Keywords: g-C3N4 Cu single atom Hollow nanospheres Charge transfer pathways Photocatalytic hydrogen production
ABSTRACT Establishing an effective charge transfer mechanism in carbon nitride (g-C3N4) to enhance its photocatalytic activity remains a limiting nuisance.Herein,the combination design of a single Cu atom with hollow g-C3N4 nanospheres (Cu-N3 structure) has been proven to offer significant opportunities for this crucial challenge.Moreover,this structure endows two pathways for charge transfer in the reaction,namely,the N atoms in the three-dimensional planar structure are only bonded with a single Cu atom,and charge transfer occurs between the plane and the layered structure due to the bending of the interlayered g-C3N4 hollow nanospheres.Notably,Cu-N3 and hollow nanosphere structures have been certified to greatly enhance the efficiency of photogenerated carrier separation and transfer between the layers and planes by ultrafast spectral analysis.As a result,this catalyst possesses unparalleled photocatalytic efficiency.Specifically,the hydrogen production rate up to 2040 μmol h-1 g-1,which is 51 times that of pure C3N4 under visible light conditions.The photocatalytic degradation performance of tetracycline and oxidation performance of benzene is also expressed,with a degradation rate of 100%,a conversion of 97.3% and a selectivity of 99.9%.This work focuses on the structure-activity relationship to provide the possibilities for the development of potential photocatalytic materials.
Photocatalysis is a promising new energy conversion technology and pollutant treatment technology [1-4].Polymeric carbon nitride (g-C3N4),as a common organic semiconductor photocatalysts,has been applied in the fields of energy and environmental remediation due to its visible light responsiveness,robust stability,high wear resistance,excellent physicochemical properties and low synthesis cost [5-9].However,the low charge separation and transfer efficiency of bulk C3N4restrict its further development [10-13].To overcome the challenge of constraints and enhance unsatisfactory performance,various effective strategies have been probed,such as the establishment of defects,doping of heterogeneous elements,construction of heterojunctions,and nanostructures [14-19].Nevertheless,it is imperative to develop and use multiple charge transport tunnels.
The ultra-high conductivity metal seems to be the best candidate for modifying g-C3N4.In particular,the introduction of atomsized metals can not only maximize the utilization of metals but also improve the charge separation and transfer efficiency of g-C3N4[20-23].For example,various noble or non-noble metal single atoms (Au,Pt,Pd,Cu,Co,etc.) have been immobilized on g-C3N4,because these metal atoms can be anchored at rich N sites[24-26].The metal atom fixed carbon nitride can effectively improve photocatalytic performance because the atom acts as an effective electron acceptor in the separation and transfer of photogenerated carriers in the plane [27-30].Thus,the introduction of metal atoms in the g-C3N4plane of the pyrazine (herbicide) conjugate can change the spatial charge transfer pathway [31].However,in-plane doping alone is insufficient to further promote photocatalytic performance,among which,the most effective challenge is to improve the charge transfer between layers (adjacent layers of g-C3N4).This is mainly because the intrinsic planar layered structure of g-C3N4is destroyed along with the weakening of electron transfer in the flat layer [32,33].The basic understanding is that the electronic structure of g-C3N4consists of sp2hybridπdelocalized electrons and lone electrons in N-pz orbit [34,35].Bending the structure of g-C3N4from the plane structure to the spherical structure will cause the electronic structure to deform and compress in the mixed intermediateness between sp2and sp3,further causing the delocalizedπelectrons to migrate from the concave to the convex [36,37].The formation of a hollow spherical structure is an effective method for bending layered g-C3N4.This will eventually result in a significant potential difference between the inner and outer surfaces of the g-C3N4hollow nanospheres due to the uneven distribution of electrons.Therefore,the potential difference of g-C3N4hollow nanospheres as the motive power will stimulate the e-to conquer the electrostatic barrier of the interlayer and shift to the outer layer of g-C3N4hollow nanospheres [38,39].In addition,the hollow nanosphere structure can enhance the light scattering and photosensitivity of g-C3N4or their composites.In a word,hollow sphere structure is an effective method to improve photocatalytic performance,which has been widely proved.
