Development of a clinical nomogram for prediction of response to neoadjuvant chemotherapy in patients with advanced gastric cancer
2024-03-11BingLiuYuJieXuFengRanChuGuangSunGuoDongZhaoShengZhongWang
Bing Liu, Yu-Jie Xu, Feng-Ran Chu, Guang Sun, Guo-Dong Zhao, Sheng-Zhong Wang
Abstract BACKGROUND The efficacy of neoadjuvant chemotherapy (NAC) in advanced gastric cancer (GC) is still a controversial issue.AIM To find factors associated with chemosensitivity to NAC treatment and to provide the optimal therapeutic strategies for GC patients receiving NAC.METHODS The clinical information was collected from 230 GC patients who received NAC treatment at the Central South University Xiangya School of Medicine Affiliated Haikou Hospital from January 2016 to December 2020. Least absolute shrinkage and selection operator logistic regression analysis was used to find the possible predictors. A nomogram model was employed to predict the response to NAC.RESULTS In total 230 patients were finally included in this study, including 154 males (67.0%) and 76 females (33.0%). The mean age was (59.37 ± 10.60) years, ranging from 24 years to 80 years. According to the tumor regression grade standard, there were 95 cases in the obvious response group (grade 0 or grade 1) and 135 cases in the poor response group (grade 2 or grade 3). The obvious response rate was 41.3%. Least absolute shrinkage and selection operator analysis showed that four risk factors significantly related to the efficacy of NAC were tumor location (P < 0.001), histological differentiation (P = 0.001), clinical T stage (P = 0.008), and carbohydrate antigen 724 (P = 0.008). The C-index for the prediction nomogram was 0.806. The calibration curve revealed that the predicted value exhibited good agreement with the actual value. Decision curve analysis showed that the nomogram had a good value in clinical application.CONCLUSION A nomogram combining tumor location, histological differentiation, clinical T stage, and carbohydrate antigen 724 showed satisfactory predictive power to the response of NAC and can be used by gastrointestinal surgeons to determine the optimal treatment strategies for advanced GC patients.
Key Words: Advanced gastric cancer; Predictor; Neoadjuvant chemotherapy; Nomogram; Tumor regression grade
INTRODUCTION
Gastric cancer (GC) is the fourth most common malignancy in terms of mortality, with approximately 770000 deaths in 2020[1]. However, early GC does not show obvious symptoms, leading to the extremely low early diagnosis rate globally[2]. For advanced GC patients, the 5-year survival rate is as low as 25%-31%[3-6]. Although gastrectomy plus D2 lymph node dissection and postoperative chemotherapy can improve the survival in advanced GC patients, their overall survival (OS) remains low.
Recently, neoadjuvant chemotherapy (NAC) has been proposed by several national and international guidelines as a critical treatment to improve the therapeutic effect in patients with advanced GC[7-9]. NAC is used for the downstaging of the tumor in the hopes of R0 resection for advanced GC patients[10]. The National Comprehensive Cancer Network guideline (version 2021.1) recommends that patients with clinical TNM stage ≥ T2N+ should receive NAC treatment[8]. The fifth edition of Japanese treatment guidelines recommend that patients with the stage from T2 to T4 and lymph node enlargement should receive NAC[9].
Although NAC can reduce tumor burden, decrease tumor stage, increase the radical resection rate, and improve survival outcomes, there are still many controversial points including chemotherapy scheme, chemotherapy frequency, and indications[11]. It was previously reported that NAC depends on the chemotherapeutic response of the tumor to achieve its survival advantage, indicating that patients with complete pathological response to NAC may show long OS and disease-free survival[12-14]. However, patients with low response to chemotherapy and no significant reduction of the tumor after chemotherapy may indicate a poor prognosis. For patients with a low objective response rate to NAC, the treatment not only delays surgery but also causes serious toxic side effects to patients. Therefore, it is very important to predict the sensitivity of NAC for patients with GC and further evaluate whether they are suitable for NAC. For those with poor sensitivity, surgery or other comprehensive treatment should be carried out immediately.
