Silencing of Jumonji domain-containing 1C inhibits the osteogenic differentiation of bone marrow mesenchymal stem cells via nuclear factor-κB signaling
2024-03-24JingYiLiTingTingWangLiMaYuZhangDiZhu
Jing-Yi Li,Ting-Ting Wang,Li Ma,Yu Zhang,Di Zhu
Abstract BACKGROUND Osteoporosis is a common metabolic bone disorder induced by an imbalance between osteoclastic activity and osteogenic activity.During osteoporosis,bone mesenchymal stem cells (BMSCs) exhibit an increased ability to differentiate into adipocytes and a decreased ability to differentiate into osteoblasts,resulting in bone loss.Jumonji domain-containing 1C (JMJD1C) has been demonstrated to suppress osteoclastogenesis.AIM To examine the effect of JMJD1C on the osteogenesis of BMSCs and the potential underlying mechanism.METHODS BMSCs were isolated from mouse bone marrow tissues.Oil Red O staining,Alizarin red staining,alkaline phosphatase staining and the expression of adipogenic and osteogenic-associated genes were assessed to determine the differentiation of BMSCs.Bone marrow-derived macrophages (BMMs) were incubated with receptor activator of nuclear factor-kappa B ligand to induce osteoclast differentiation,and osteoclast differentiation was confirmed by tartrate-resistant acid phosphatase staining.Other related genes were measured via reverse transcription coupled to the quantitative polymerase chain reaction and western blotting.Enzyme-linked immunosorbent assays were used to measure the levels of inflammatory cytokines,including tumor necrosis factor alpha,interleukin-6 and interleukin-1 beta.RESULTS The osteogenic and adipogenic differentiation potential of BMSCs isolated from mouse bone marrow samples was evaluated.JMJD1C mRNA and protein expression was upregulated in BMSCs after osteoblast induction,while pnuclear factor-κB (NF-κB) and inflammatory cytokines were not significantly altered.Knockdown of JMJD1C repressed osteogenic differentiation and enhanced NF-κB activation and inflammatory cytokine release in BMSCs.Moreover,JMJD1C expression decreased during BMM osteoclast differentiation.CONCLUSION The JMJD1C/NF-κB signaling pathway is potentially involved in BMSC osteogenic differentiation and may play vital roles in the pathogenesis of osteoporosis.
Key Words: Osteoporosis;Mesenchymal stem cells;Osteogenesis;Jumonji domain-containing 1C;Nuclear factor-κB
INTRODUCTION
Osteoporosis is a common bone disease worldwide characterized by low bone mineral density that can cause fractures in affected bones[1].This condition is prevalent among elderly individuals,particularly women of postmenopausal age[2].Osteoporosis is elicited by the disequilibrium of osteoblastic bone formation and osteoclastic bone resorption[3].Current therapies for osteoporosis mainly focus on the regulation of bone remodeling;nevertheless,these treatments have certain adverse effects and limitations[4].Further elucidation of the molecular mechanisms underlying osteoporosis is important for developing effective therapeutic strategies for this disease.
Bone mesenchymal stem cells (BMSCs),progenitor cells that can be isolated from bone marrow,can differentiate into various types of cells,such as adipocytes and osteoblasts[5].As the progenitor cells of adipocytes and osteoblasts,BMSCs play a critical role in bone homeostasis[6].Recent methods and current developments in utilizing BMSCs for the repair of bone fractures resulting from osteoporosis have been reported in many studies[7].Moreover,previous studies have reported that osteoporotic BMSCs exhibit impaired osteogenic differentiation potential[8-10].
Jumonji domain-containing (JMJD) proteins,which have histone lysine demethylase (KDM) activities,constitute a large protein family with more than 30 members[11].One subfamily of JMJD proteins comprisesKDM3A/JMJD1A,KDM3B/JMJD1BandKDM3C/JMJD1C[12].In oral inflammatory lesions,JMJD1C deficiency results in elevated alveolar bone loss.Moreover,loss ofJMJD1Caccelerates bone marrow-derived macrophage (BMM) differentiation into osteoclastsin vitro[13].Database analysis revealed thatJMJD1Cexpression is downregulated in bone marrow stromal cells (BMSCs) from patients with osteoporosis.JMJD1Clevels are upregulated in osteogenic induction medium and BMSC growth medium supplemented with modified extracellular matrix[14].However,whetherJMJD1Cis involved in osteoblast differentiation of BMSCs during osteoporosis is still unclear.