Therefore,we prepared hollow g-C3N4nanospheres (HC3N4)and loaded Cu single atoms as electron acceptors on the surface of Cu-HC3N4.Multiple testing methods demonstrated that single Cu atoms were embedded in the C3N4plane,and had an enormous capacity to enhance charge separation and transfer within the plane.The hollow nanosphere structure enhanced charge separation and transfer in the interlayer.Therefore,under visible light irradiation,Cu single atom-modified,hollow g-C3N4nanospheres exhibited excellent performance in the selective oxidation of benzene to phenol and photocatalytic hydrogen evolution.The molecular structure engineering strategy of introducing the bending of the delocalizedπelectrons of g-C3N4provided a new option for promoting oriented charge transfer between nanolayers and is applicable to other single-atom functional materials.
As shown in Fig.1a,we used SiO2as the hard template to synthesize HC3N4nanospheres by thermal polymerization.Then,monoatomic copper is loaded,and finally hollow carbon nitride loaded with monoatomic copper is formed.Single atoms of copper show bright spots in high resolution high angle ring dark field(HAADF) to determine whether it exists.As shown in Fig.1b,multiple bright spots with uniform size and uniform distribution are presented,proving that the product contains Cu in the form of a single atom.Furthermore,the synthesized HC3N4is composed of C,N and Cu elements and proved by element mapping.More excitingly,it can be observed that Cu is uniformly distributed on the entire carbon nitride carrier.The loaded copper content was monitored as 0.50±0.02 wt% by inductively coupled plasma atomic emission spectrometry (ICP-AES).As displayed in Fig.S2 (Supporting information),the Cu-HC3N4is the nanospheres structure.Furthermore,it can be seen from the mapping image and Fig.1c that the prepared sample is a hollow nanosphere structure.And the Cu element is evenly distributed in the hollow spherical shell.
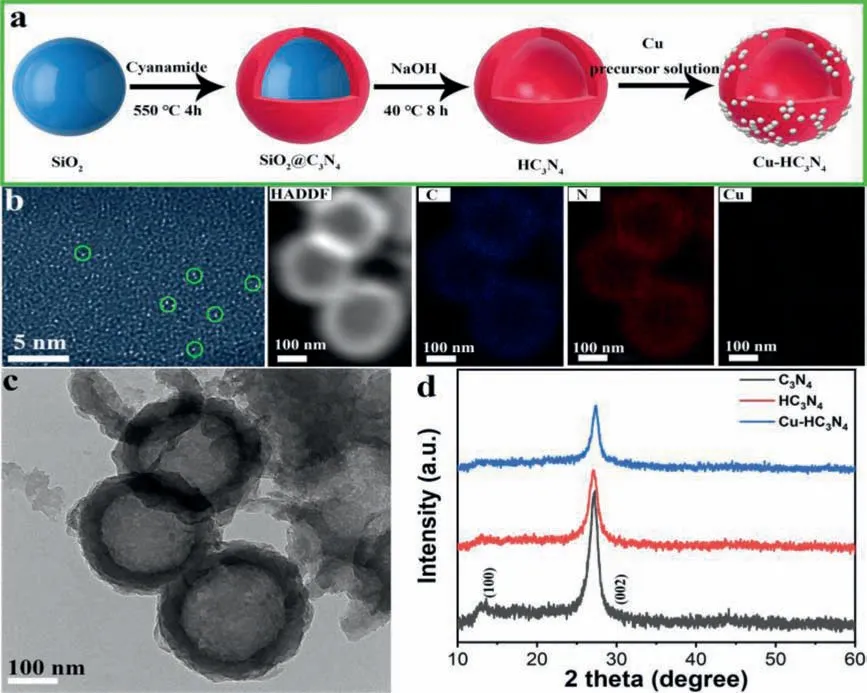
Fig.1.(a) Scheme of Cu single atom doted HC3N4.(b) HAADF-STEM,HADDF,mapping image of Cu single atom HC3N4.(c) TEM of Cu-HC3N4 composite.(d) XRD patterns for C3N4,HC3N4 and Cu-HC3N4 composite.