Recently, many studies have been conducted to identify predicting factors for NAC response, and nomogram models have been used for the prediction of advanced GC prognosis after NAC[15-19]. Recently, researchers have built a deep learning radiomic nomogram based on a computed tomography (CT) scan before treatment to solve this problem[20]. Compared with the traditional segmented models, these nomograms showed superior performance. However, most studies have only discussed the prognosis of patients and postoperative complications after NAC. Only a few studies identified some predictors that could predict the effect of NAC before chemotherapy.
Therefore, in this study, we retrospectively analyzed the tumor biological characteristics and clinical parameters that may affect the effect of NAC in patients with advanced GC and established a nomogram model to predict the response of NAC, aiming to provide individualized treatment strategies and maximize the benefits for patients with advanced GC.
MATERIALS AND METHODS
Patients and data collection
This retrospective study was approved by the Research Ethics Committee of Haikou Hospital affiliated to Xiangya Medical College of Central South University. From January 2016 to December 2020, clinical information was extracted from the medical records of 259 patients with advanced GC who received NAC treatment in Haikou Hospital affiliated to Xiangya Medical College of Central South University. Then, the extracted information was analyzed retrospectively. Inclusion criteria were as follows: (1) Patients were diagnosed with GC through gastroscopy and biopsy; (2) GC patients with clinical stage T2N + M0 or T3-4N0/ + M0; (3) Patients who had completed NAC; (4) GC patients received radical gastrectomy after NAC; (5) The chemotherapy regimen was XELOX (capecitabine plus oxaliplatin); and (6) Patients were aged between 18 and 80. The exclusion criteria included: (1) Preoperative chemotherapy was not completed as planned (< 3 cycles); (2) In addition to GC, the patient also suffered from other malignant tumors; (3) Patients with gastric stump cancer; (4) Patients had received radiotherapy, traditional Chinese medicine, or other anti-tumor treatment; (5) Clinical data were incomplete; and (6) Postoperative pathology examination was not adenocarcinoma.
Treatment process
The patients whose clinical stage was T2N + M0 or T3-4N0/+ M0 were treated with laparoscopic exploration. If no distant metastasis such as intraperitoneal metastasis was found during the operation and the tumor could be resected, then chemotherapy was given for 3 cycles on the 1stor 2ndday after the laparoscopic exploration. Adjustments to dosage were made based on the effectiveness and patient tolerability. Two weeks after the completion of NAC, the resectability of the primary tumor site was confirmed again according to endoscopy and enhanced CT examination. Then, the surgery was performed. All enrolled patients received curative tumor resection (total or subtotal gastrectomy, open or laparoscopic surgery) with D2 lymphadenectomy.
Data collection
The clinical data collected before NAC in this study included age, sex, body mass index, blood group, tumor markers [carcinoembryonic antigen, carbohydrate antigen (CA) 125, CA199, CA724], tumor location, tumor size, depth of invasion, lymph node metastasis, pathological classification, albumin, platelet count, lymphocytes, neutrophils, monocytes, and smoking history. Tumor size, depth of invasion, and lymph node metastasis were evaluated on the basis of enhanced CT with laparoscopic exploration before NAC. The curative effect evaluation standard of NAC was based on the TRG standard as proposed by the National Comprehensive Cancer Network guidelines in 2021[8]. Grade 0 (complete response) is defined as no viable cancer cells, including lymph cells. Grade 1 (near complete response) is defined as single cells or rare small group of cancer cells. Grade 2 (partial response) was interpreted as residual cancer cells with evident tumor regression but more than single cells or rare small groups of cancer cells. Grade 3 (poor or no response) was defined as intermediate extensive residual cancer with no evident tumor regression. We classified grade 0 and grade 1 as obvious response. Grade 2 and grade 3 were classified as poor response. Postoperative complications were defined as events occurring within 30 d after surgery, which were assessed by the Clavien-Dindo classification system[21,22]. The adverse events of NAC were based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0).
Statistical analysis
All statistical analyses were performed by SPSS software ver. 22.0 (IBM, Armonk, NY, United States) and R version 4.0.3 software (The R Foundation for Statistical Computing, Vienna, Austria. www.r-project.org).
Univariate analysis:Parameters that were not normally distributed were expressed in the form of median (25% to 75% interquartile range) and were analyzed by the Mann-Whitney test, while normally distributed parameters were expressed in the form of mean ± standard deviation and were analyzed by Student’st-test. Categorical variables were analyzed by theχ2test. The test level α = 0.05.