Nuclear factor-κB (NF-κB) is a transcription factor that modulates the expression of many genes implicated in the immune response and inflammation[15].Previous findings also revealed that NF-κB is an essential factor that contributes to impaired bone formation during osteoporosis[16].Moreover,NF-κB suppression improved osteopenia and promoted bone formation in a mouse model of osteoporosis[17].Moreover,repression of the NF-κB pathway induces osteogenic differentiation in MSCs[18].Importantly,complete knockout and transient knockdown ofJMJD1Cled to the activation of the NF-κB subunit p65 in both mouse and human macrophages[13].
Here,this study explored the role ofJMJD1Cin BMSC osteogenic differentiation and the potential underlying mechanisms.We demonstrated thatJMJD1Cexpression was increased while NF-κB signaling activation was not affected during BMSC osteogenic differentiation.Silencing ofJMJD1Csuppressed osteogenic differentiation and promoted NF-κB activation in BMSCs.Therefore,this study might lead to the identification of an innovative target for treating osteoporosis.
MATERIALS AND METHODS
Cell culture
C57BL/6 mice (8 wk old) obtained from Beijing Weitong Lihua Experimental Animal Technology Co.,Ltd.,were housed in pathogen-free facilities under a light/dark cycle of 12/12 h.The animal experiments were approved by the Ethics Committee of Beijing Tiantan Hospital Affiliated with Capital Medical University.Primary BMSCs were isolated as described previously.In brief,after anesthesia,the mice were sacrificedviacervical dislocation.Bone marrow cells were collected from the tibiae and femurs of the mice.The cells were maintained in α-MEM (Gibco,Grand Island,NY,United States) supplemented with 1% penicillin/streptomycin (Gibco) and 10% fetal bovine serum (FBS) (Gibco) in an incubator with 5% CO2at 37 °C.When the cells reached 80% confluence,they were digested and cultured.Cells (5-9 passages) were collected for the next assays.BMMs from mice were cultured in α-MEM supplemented with macrophage colonystimulating factor (M-CSF,5 ng/mL),1% penicillin/streptomycin and 10% FBS.The BMSCs were randomly divided into five groups: Control (without any treatment),osteoblast induction (maintained in osteogenic induction medium for the indicated time periods),adipocyte induction (cultured in adipogenic induction medium for 14 d),short hairpin RNAs(shRNAs)-NC (transfected with shRNA-NC for 48 h) and shRNA-JMJD1C(transfected with shRNA-JMJD1Cfor 48 h).In addition,the BMMs were randomly divided into two groups: Control (BMMs without any treatment) and osteoclast induction [BMMs treated with receptor activator of nuclear factor-kappa B ligand (RANKL) for 7 d] groups.
Flow cytometry
BMSC surface markers were detectedviaflow cytometry.Briefly,after being rinsed by phosphate buffered saline (PBS),the BMSCs were collected with trypsin.After centrifugation at 1500 rpm,the cells were incubated for 0.5 h with primary antibodies against CD29,CD31,CD34,CD45,CD90,MHCII,SCA-1 and CD11b (Biolegend,California,San Diego,United States and Becton Dickinson,Franklin Lakes,NJ,United States).Afterward,flow cytometry (Becton Dickinson,Franklin Lakes,NJ,United States) was used to assess the cells.
Osteoblast differentiation
BMSCs were maintained in osteogenic induction medium (α-MEM supplemented with 10-7M dexamethasone,10 mmol/L β-glycerophosphate,0.2 mmol/L ascorbic acid,1% penicillin/streptomycin and 10% FBS) to induce osteogenic differentiation.The osteogenic induction medium was replaced every 3 d.
Alkaline phosphatase staining
In accordance with the manufacturer’s instructions,alkaline phosphatase (ALP) staining of cells was performed using a BCIP/NBT ALP color development kit (Beyotime,Jiangsu,China) following 7 d of osteogenic induction.Briefly,after rinsing with PBS,the BMSCs were fixed in paraformaldehyde (4%).Afterward,the cells were treated with BCIP/NBT staining solution.The color development reaction was terminated using deionized water.A light microscope (Olympus,Tokyo,Japan) was used to observe the results.