Fig.1d shows the XRD patterns of HC3N4with Cu single atom loadings,C3N4and HC3N4.For C3N4,a very distinct XRD diffraction peak situated at 27.7°,which is attributed to the interlayer stacked reflectance of the conjugated aromatic hydrocarbon system[5].The signature peak at 2θis the typical (002) peak to indicate the graphite material [40].Otherwise,for HC3N4,the peak with intensity slightly higher than the noise appears at 2θof 13.3°,showing an in-plane repeated structural unit of tri-s-triazine.It can be seen that compared with C3N4,hollow g-C3N4nanospheres have a less porous and layered structure.Compared with the former two,there is almost no (100) peak in Cu-HC3N4,which proves that the aromatic structure is destroyed [9].Fig.S3 (Supporting information) gives the FTIR spectroscopy to clarify the structural composition of the synthesized photocatalyst.It is obvious that all the synthesized photocatalysts display the analogous FT-IR signals.Specifically,the characteristic peak loaded at 813 cm-1represents the condensed heptazine heterocyclic ring (C6N7).The peaks at 1545 cm-1,1328 cm-1,and 1232 cm-1correspond to C=N(sp2),C-N(sp2) in the heptazine unit and the C-NH-C bond in melamine,respectively,which prove the composition of the basic heptazine unit of g-C3N4[41-45].The pronounced broad spectral band is in the range of 3000-3800 cm-1,which is mainly due to the N-H band formed by the uncondensed amine group and the ·OH formed by the adsorption of H2O on the surface [5].
X-ray absorption spectroscopy (XAS) was used to determine the valence structure and coordination environment of Cu in the synthesized Cu-HC3N4sample.As displayed in Fig.2a,the absorption edge of the Cu-HC3N4sample is located between the absorption edges of CuO and Cu2O (Cu K-edge X-ray absorption near edge structure spectrum,XANES),demonstrating the presence of Cu+(the predominant oxidation state) and Cu2+in Cu-HC3N4[20,31].The absorption edge of the Cu-HC3N4migrated to a higher energy than that of Cu foil,indicating that Cu is oxidized in Cu-HC3N4[26].The FT value of the EXAFS results at the Cu K edge shows that a distinct symmetrical peak of the Cu foil at ≈2.2 ˚A is attributed to the Cu-Cu bond (Fig.2b),which is not found in Cu-HC3N4.On the contrary,a very obvious peak appears at ≈1.4 ˚A,which is attributed to the Cu-N bond.The chemical structure of the Cu-HC3N4sample was further determined by X-ray photoelectron spectroscopy (XPS).Fig.2c presents the survey XPS spectra of all synthesized material,and it is detected that the material has two characteristic peaks of C and N elements.It is worth noting that a slight or negligible Cu signal is present in Cu-HC3N4material,proving that the Cu element content in the sample is low.Two significant characteristic peaks of 284.8 and 288.3 eV appeared in the C 1s high-resolution XPS spectrum (Fig.2d),indicating the presence of C-C and sp2bond carbon (N-C=N) in the g-C3N4skeleton,respectively [9].The high-resolution spectrum of N 1s shows characteristic peaks at 401.3,400.2,and 398.6 eV in the g-C3N4sample (Fig.2e),corresponding to graphical nitrogen,pyrrole nitrogen,and pyridine nitrogen,respectively [9].Not surprisingly,the N 1s binding energy of Cu-HC3N4is slightly higher than that of C3N4,indicating the slight decrease in the electron density of the pyridine N atom,which is attributed to its interaction with the Cu atom.Hence,the Cu atom is immobilized on the pyridine N atom in g-C3N4to form a stable structure.For Cu-HC3N4,in the Cu 2p high-resolution spectrum,in addition to containing Cu+,it also shows two characteristic peaks of Cu2+corresponding to 935.0 and 955.0 eV,and the ratio of Cu2+to Cu+is~2 (Fig.2f) [28].The above results indicate the existence of single-atom copper.