Multivariate analysis:The least absolute shrinkage and selection operator (LASSO) method was used to select the most useful predictive factors for outcomes of NAC response (P< 0.05). The regression coefficient and odds ratio with 95% confidence intervals were estimated.
Nomogram construction:To predict the response of NAC, a nomogram including significant prognostic factors was constructed based on logistic regression analysis using glm R package (version 4.0.3). The consistency index was calculated. Decision curve analysis and correction curve were drawn to evaluate the predictive efficiency of the nomogram.
RESULTS
Baseline and patient characteristics
Patient information is listed in Table 1. Due to incomplete clinical data, receiving targeted therapy, or pathological results for non-adenocarcinoma, 29 patients were excluded. A total of 230 patients entered the study, consisting of 154 males (67.0%) and 76 females (33.0%). All patients were aged 24-80 years (average, 59.37 ± 10.60). In line with the TRG standard, 95 patients were assigned to the obvious response group (grades 0-1), whereas 135 patients were assigned to the poor response group (grades 2-3), with the obvious response rate being 41.3%. The cases of depth of invasion T2 or T3 were 71, and T4 were 159. There were 83 patients (36.1%) whose tumors were at the esophagogastric junction. In total, 180 patients showed positive lymph node metastasis, accounting for 78.3%.

Table 1 Characteristics of patients in the primary and P value of univariate analysis
Factors of NAC response