Alizarin red staining
According to the manufacturer’s protocol,osteogenic differentiation was evaluated by Alizarin red staining following osteogenic induction for 21 d.In brief,the cells were washed with PBS.After fixation with paraformaldehyde (4%),500 μL of Alizarin red staining solution was added to the cells (1%,Sigma,St.Louis,MO,United States).Fifteen minutes later,the staining solution was discarded.A light microscope (Olympus) was used to observe the results after the cells were washed three times with PBS.
Adipogenic differentiation
For induction of adipogenic differentiation,BMSCs (2.5 × 106cells per well) were cultured for 14 d in 6-well plates with adipogenic induction medium (1 μmol/L dexamethasone,0.5 mmol/L 3-isobutyl-1-methylxanthine,5 μg/mL insulin,and 10% FCS in α-MEM).The culture medium was changed every other day.
Oil red O staining
After adipogenesis induction for 14 d,mature adipocytes were distinguished from preadipocytes using Oil Red O staining.In brief,after rinsing with PBS,the cells were fixed in paraformaldehyde (4%) at room temperature for 30 min.The cells were then washed with PBS and stained with Oil Red O.Twenty minutes later,a light microscope (Olympus)was used to observe the cells.
Osteoclast differentiation
For osteoclast differentiation,10 ng/mL RANKL obtained from R&D Systems (Minneapolis,MN,United States) was used to treat the BMMs for 7 d.
Tissue-resistant acid phosphatase staining
Osteoclast differentiation of BMMs was confirmed by using tissue-resistant acid phosphatase (TRAP) staining.In brief,the cells were fixed in paraformaldehyde (4%).TRAP staining was performed instantly in the dark for 30 min at 37 °C.The images were obtained by using an optical microscope (Olympus).TRAP-positive cells containing at least 3 nuclei were regarded as osteoclasts.
Cell transfection
A lentivirus containing short hairpin RNA againstJMJD1C(sh-JMJD1C) was obtained from Sangon Biotech (Shanghai,China) forJMJD1Cknockdown in BMSCs,and the lentivirus was used to transfect the cells following the manufacturer’s instructions for viral infection.The cells were collected for subsequent assays after 48 h of transfection.
Reverse transcription coupled to the quantitative polymerase chain reaction
TRIzol (Invitrogen,Carlsbad,CA,United States) was used to extract total RNA.A PrimeScript RT reagent kit (TaKaRa,Tokyo,Japan) was used to transcribe the extracted RNA into cDNA.A CFX96™ Real Time RT-PCR System was used for analysis with SYBR Premix Ex Taq™ II (TaKaRa).Glyceraldehyde 3-phosphate dehydrogenase was used for normalization.The synthesized primers are shown in Table 1.The relative gene expression levels were analyzed with the 2-ΔΔCTmethod.

Table 1 The primer sequences used in reverse transcription coupled to the quantitative polymerase chain reaction
Western blot
RIPA buffer (Thermo Fisher Scientific) with protease and phosphatase inhibitors was used for extraction of total protein at 4 °C for 0.5 h.A BCA protein assay reagent kit (Thermo Fisher Scientific) was used to determine the protein concentrations in the cell lysates.Next,the protein samples were separatedvia10% sodium-dodecyl sulfate gel electrophoresis(Bio-Rad,Hercules,CA,United States) before being transferred to polyvinylidene fluoride membranes (Millipore,Bedford,MA,United States).Five percent BSA was used to block the membranes at room temperature for 1 h.Then,the membranes were incubated at 4 °C overnight with primary antibodies against JMJD1C (Invitrogen),p-NF-κB (Abcam),NF-κB (Abcam) and β-tubulin (Abcam).Next,the sections were incubated with secondary antibodies.Ultimately,enhanced chemiluminescence reagent (Beyotime) was used to detect the immunoreactive bands.Image Lab (Bio-Rad,Hercules,CA,United States) was used to quantify the band intensity.
Enzyme-linked immunosorbent assay
In accordance with the manufacturer’s instructions,enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems,Minneapolis,MN,United States) were used to measure the levels of cytokines,including tumor necrosis factor alpha(TNF-α),interleukin-6 (IL-6) and IL-1β,in the supernatants.A microplate reader was used to determine the absorbance value of each sample.
Statistical analysis
The data of this study were accessed by GraphPad Prism 8.0 software.Analysis of variance (ANOVA) or Student’sttest was used to analyze the results.P< 0.05 was regarded as significant,and the outcomes are presented as the means ± SD.