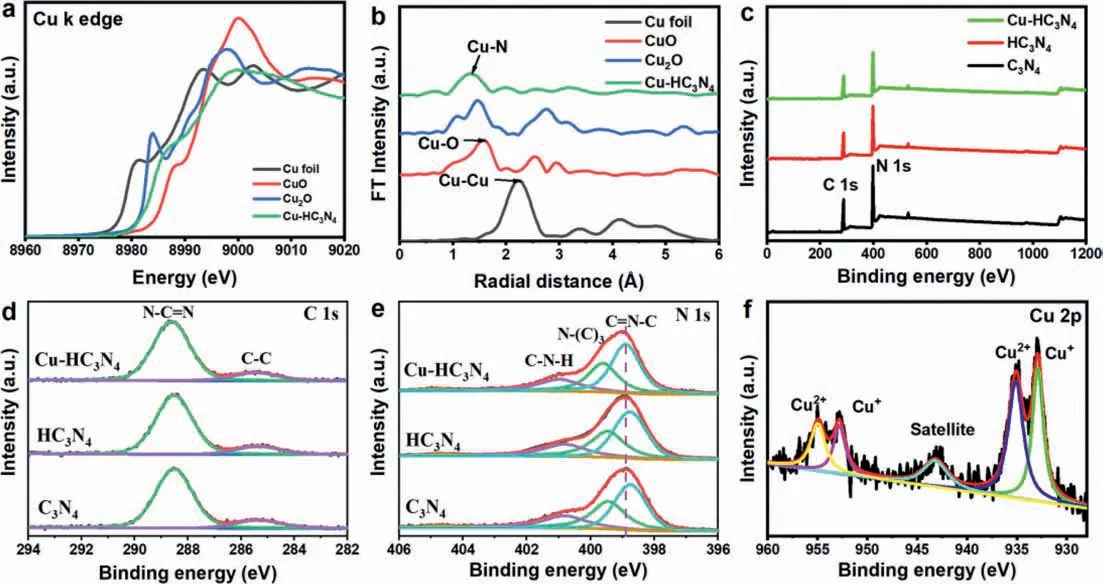
Fig.2.(a) Cu K-edge XANES spectra.(b) FT-EXAFS spectra.(c) XPS survey spectra and corresponding high-resolution XPS spectra of (d) C 1s,(e) N 1s and (f) Cu 2p.
The optical properties and light harvesting ability of C3N4,HC3N4and Cu-HC3N4were studied by UV-vis diffuse reflectance spectroscopy.As presented in Fig.3a,the UV-vis absorption edges of all samples are analyzed as follows: The absorption edge of C3N4is located at 458 nm,while the HC3N4and Cu-HC3N4are redshifted to 465 and 468 nm,respectively.In addition,all materials show tailing absorption in the visible region,which is attributed to the Cu single atom and hollow structure.The reaction of Cu atoms on the surface properties of hollow g-C3N4nanospheres was investigated by N2adsorption tests.As shown in Fig.3b,HC3N4and Cu-HC3N4samples express the traditional type IV isotherms and have high adsorption capacity,especially at high (P/P0>0.8) relative pressure.The BET specific surface areas of HC3N4and Cu-HC3N4samples are 45.2 and 100.4 m2/g,which are 4.8 and 10.6 times that of g-C3N4(9.4 m2/g),respectively.The Cu-HC3N4sample with such a high specific surface area is derived from its unique hollow nanospheres morphology,which provides more active sites for utilization.
