Additive-free synthesis of S-substituted isothioureas via visible-light-induced four-component reaction of α-diazoesters,aryl isothiocyanates,amines and cyclic ethers
2024-04-05YufenLvHongyuDingJinmaoYouWeiWeiDongYi
Yufen Lv ,Hongyu Ding ,Jinmao You ,Wei Wei ,Dong Yi
a Key Laboratory of Catalytic Conversion and Clean Energy in Universities of Shandong Province,School of Chemistry and Chemical Engineering,Qufu Normal University,Qufu 273165,China
b College of Chemistry and Chemical Engineering,Shaoxing University,Shaoxing 312000,China
c School of Pharmacy,Southwest Medical University,Luzhou 646000,China
Keywords: Visible-light Additive-free Four-component reaction S-Substituted isothioureas
ABSTRACT An additive-free and environmentally friendly strategy has been realized for the construction of S-substituted isothioureas through visible-light-induced multicomponent reaction starting from αdiazoesters,aryl isothiocyanates,amines and cyclic ethers.This methodology features simple operation,mild reaction conditions,favorable functional group tolerance,easily available starting materials and high efficiency.
Sulfur-containing molecules have exhibited wide applications in materials science,organic synthesis,and pharmaceutical chemistry [1-8].Especially,S-substituted isothioureas as highly valuable sulfur-containing compounds have attracted a great deal of research interest from chemists owing to their diverse biological and medicinal activities such as antiviral [9],antifungal [10],antibacterial [11] and antitumor [12].As shown in Fig.1,S-substituted isothiourea-containing molecules have been increasingly designed and synthesized as NOS inhibitor,anti-cancer agent,calcium ion channel blocker,or insecticide in the agricultural and pharmaceutical industries [13-16].Furthermore,isothioureas as important synthetic intermediates have also been elegantly utilized for the construction of guanidines in synthetic chemistry [17-19].

Fig.1.Representative examples of bioactive S-substituted isothioureas containing molecules.