RESULTS
Osteogenic and adipogenic differentiation of BMSCs
First,we separated BMSCs from mouse bone marrow tissues.Flow cytometry was conducted to identify the BMSCs.As displayed in Figure 1A,BMSCs highly expressed specific stem cell markers,including CD29,CD90 and SCA-1 but weakly expressed CD31,CD45,CD34,MHC2 and CD11b.The results indicated that the BMSCs were successfully separated from the mouse bone marrow.Osteogenic differentiation of BMSCs was induced by incubating the cells with osteogenic induction medium.ALP staining revealed a high level of ALP in BMSCs on day 14 (Figure 1B).Alizarin red staining revealed calcium deposition in the BMSCs on day 21 (Figure 1C).The expression of several osteogenesis-related genes,including Osteocalin,Runt-related transcription factor 2 and ALP,increased after osteogenic induction (Figure 1D).These data suggested successful osteogenic differentiation.Next,the BMSCs were maintained in adipogenic induction medium.The results from Oil Red O staining revealed various lipid drops within the differentiated cells after 14 d of adipogenic differentiation (Figure 1E).In addition,the expression of adipogenesis-related genes,including peroxisome proliferatoractivated receptor gamma and CCAAT enhancer-binding protein alpha,was significantly increased in BMSCs after adipocyte induction (Figure 1F).These results indicated successful adipogenic differentiation of BMSCs.
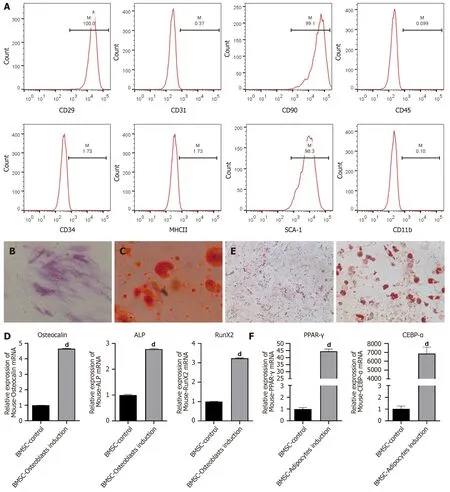
Figure 1 Osteogenic and adipogenic differentiation of bone mesenchymal stem cells. A: Bone mesenchymal stem cells (BMSCs) were isolated from mice bone marrow tissues.Flow cytometry was used to detect the expressions of CD29,CD31,CD90,CD45,CD34,MHCII,SCA-1 and CD11b;B-D: BMSCs were induced towards osteogenic differentiation.ALP staining was used to evaluate alkaline phosphatase (ALP) activity (B);calcium deposits were visualized by Alizarin red staining (C);the mRNA levels of osteogenic genes,including Osteocalin,Runt-related transcription factor 2 and ALP,were detected by reverse transcription coupled to the quantitative polymerase chain reaction (RT-PCR) (D);E and F: BMSCs were induced towards adipogenic differentiation.Representative images of Oil red O staining (E),the mRNA levels of adipogenic genes,such as peroxisome proliferator-activated receptor gamma and CCAAT enhancer-binding protein alpha,were measured by RT-PCR (F).All values are shown as mean ± SD.dP < 0.0001.n=3.BMSC: Bone mesenchymal stem cell;ALP: Alkaline phosphatase;RunX2:Runt-related transcription factor 2;CEBPα: CCAAT enhancer-binding protein alpha;PPARγ: Peroxisome proliferator-activated receptor gamma.
JMJD1C was upregulated but NF-κB activation was not affected during the osteogenic differentiation of BMSCs
To determine the role ofJMJD1Cin BMSC osteogenic differentiation,we induced osteogenic differentiation in BMSCs.The reverse transcription coupled to the quantitative polymerase chain reaction results revealed thatJMJD1CmRNAexpression was significantly increased in BMSCs after osteoblast induction (Figure 2A).Consistently,theJMJD1Cprotein level was increased after osteoblast induction,as shown by western blot analysis (Figure 2B and C).These data indicated thatJMJD1Cwas upregulated during BMSC osteogenic differentiation.In addition,the protein expression of p-NF-κB was not significantly altered in BMSCs after osteoblast induction (Figure 2B and D).Moreover,the cytokine secretion levels of IL-1β,IL-6 and TNF-α were not significantly altered in BMSCs after osteoblast induction (Figure 2E-G).