Table 1 displays univariable associations between the clinical parameters and response of NAC. Significant factors (P< 0.05) included tumor location, differentiation, clinical T stage, and CA724. The results showed that tumors in the esophagogastric junction displayed better efficacy than that of non-esophagogastric junction tumors. Greater differentiation level (well/moderatevspoor differentiation), lower T stage (T2/T3vsT4 stage), and lower CA724 level were associated with a better NAC efficacy.
To avoid the multicollinearity problem in regression analysis, the distribution coefficient was analyzed by LASSO regression with an elastic net penalty. The results of the LASSO regression analysis were the same as those of the univariate analysis. Four independent predictors including tumor location, differentiation, clinical T stage, and CA724 were included in the final model, as shown in Figure 1. The model incorporating the above independent predictors was developed and presented as the nomogram (Figure 2). The C-index for the prediction nomogram was 0.806, indicating that the prediction performance of this nomogram has good feasibility. The calibration curve of the NAC nomogram demonstrated a good consistency between prediction and actual observations in the primary cohort (Figure 3). The value of the nomogram and its use in the clinic was evaluated by the decision curve analysis, evaluating the value in terms of clinical application for the NAC nomogram (Figure 4).

Figure 1 Screening of variables based on least absolute shrinkage and selection operator regression. A: Variation characteristics of the coefficient of variables; B: Selection process of the optimum value of the parameter λ in the least absolute shrinkage and selection operator regression model by cross-validation method.