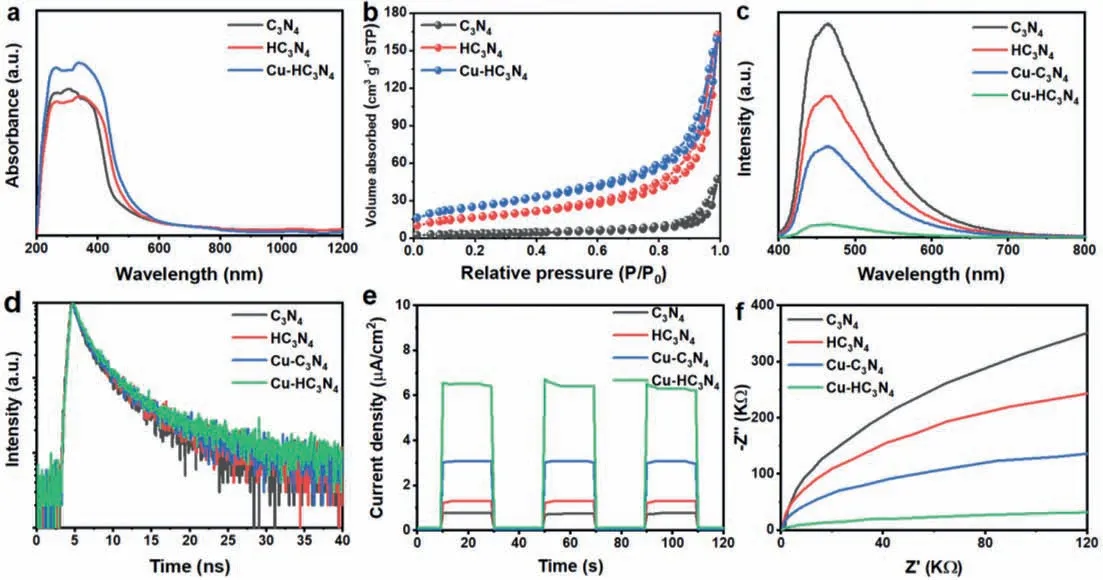
Fig.3.(a) UV-vis DRS of C3N4,HC3N4,and Cu-HC3N4.(b) Nitrogen adsorption desorption isotherm,(c) steady-state PL spectra,(d) time-resolved PL spectra,(e) transient photocurrent responses and (f) Nyquist plots of C3N4,HC3N4, Cu-C3N4,and Cu-HC3N4.
As shown in Fig.3c,a significant photoluminescence (PL)quenching occurs in the steady-state PL emission spectra of the Cu-HC3N4heterostructure relative to Cu-C3N4,HC3N4,and C3N4,indicating the intrinsic radiative recombination of the photogenerated electron-hole pairs in C3N4,HC3N4,and Cu-C3N4.It follows that the PL quenching on C3N4and HC3N4may be caused by the introduction of Cu atoms.In addition,compared with C3N4,the PL intensity of HC3N4also decreases,which indicates that C3N4can effectively improve the charge transport between the interface layers after surface bendingviahollow structure,and effectively inhibit the carrier recombination.Furthermore,as seen in Fig.3d and Table S4 (Supporting information),the time-resolved transient PL decay spectra by the position of the corresponding steady-state emission peaks show that the Cu-HC3N4heterostructure has a longer charge carrier average lifetime (τavg) relative to Cu-C3N4,HC3N4,and C3N4,which indicates the presence of more efficient charge transfer in Cu-HC3N4.By using a pump pulse at 400 nm wavelength,electrons are effectively promoted from the valence band to the conduction band of g-C3N4.As shown by the results,a very similar TA spectrum characterized by the detection of bleaching signals appears in the detection range of 450-760 nm wavelengths.A global fitting procedure to retrieve the characteristic relaxation time constants was performed to eliminate the avoidable effects of different probe wavelengths on the recovery kinetics.The average time constants for C3N4,HC3N4,Cu-C3N4,and Cu-HC3N4are 112,93,86,and 68 ps,respectively,as shown in Fig.S4 (Supporting information).The fit results converge to a long-lived plateau with a certain amplitude,which reflects the longer lifetime of the charge recombination process on the nano-microsecond time scale.And the average recovery lifetime is an obvious indicator to assess the separation/transfer efficiency of photogenerated charge carriers.By comparing the average recovery lifetime cases of HC3N4and Cu-HC3N4,a decrease can be observed.This may be due to the fact that Cu-N3opens an additional in-plane channel for electron transfer which shortens the average recovery lifetime.There is also a decrease in the average recovery lifetime of Cu-HC3N4compared to Cu-C3N4,which may be due to the hollow nanosphere structure opening an additional interlayer channel for electron transfer.
Furthermore,chrono-measuredI-tcurves under chopper illumination conditions were used to characterize the photo-response of C3N4,HC3N4,Cu-C3N4,and Cu-HC3N4catalysts.As shown in Fig.3e,the Cu-HC3N4catalyst has a higher photocurrent value relative to the pristine HC3N4and Cu-C3N4,indicating that the Cu-HC3N4catalyst has a higher electron-hole separation efficiency.A rapid improvement in charge transfer capability was also confirmed by electrochemical impedance spectroscopy (EIS).The small arc radius of the Nyquist plot of the Cu-HC3N4electrode was used to characterize the rapid interfacial charge transfer between Cu-HC3N4and the dielectric (Fig.3f).The results shown above further demonstrate that the hollow structure has the positive effect of promoting the separation of photogenerated carriers within the face and between the layers,thereby also improving the performance of single-atom Cu-HC3N4photocatalysts.