Figure 2 Jumonji domain-containing 1C was upregulated while nuclear factor-κB activation were not affected during bone mesenchymal stem cells osteogenic differentiation. A: Jumonji domain-containing 1C (JMJD1C) mRNA level in bone mesenchymal stem cells after osteoblast induction was measured by reverse transcription coupled to the quantitative polymerase chain reaction;B-D: The protein expressions of JMJD1C and p-nuclear factor-κB were determined by western blot;E-G: The levels of inflammatory cytokines [interleukin (IL)-1β,IL-6 and tumor necrosis factor alpha] were detected using enzyme-linked immunosorbent assay.All values are shown as mean ± SD.aP < 0.05,dP < 0.0001,NS: No significance.n=3.JMJD1C: Jumonji domain-containing 1C;TNF-α:Tumor necrosis factor alpha;IL: Interleukin;NF-κB: nuclear factor-κB.
JMJD1C knockdown inhibited osteogenic differentiation and promoted NF-κB activation in BMSCs
To further explore the role ofJMJD1Cin BMSC osteogenesis,we knocked downJMJD1Cin BMSCs by infection with a lentivirus harboring sh-JMJD1C.Compared with that in the control group,theJMJD1Cprotein level was notably lower in the cells transfected with shRNA-JMJD1C(Figure 3A and B),indicating successful knockdown ofJMJD1C.Depletion ofJMJD1Cnotably upregulated the protein expression of p-NF-κB (Figure 3A and C),which indicated thatJMJD1Cknockdown facilitated NF-κB activation in BMSCs.Moreover,knocking downJMJD1Cinhibited stem cell osteogenic differentiation (Figure 3D and E).Moreover,the secretion of the cytokines IL-1β,IL-6 and TNF-α was significantly increased (Figure 3F-H).These findings indicated thatJMJD1Cpromotes cell osteogenic differentiation by negatively regulating p-NF-κB expression in BMSCs.

Figure 3 Jumonji domain-containing 1C knockdown inhibited osteogenic differentiation and upregulated p-nuclear factor-κB expression in bone mesenchymal stem cells. Bone mesenchymal stem cells (BMSCs) were transfected with sh-NC or short hairpin RNA against Jumonji domaincontaining 1C (sh-JMJD1C).A-C: Western blot was adopted to measure JMJD1C and p-nuclear factor-κB protein levels;D and E: Detection of osteogenic differentiation ability of BMSCs using alizarin red staining;F-H: Cytokines secretion level of interleukin (IL)-1β,IL-6 and tumor necrosis factor alpha were detected by enzyme-linked immunosorbent assay.All values are shown as mean ± SD.bP < 0.01,cP < 0.001,dP < 0.0001,n=3.JMJD1C: Jumonji domain-containing 1C;TNF-α: Tumor necrosis factor alpha;IL: Interleukin;NF-κB: nuclear factor-κB.
JMJD1C was downregulated during RANKL-induced osteoclast differentiation in BMMs
Osteoclast differentiation was induced in BMMs by treatment with RANKL and M-CSF.The number of TRAP+multinucleated osteoclasts was significantly greater after 7 d of stimulation than that in the control group,as shown by TRAP staining (Figure 4A),which indicated successful osteoclast differentiation of BMMs induced by RANKL and M-CSF.Compared with those in the control group,JMJD1CmRNA and protein levels were notably lower in cells undergoing osteoclast differentiation (Figure 4B-D).Taken together,these data suggested thatJMJD1Cwas downregulated during BMM osteoclast differentiation.