Fig 2.On/off experiments.
In term of their importance in pharmaceuticals and synthetic chemistry,numerous synthetic methods have been exploited for the preparation ofS-substituted isothioureas [20-33].Classical synthesis strategy involves the reaction of amine with aryl isothiocyanate and subsequent alkylation of thioureas with RX in the presence of base or microwave (Scheme 1a) [22-24].This twostep reaction requires the use of stoichiometric amounts of reaction auxiliaries and extra operation for the purification of thiourea.During the past several years,some alternative methods have been developed for the synthesis ofS-substituted isothioureasviathe cross-coupling reactions of mercaptothioureas with aryl halides[25-27] or aryl boronic acids [28] orS-alkylation of arylthioureas with tetraalkylammonium salts (Scheme 1b) [29].Nevertheless,there somehow exist some drawbacks such as the use of large amounts of additives and expensive reagents,low yields,and relatively harsh reaction conditions.
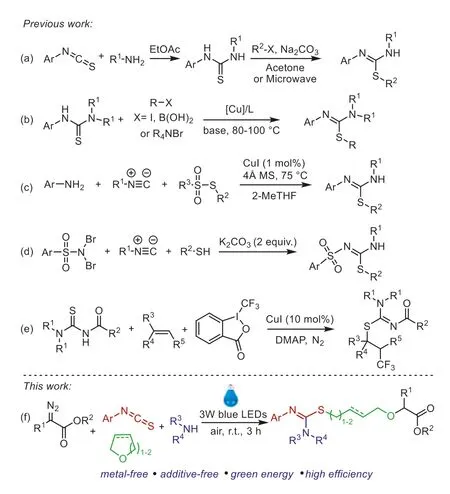
Scheme 1.The procedures for the synthesis of S-substituted isothioureas.
Multicomponent tandem reactions could provide atom-and step-economic approach to access various diverse functionalized molecules in a one-pot operation [30-33].In this context,Maeset al.have recently developed a multicomponent strategy for the synthesis of isothioureas from thiosulfonates,iso-cyanides,and (hetero)aromatic amines (Scheme 1c) [34].In 2021,Phukan and coworkers reported K2CO3-promoted procedure for the synthesis of isothiourea fromN,N-dibromoarylsulfonamides,isonitriles,and thiols (Scheme 1d) [35].In 2022,Jiang described a copper-catalyzed multicomponent reaction of thioureas,alkenes,and Togni reagent II leading to CF3-containing isothioureas (Scheme 1e) [36].Although significant progress has been achieved,the use of metal reagents and stoichiometric amounts of additives still limited their wide application.Therefore,the development of mild,green,and additive-free multicomponent reaction strategy is still highly desirable.In recent years,visible-light-promoted multicomponent tandem reactions have been increasingly utilized in synthetic chemistry to construct various functional complex molecules owing to their mild and environmentally friendly features [37-45].With our continued research interests in photoinduced multicomponent reactions and synthesis of sulfur-containing compounds [46-50],we describe herein a new visible-light-promoted strategy for the construction ofS-substituted isothioureasviafour-component reaction ofα-diazoesters,aryl isothiocyanates,amines,and cyclic ethers(Scheme 1f).This reaction proceeds under mild conditions to afford severalS-substituted isothioureas in moderate to good yields with favorable functional group tolerance without using any metal catalyst and additives.
Initially,the reaction of 1-methyl phenyldiazoacetate (1a),phenyl isothiocyanate (2a),and morpholine (3a) was investigated in THF with the irradiation of 3 W blue LED lamps.When the model reaction was conducted in the presence of Cs2CO3(100 mol%) at room temperature,the four-component product5aawith ring-opening of THF was obtained in 85% yield (Table 1,entry 1).Then,a series of bases including DABCO,DBU,Et3N,K3PO4,Na2CO3,and KOH were screened to optimize the reaction effi-ciency,and the results showed that yield of5aawas not obviouslyincreased (Table 1,entries 2-7).Furthermore,the addition of photocatalyst (2 mol%) such as Ru(bpy)Cl2,Rose Bengal,Eosin Y,or Rhodamine B did also not improve the reaction efficiency (Table 1,entries 8-11).Notably,the product5aawas still obtained in 90%yield when the reaction was carried out in the absence of any additive (Table 1,entry 12).Next,the light sources were further investigated without the use of any additive,and results demonstrated that 3 W blue LEDs was the best choice and other light sources such as purple LEDs,green LEDs,and white LEDs would lead to the relatively lower reaction efficiency (Table 1,entries 13-15).It should be noted that the transformation did not occur in the absence of visible light irradiation (Table 1,entry 16).Solvent effect on the reaction efficiency was also examined.The mixture of THF with other organic solvents (v/v=1/1) such as EtOAc,CH2Cl2,DCE and CH3CN also gave the product5aain good yield (Table 1,entries 17-20),and the highest yield of 93% could be obtained by using toluene/THF (1/1) as the reaction medium (Table 1,entry 22).Further reducing THF loading would lead to lower reaction efficiency (Table 1,entries 23-26).When the reaction was performed under N2,the desired product5aawas only obtained in 66% yield(Table 1,entry 27).The reaction did not occur at 60 °C in the absence of light irradiation (Table 1,entry 28).

Table 1Screening of the reaction conditions.a
Having established the optimal reaction conditions in hand,we further explored the scope of this visible-light-mediated fourcomponent reaction ofα-diazoesters,amines,isothiocyanates,and cyclic ethers.As demonstrated in Scheme 2,α-diazoesters with a number of substituents at the aromatic ring were successfully transformed into the desired products5aa-5amin moderate to excellent yields.Generally,electron-effect has little influence on the reaction efficiency,both electron-donating and electronwithdrawing group substituted substrates were all suitable for this reaction.On the contrary,steric hindrance has a marked effect on the activities ofα-diazoesters.In comparison with substituents atpara-andmeta-position of phenyl ring,ortho-substitutedα-diazoesters would lead to lower reaction efficiency,and the corresponding products5aiand5ajwere obtained in 46% and 60% yields,respectively.It should be noted that functional groups such as halogen,C(O)OMe,CN,and NO2substituents were not an obstacle for this reaction,which provided a chance for subsequent structural modification.Furthermore,the scope ofα-diazoesters with a variety of substituents at ester group was also investigated.In addition to methyl group,other alkyl groups including ethyl,isopropyl,benzyl,2-phenylethyl,and isopentyl groups were well tolerated in this process to afford the corresponding products5an-5arin 77%-97% yields.