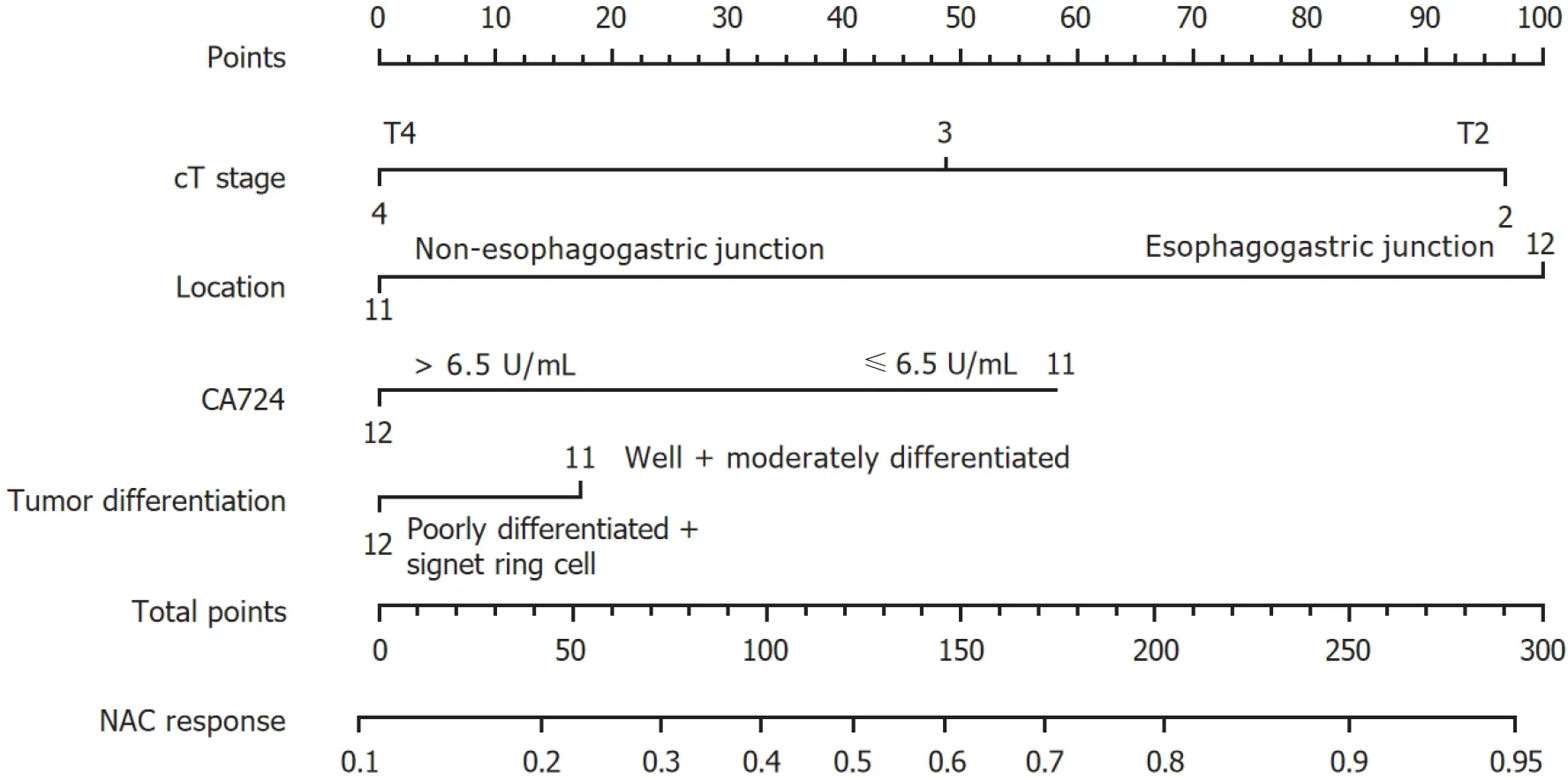
Figure 2 Nomogram for predicting response to neoadjuvant chemotherapy. CA724: Carbohydrate antigen 724; NAC: Neoadjuvant chemotherapy.
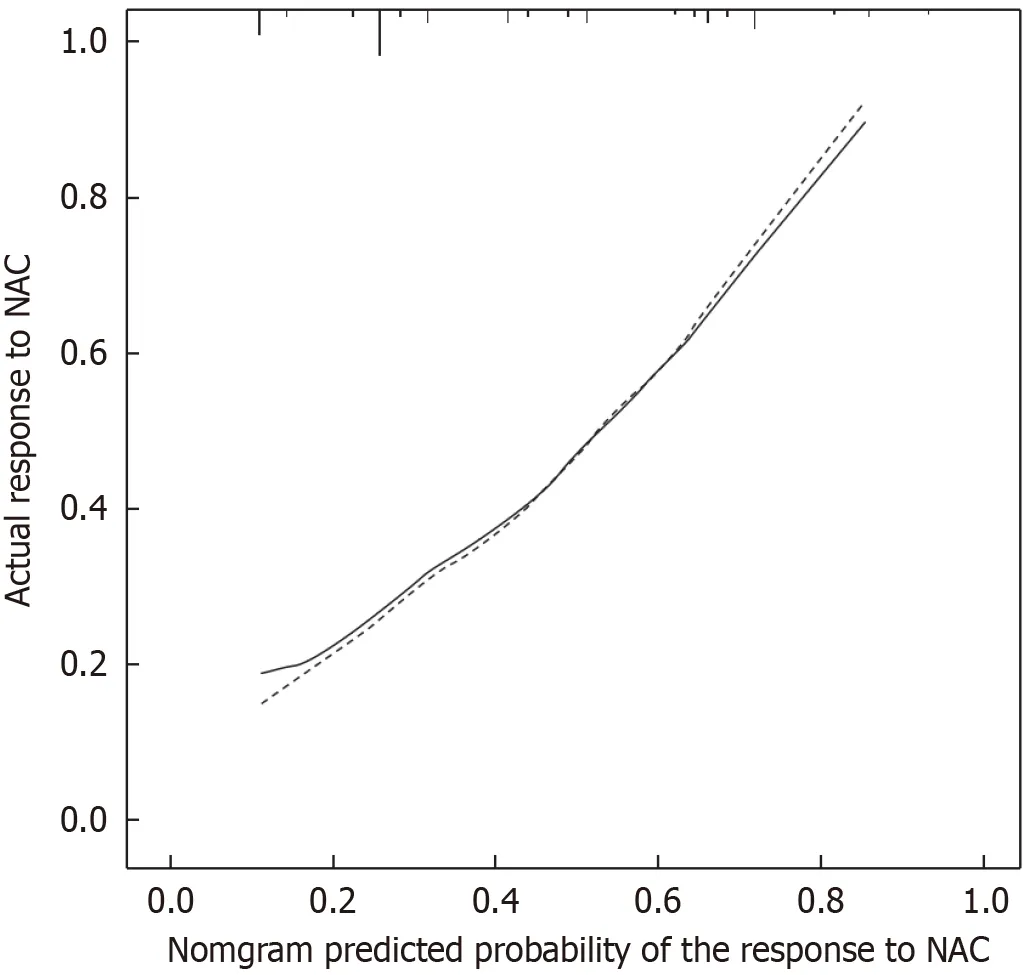
Figure 3 Calibration curve for the nomogram model. NAC: Neoadjuvant chemotherapy.

Figure 4 Decision curve analysis analyzed clinical utility of the nomogram. The y-axis represented net benefits, and the x-axis measured threshold probability. The horizontal solid line indicated the advantage for patients not receiving neoadjuvant chemotherapy (NAC), the oblique solid line represented the advantage for patients receiving NAC, and the diagonal dotted line (nomogram) indicated survival based on nomogram scores to resolve whether a patient should receive NAC. A treatment strategy was superior if it had the highest value compared to other models, including two simple strategies, such as performing NAC for all patients (sloping solid line) or performing primary surgery first (horizontal solid line).
Toxicity of NAC
Based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0, the overall incidence of NAC adverse events was 85.7%, and the rate of grade 3/4 toxicity was 33.5%. The main side effects were hematological toxicity and gastrointestinal reaction. Anemia (15.7%) was the most common grade 3/4 adverse event (Table 2). In addition, we found that in the gastrointestinal, hematological, and neurological systems, the incidence of adverse reaction in the group with poor response was slightly higher than that in the group with obvious response, even though the differences were not statistically significant (P> 0.05), as shown in Table 3.