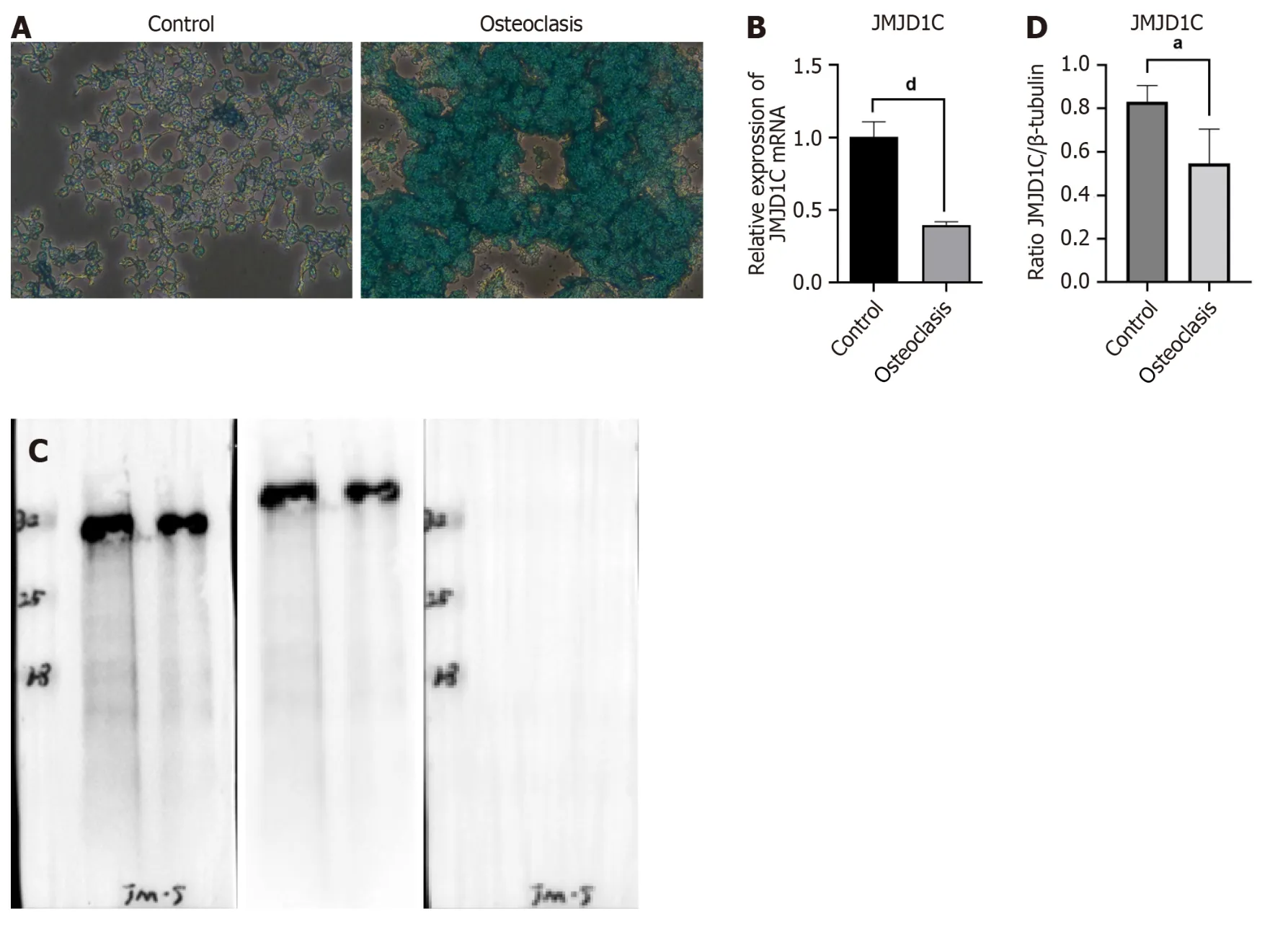
Figure 4 Jumonji domain-containing 1C was downregulated after bone marrow-derived macrophages osteoclast differentiation. Bone marrow-derived macrophages were incubated with RANKL for osteoclast differentiation.A: Representative images of tissue-resistant acid phosphatase staining were presented;B-D: Jumonji domain-containing 1C mRNA and protein levels in MMCs after osteoclast induction were measured by reverse transcription coupled to the quantitative polymerase chain reaction and western blot.All values are shown as mean ± SD.aP < 0.05,dP < 0.0001,n=3.JMJD1C: Jumonji domain-containing 1C.
DISCUSSION
Osteoporosis is a common bone disorder that arises from an imbalance in bone homeostasis[19].Impaired BMSC osteogenesis,decreased bone formation and increased marrow adiposity were observed in osteoporosis[20].Herein,BMSCs isolated from the bone marrow of mice were characterized for their ability to differentiate into osteoblasts and adipocytes.JMJD1Cwas increased while NF-κB activation was not altered in BMSCs during osteogenic differentiation.Moreover,JMJD1Cdepletion suppressed osteogenic differentiation and facilitated the activation of NF-κB signaling in BMSCs.