The photocatalytic hydrogen evolution experiments were implemented by using methanol as an electron donor and platinum as a co-catalyst under visible light (λ>420 nm) irradiation.Hydrogen evolution rates were about 20 μmol/h for C3N4,68 μmol/h for HC3N4,378 μmol/h for Cu-C3N4,and 1020 μmol/h for Cu-HC3N4(Figs.4a and b).Cu-HC3N4has a relatively good activity as a good performing photocatalyst over a longer period of time (Fig.4c).It is because of the high planar and interlayer conductivity generated by Cu-N3and hollow nanostructures,which allow rapid migration of photogenerated electron-hole pairs and inhibit charge recombination,the photocatalytic activity of Cu-HC3N4is higher than that of C3N4,HC3N4and Cu-C3N4.Besides,the effect of the number of Cu single atoms on the product hydrogen evolution can also be seen by Fig.S5 (Supporting information),where the loading of Cu should not exceed 0.50 wt%,otherwise there is a significant decrease in activity,which is due to the aggregation of copper atoms making the utilization of copper atoms less efficient.
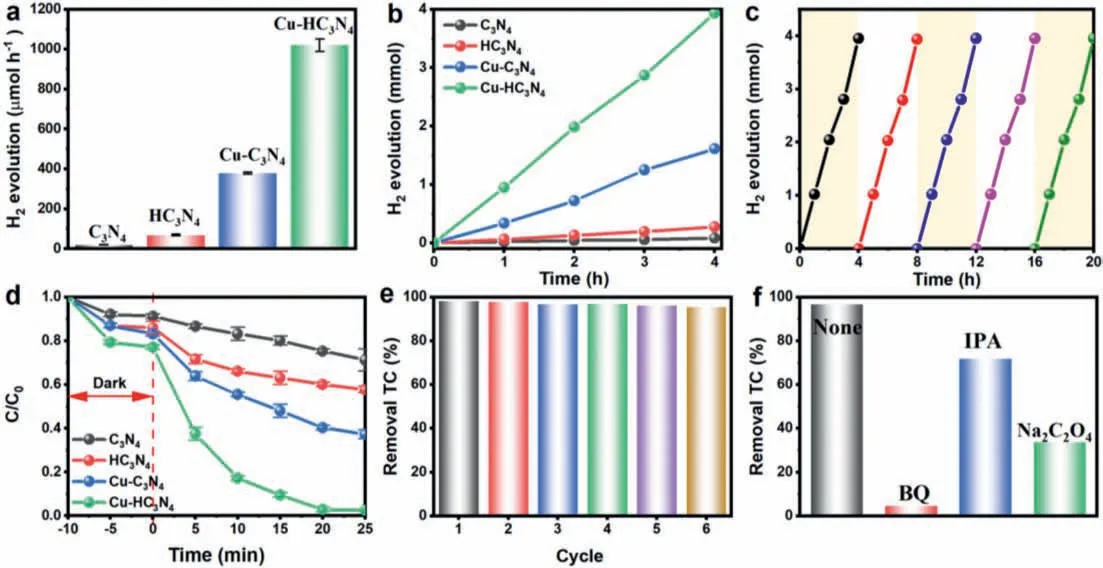
Fig.4.(a) Average hydrogen production rates and (b) H2 evolution under visible light irradiation.(c) Cycling tests of photocatalytic hydrogen generation of Cu-HC3N4.(d) Photocatalytic TC degradation.(e) Cycling tests of photocatalytic TC degradation of Cu-HC3N4.(f) The effects of scavengers on the photocatalytic TC degradation (catalysts=50 mg,scavenger=10 mmol/L).