Scheme 2.Substrate scope.Reaction condition: α-diazoester 1 (0.2 mmol),phenyl isothiocyanate 2a (0.2 mmol),morpholine 3a (0.1 mmol),THF (1 mL),toluene (1 mL),3 W blue LED lamps,r.t.,air,3 h.Isolated yields based on 3a.
Then,the scope and limitation of isothiocyanates,amines,and cyclic ethers have also been investigated under the standard conditions (Scheme 3).In general,aryl isothiocyanates having either electron-donating or -withdrawing groups onortho-,meta-andpara-position of aryl rings have shown good reactivities,and the corresponding four-component products (5ba-5ja) were obtained in good yields.With respect to amines,in addition to morpholine,a series of secondary aliphatic amines such as dimethylamine,piperidine,4-methylpiperidine,cis-2,6-dimethylmorpholine,Nisopropylcyclohexanamine,andN-methyl-1-phenylmethanamine were all suitable substrates to produce the desired products5ka-5pain moderate to excellent yields.Nevertheless,when secondary aromatic amine such as diphenylamine was used in this reaction system,none of desired product5qawas observed.Finally,the possibility of using other cyclic ethers was investigated under the standard conditions.It was found that other cyclic ethers such as tetrahydropyran and 2,5-dihydrofuran were also able to compatible with this procedure,providing the corresponding products5raand5sain moderate yields.However,the desired products were not obtained when acyclic ethers such as diethyl ether andn-butyl ether were employed in this procedure.

Scheme 3.Substrate scope.Reaction condition: α-diazoester 1a (0.2 mmol),isothiocyanate 2 (0.2 mmol),amine 3 (0.1 mmol),cyclic ether 4 (1 mL),toluene (1 mL),3 W blue LED lamps,r.t.,air,3 h.Isolated yields based on 3.
The scalability and practicality of this reaction was further investigated.When the model reaction was conducted on a 2 mmol scale,the reaction still maintained a good reaction efficiency and the product5aacould be isolated in 87% yield (Scheme 4).
Several control experiments were further conducted to understand the possible reaction mechanism (Scheme 5).Firstly,thiourea6awas isolated in 99% yield by treatment of phenyl isothiocyanate (2a) with morpholine (3a) in the absence of visiblelight irradiation (Scheme 5a).Furthermore,the desired product5aawas isolated in 92% yield when the reaction of 1-methyl phenyldiazoacetate (1a),thiourea (6a),and THF was performed under standard conditions (Scheme 5b).These results indicated that thiourea6ashould be a key intermediate in this reaction system.Moreover,when radical scavenger TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) or BHT (2,6-di-tert-butyl-4-methylphenol) was added in this reaction system,the model reaction was not inhibited and the product5aawas still isolated in 90% and 86% yields,respectively (Scheme 5c).This result suggested that a radical process should not be involved in this multi-component reaction.In addition,on/off light-irradiation result demonstrated that the continuous visible-light illumination is important for promoting this transformation (Fig.2).

Scheme 5.Control experiments.
Based on the above results and referring to previous reports [51-57],we proposed a possible mechanism as shown in Scheme 6.Firstly,α-diazoester1awas irradiated by blue light to generate an excited state1a*,Then,the decomposition of an excited state1a*would lead to the formation of carbene intermediate8a,which was trapped by THF to give oxonium ylide intermediate9a.While the addition of morpholine (3a) with phenyl isothiocyanate (2a) afforded thiourea6a,which rapidly tautomerized to yield intermediate7a.Next,the nucleophilic attack of intermediate7ato oxonium ylide intermediate9aand further ring-opening provided10a.Finally,the intramolecular proton transfer occurred to afford the final product5aa.

Scheme 6.Possible reaction pathway.
In conclusion,we have developed a facile and green synthetic method for the assembly ofS-substituted isothioureasviavisiblelight-induced four-component reaction ofα-diazoesters,isothiocyanate,amines,and cyclic ethers.Under mild conditions,a series of substrates with different functional groups were applicable in this multi-component tandem reaction.The simple operation,green energy source,and additive-free conditions for constructingS-substituted isothioureas are valuable features,which may find potential application in the pharmaceutical synthesis.Further investigation on synthetic applications and mechanistic details are ongoing in our group.
Acknowledgments
This work was financially supported by Natural Science Foundation of Shandong Province (No.ZR2021MB065),the major innovation fund of Shandong Province (No.2021ZDSYS23),and the National Natural Science Foundation of China (Nos.21976105,22101237).
杂志排行
Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