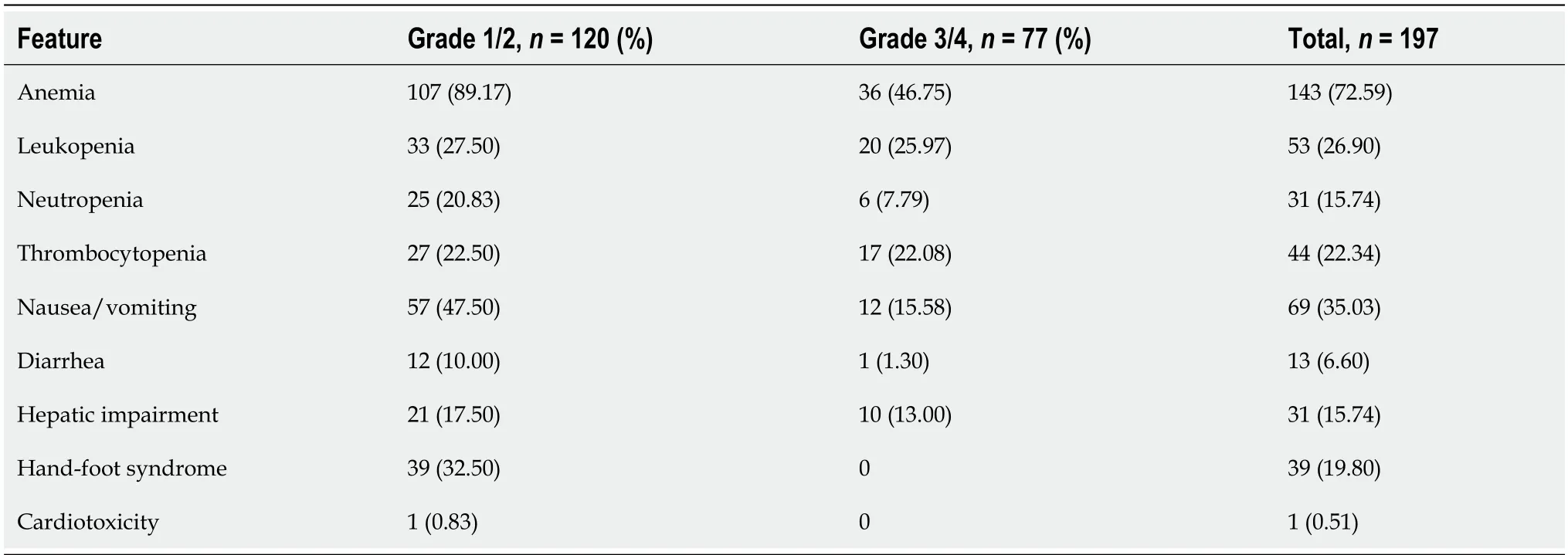
Table 2 Toxicity of neoadjuvant chemotherapy

Table 3 Comparison of toxicity between the obvious response group and the poor response group

Table 4 Postoperative complications after neoadjuvant chemotherapy (Clavien-Dindo classification)

Table 5 Comparison of postoperative complications between the obvious response group and the poor response group
Postoperative complications
In this study, 51 patients (22.2%) suffered from postoperative complications, and most of them were Clavien-Dindo grade 2 complications. The most common complications were pulmonary infection and pleural effusion (15.2%). One patient died of anastomotic leakage and abdominal hemorrhage. There was no statistical difference in the incidence of each complication between the obvious response group and the poor response group. Detailed information was listed in Tables 4 and 5.
DISCUSSION
Surgery is the most vital treatment for GC. More than 60% of patients have reached the advanced stage at the time of diagnosis, which leads to a low radical resection rate. Therefore, an efficient method for increasing the radical resection rate is urgently needed in the clinic[23].
Previous studies have indicated that surgery can induce tumor cells to transform into drug-resistant clones and increase the production of tumor growth stimulating factors, which can promote tumor cell proliferation. In the early stage, cell proliferation and DNA replication are active with the small number of tumor cells; at this time, tumor cells are more sensitive to chemotherapeutic drugs[24]. Therefore, giving chemotherapy drugs before tumor resection can not only kill the primary tumor but also inhibit the growth stimulating factors of cancer cells, which is also effective for micrometastases. It indicates that the earlier chemotherapy is administered, the fewer drug-resistant cell lines[12]. This highlights the importance of NAC.
At present, preoperative chemotherapy is receiving increasing attention. The role of NAC is to help surgeons decrease the primary tumor size and stage, eliminate micrometastasis, alleviate tumor related symptoms, improve curative resection rate, and reduce postoperative recurrence rate. However, some patients who are not sensitive to chemotherapy drugs cannot benefit from NAC, causing tumor progression and delaying the time to surgical resection. Studies have shown that approximately 15% of patients receiving preoperative neoadjuvant therapy have the risk of tumor progression[25]. Moreover, patients often suffer from side effects of NAC including cardiotoxicity, hepatotoxicity, and nephrotoxicity, increasing the risk of complications and mortality during surgery. Therefore, it is particularly important to predict the efficacy of NAC. Thus, we performed an exploratory study to identify pretreatment parameters that can predict NAC sensitivity, aiming to provide the basis for individualized treatment of GC patients. For patients with promising responsiveness to NAC, NAC should be considered. Otherwise, surgery or other comprehensive treatment should be performed as soon as possible.