As shown in Fig.4d,the photocatalytic performance of the Cu-HC3N4sample was verified by tetracycline (TC) photocatalytic degradation in an aqueous solution under visible light irradiation,where the photoelectrons generated by the photocatalyst were transferred to copper ions for efficient copper cycling,enabling the continuation of the reaction.In addition to this,the holes generated by light can also produce certain radicals.Therefore,singleatom copper and hollow structure modified photocatalytic materials can generate free radicals better due to their higher charge separation properties.Under dark conditions,the adsorption of the model pollutant TC on the surfaces of C3N4,HC3N4,Cu-C3N4and Cu-HC3N4after 10 min was not different compared to the results of BET.Under visible light irradiation,the absorption intensity of TC decreases with time,indicating the appearance of photo-oxidation of TC,and suggesting that Cu-HC3N4has the best photocatalytic degradation,which is caused by the combined effect of the unitary copper and hollow nanosphere structure.The total organic carbon removal rate is displayed in Fig.S6 (Supporting information),and the results indicate that the removal of organic carbon is similar to the degradation outcomes,with Cu-HC3N4exhibiting the highest removal rate.This suggests that it has superior photocatalytic degradation performance.
The stable photocatalytic performance of Cu-HC3N4was demonstrated by stability testing (Fig.4e),proving the successful preparation of a single-atom photocatalyst with excellent performance.XRD and FTIR tests were performed for the samples recovered after the test (Fig.S7 in Supporting information).As shown in Fig.S7b,there was no significant change in the spectra before and after the tests,which further verified that the photocatalyst has good stability.Furthermore,as shown in Fig.4f,it can be seen that BQ has the greatest impact on performance,indicating that·O2-is the main reactive oxygen species.As benzene is an important reagent for the synthesis of many chemicals,the photocatalytic oxidation activity of Cu-HC3N4can be tested by whether it can selectively oxidize benzene to phenol,and the conversion of benzene to phenol cannot occur in the absence of the catalyst (Fig.S8 in Supporting information).Compared to C3N4,HC3N4and Cu-C3N4,the conversion and selectivity of Cu-HC3N4were improved to 97.3%and 99.9%,respectively,under visible light conditions,providing a more excellent performance.The sample recovery was tested after the experiment (Fig.S9 in Supporting information),and Cu-HC3N4also has good stability for phenol production,as evidenced by hydrogen evolution and TC degradation experiments.
In general,the photocatalytic process involves several steps:First,light is absorbed by the prepared Cu-C3N4photocatalyst,generating an excited state called an electron-hole pair.Second,the electron and hole are separated and migrate to a reaction site on the photocatalyst surface,where the hollow sphere structure and Cu single atoms can effectively promote the separation of photogenerated charge carriers (Fig.S10 in Supporting information).Third,the reactant molecules or ions adsorb onto the photocatalyst surface,and the larger specific surface area of the hollow sphere structure can increase the adsorption of reactant molecules.Fourth,the electrons on the photocatalyst surface react with the adsorbed reactant molecules or ions,causing them to undergo redox reactions.Fifth,the products of the redox reactions desorb from the photocatalyst surface,completing the photocatalysis process.
In summary,we propose a simple pre-assembly strategy to prepare porous and hollow nanospheres g-C3N4photocatalysts with unique Cu-N3species and high active site accessibility.Interestingly,compared with HC3N4and Cu-C3N4,the developed Cu-HC3N4catalyst exhibits excellent photocatalytic performance,especially in the photocatalytic degradation of TC and the selective oxidation of benzene to phenol.The atomically dispersed,anchored peculiar Cu-N3species is responsible for the prominent catalytic behavior.This study not only affords a neoteric and highly active catalyst for photocatalytic hydrogen production,but also provides a new way to design and fabricate single-atom catalysts in various g-C3N4micro/nanostructures by changing the metal and/or adjusting the structure of pre-organized supramolecular aggregates.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Hainan Province Science and Technology Special Fund (No.ZDYF2022SHFZ094),National Natural Science Foundation of China (No.22166016),Hainan Provincial Key Research and Development Program (No.ZDYF2020222),and the open-ended fund of Key Laboratory of Agro-Forestry Environmental Processes and Ecological Regulation of Hainan Province (No.AFEPER202205).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108568.
杂志排行
Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