During osteoporosis development,BMSCs exhibit an increased ability to differentiate into adipocytes and a decreased capability to differentiate into osteoblasts,leading to an increase in fat accumulation and a decrease in bone formation[21].An imbalance in BMSC differentiation has been demonstrated to be a critical mechanism underlying osteoporosis pathogenesis.For example,transcriptionally regulating BMSC differentiation by DEPTOR exacerbates the imbalance of bone fat in osteoporosis[22].In age-related osteoporosis,the adipogenic differentiation of BMSCs is suppressed,and the osteoblastic differentiation of BMSCs is promoted by the overexpression of miR-130a[23].The promoted BMSC osteogenesis induced by Foxf1 knockdown contributes to the prevention of ovariectomy-elicited bone loss[24].Investigating the molecular mechanism modulating adipogenic differentiation and osteogenic differentiation in BMSCs is critical for comprehending the development of osteoporosis and identifying innovative treatment approaches.In the present study,BMSCs cultured in osteogenic induction medium exhibited obvious calcium deposition and osteogenic differentiation,which was in line with the findings of previous studies.
Recent studies onJMJD1Chave focused predominantly on tumors and cancers[25,26].In addition,JMJD1Cis involved in regulating cell differentiation in many cells.For example,JMJD1Cknockdown is sufficient to trigger neural differentiation of human embryonic stem cells[27];JMJD1Csilencing leads to the induction of differentiation of mouse embryonic stem cells[28].However,whetherJMJD1Cis closely associated with osteogenic differentiation is still unclear.A previous study confirmed that in murine 3T3-L1 preadipocyte cells,knockdown of JMJD1C can impair adipogenesis[29].BMMs isolated fromJMJD1Cknockout mice exhibit enhanced osteoclastogenesis[13].Furthermore,KDM3B/JMJD1Bhas been identified as a potential modulator of osteogenic differentiation[30].Herein,this study revealed thatJMJD1CmRNA and protein levels were elevated during the osteogenic differentiation of BMSCs.In addition,JMJD1Cexpression decreased in BMMs after osteoclast differentiation.Moreover,we found thatJMJD1Cknockdown suppressed BMSC osteogenic differentiation.Our study demonstrated the involvement ofJMJD1Cin regulating BMSC osteogenesis.
NF-κB,an essential transcription factor,is implicated in numerous cellular pathophysiological activities.Moreover,many studies have revealed that NF-κB signaling plays a vital role in osteoblast differentiation.For example,taxifolin promotes BMSC osteogenic differentiation partially through the NF-κB pathway[31].Melatonin alleviates inflammation and facilitates osteoblast differentiation of BMSCs by repressing the NF-κB pathway[32].Moreover,in BMSCs from systemic lupus erythematosus patients,activated NF-κB suppressed osteogenic differentiation through downregulation of Smad signaling[33].TRIM38 acts as a negative modulator of NF-κB during the differentiation of osteoblasts,playing an essential role in bone remodeling[34].Taken together,these findings suggest that NF-κB may play an essential role in osteoporosis development.Here,we discovered that p-NF-κB expression and inflammatory cytokines,including TNF-α,IL-6 and IL-1β,were not altered in BMSCs during osteogenic differentiation.
Recent studies have revealed the involvement of the NFκB pathway in the functional roles of JMJD proteins in various pathological processes.JMJD3 was found to regulate osteoclastogenesisviaRANKL and Ephrin receptor B4 signaling[35].Inhibition ofJMJD1A/KDM3Aameliorates hyperglycemia-mediated myocardial injury by modulating NF-κB/p65[36].JMJD1A/KDM3Agene silencing mitigates the phosphorylation of MAPKs and NF-κB/p65 activation to attenuate vascular smooth muscle cell injury[37].Furthermore,in mouse BMMs,JMJD1Csilencing promoted NF-κB activation and translocation and resulted in enhanced secretion of IL-6,IL-1β and TNF-α[13].Similarly,in the present study,JMJD1C knockdown in BMSCs upregulated p-NF-κB expression.Thus,our findings further confirm the negative regulatory effect ofJMJD1Con the expression of p-NF-κB.Moreover,JMJD1Cknockdown promoted the release of inflammatory cytokines(IL-6,IL-1β,and TNF-α) in BMSCs.Additionally,JMJD1Cknockdown suppressed BMSC osteogenic differentiation.Taken together,our study suggested that JMJD1C may play a role through the NF-κB pathway during the osteogenic differentiation of BMSCs.However,the role ofJMJD1C/NF-κB signaling in BMSC osteogenic differentiation requires further exploration.
CONCLUSION
In conclusion,during osteogenic differentiation,JMJD1Cwas upregulated,while NF-κB activation was not altered in BMSCs.JMJD1Cknockdown inhibited osteogenic differentiation and enhanced the activation of NF-κB signaling in BMSCs.Therefore,theJMJD1C/NF-κB pathway may modulate the osteoblast differentiation of BMSCs,which is possibly involved in the pathogenesis of osteoporosis.
ARTICLE HIGHLIGHTS
Research background
Osteoporosis,particularly women of postmenopausal age is elicited by the disequilibrium of osteoblastic bone formation and osteoclastic bone resorption.Elucidation of the molecular mechanisms underlying osteoporosis is important for developing effective therapeutic strategies for this disease.
Research motivation
Bone mesenchymal stem cells (BMSCs) have certain characteristics of differentiation into various types of cells,such as adipocytes and osteoblasts.So that,BMSCs are playing a critical role in bone homeostasis and munch more research groups are utilizing BMSCs for the repair of bone fractures resulting from osteoporosis.The researchers found that Jumonji C domain-containing 1C (JMJD1C) deficiency results in elevated alveolar bone loss in oral inflammatory lesions and loss ofJMJD1Caccelerates bone marrow-derived macrophage (BMM) differentiation into osteoclastsin vitro.
Database analysis revealed thatJMJD1Cexpression is downregulated in BMSCs from patients with osteoporosis.Furthermore,researchers revealed thatJMJD1Clevels are increased in osteogenic induction medium and BMSC growth medium supplemented with modified extracellular matrix.
Research objectives
To investigate whetherJMJD1Cis involved in osteoblast differentiation of BMSCs during osteoporosis.
Research methods
We isolated BMSCs from C57/BL6 suckling mice bone marrow tissues.We assessed the differentiation of BMSCs with Oil Red O staining,Alizarin red staining,alkaline phosphatase staining and reverse transcription coupled to the quantitative polymerase chain reaction.We isolated BMMs and incubated with receptor activator of nuclear factor-kappa B ligand to induce osteoclast differentiation.The tartrate-resistant acid phosphatase staining were used to confirm the effect of osteoclast differentiation.We used enzyme-linked immunosorbent assay to measure the levels of inflammatory cytokines,including tumor necrosis factor alpha (TNF-α),interleukin-6 (IL)-6 and IL-1β.
Research results
JMJD1CmRNA and protein expression was increased in BMSCs after osteoblast induction.SilenceJMJD1Crepressed osteogenic differentiation and enhanced nuclear factor-κB (NF-κB) activation and inflammatory cytokine release in BMSCs.JMJD1Cupregulation decreased during BMM osteoclast differentiation.
Research conclusions
We found that the signaling pathway ofJMJD1C/NF-κB is potentially involved in BMSC osteogenic differentiation and may play vital roles in the pathogenesis of osteoporosis.
Research perspectives
R&D of MSCs (BMSC,adipose-derived SC,human umbilical cord MSC and embryonic SC,etc.) and their preparations in the field of osteoporosis treatment.
FOOTNOTES
Author contributions:Li JY contributed independently to this work,and performed most of the research;Li JY,Wang TT,Ma L,Zhang Y,and Zhu D designed the research study;Li JY,Wang TT,Ma L,and Zhang Y contributed new reagents and analytic tools,analyzed the data and wrote and revised the manuscript;and all authors have read and approve the final manuscript.
Supported by2018 Henan Medical Science and Technology Research Plan Project,China,No.SBGJ2018019.
Institutional review board statement:This study was approved by the Ethics Committee of Beijing Tiantan Hospital Affiliated with Capital Medical University.
Institutional animal care and use committee statement:The animal experiments were approved by the Ethics Committee of Beijing Tiantan Hospital Affiliated with Capital Medical University.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
ARRIVE guidelines statement:The authors have read the ARRIVE guidelines,and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Di Zhu 0000-0003-3020-2503.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Zhao S
杂志排行
World Journal of Stem Cells的其它文章
- Human dental pulp stem/stromal cells in clinical practice
- VX-509 attenuates the stemness characteristics of colorectal cancer stem-like cells by regulating the epithelial-mesenchymal transition through Nodal/Smad2/3 signaling
- Extracellular vesicles derived from mesenchymal stem cells mediate extracellular matrix remodeling in osteoarthritis through the transport of microRNA-29a
- High quality repair of osteochondral defects in rats using the extracellular matrix of antler stem cells
- Effects of different concentrations of nicotinamide on hematopoietic stem cells cultured in vitro
- Advances in the differentiation of pluripotent stem cells into vascular cells