Our data showed that the obvious response rate of NAC for advanced GC was 41.3%, which further indicated that only a portion of patients can benefit from NAC, thereby emphasizing the importance of predicting the responses to NAC. According to the results of the univariate and multivariate analysis, we found that tumor location, differentiation, depth of invasion, and CA724 were significant influencing factors for predicting the response of NAC. Using the four factors, we constructed a nomogram to predict the NAC response before performing gastrectomy with lymph node dissection.
A German retrospective cohort study including 410 patients indicated that a tumor in the upper two-thirds of the stomach tended to have a better response to NAC[26]. Another study performed by Liet al[27] also showed a similar finding. This was consistent with our result that the obvious response rate of NAC in patients with tumors located in the esophagogastric junction (63.86%) was higher than that in patients with tumors elsewhere (28.57%). The difference was statistically significant (P< 0.05).
Many studies have shown that serum tumor markers were associated with diagnosis, prognosis, and the therapeutic effect of preoperative or postoperative chemotherapy in GC[28,29]. Another study had indicated that CA724 was an independent factor for efficacy of NAC in GC[30]. Consistently, this work suggested that an increased CA724 level was related to the poor NAC response. Nonetheless, as reported in another study, CA724 only achieved a 45.0% sensitivity[31]. Additionally, CA724 was related to environmental factors andHelicobacter pyloriinfection[32,33]. Based on the above findings, a bias might exist in evaluating the patient condition according to CA724 alone, and many studies are needed to solve this problem.
Patients with a well-differentiated tumor had better survival than those with poor differentiation in GC[34,35], and previous studies suggested that differentiation is a vital predictor of pathological response[36,37], conforming to our study. However, in contrast to a previous study[38], our results showed that patients with a lower T stage (T2, T3) had a better response to NAC than advanced T stage (T4). The reason is that NAC regimens bring relatively serious toxicity and side effects in patients, damaging hematological, digestive, and nervous systems[10]. In this study, the overall incidence of NAC adverse reactions was 85.7%, and the rate of grade 3/4 toxicity was 33.5%. Therefore, it is important to select the optimal treatment options for different patients. We suggest that for these patients who are not sensitive to NAC, one solution is to apply other regimens of NAC, such as fluorouracil, leucovorin, oxaliplatin, docetaxel, resulting in superior OS compared with cisplatin and capecitabine[39]. The other is to implement surgery as soon as possible to avoid the time interval of chemotherapy.
Recent articles have concentrated on the relationship of the tumor with serum inflammatory factors, suggesting that lymphocytes, neutrophils, and platelets within the tumor microenvironment are associated with tumor metastasis and progression because inflammatory chemokines and cytokines are produced[40-45]. Typically, the increased neutrophil/platelet proportion and the decreased lymphocyte proportion suggests a damaged immune response and strong inflammatory response, thereby promoting cancer cell proliferation, distant organ metastasis, lymph node metastasis, and invasion. However, our study suggests that inflammatory factors such as platelets, neutrophils, and lymphocytes are not independent predictors of chemosensitivity.
Although a nomogram predicting the response of NAC had been established with a C-index of 0.767[10], our study achieved a C-index of 0.806, indicating a better performance for prediction than a previously reported study. LASSO analysis was used to find significant clinical factors in this study, while other similar articles mostly used logistic regression analysis. All patients were treated with XELOX, and thus the results are more reliable. Meanwhile, we also discussed the adverse reactions and postoperative complications of NAC, which further demonstrate the importance of predicting response to NAC.
However, this study has the following limitations. The results may be biased due to the retrospective design. In addition, because most patients enrolled in this study were in the most recent 2 years, there were insufficient survival events to analyze the impact of the predictor and chemosensitivity on OS rate. Therefore, high-quality studies with a larger cohort of patients are warranted to address this issue.
CONCLUSION
To conclude, four risk factors significantly related to response of NAC included tumor location, differentiation, clinical T stage, and CA724. The established nomogram exhibited a favorable prediction performance in predicting NAC response, which can be applied in identifying the best therapeutic strategies in advanced GC patients by gastrointestinal surgeons.
ARTICLE HIGHLIGHTS
Research background
Neoadjuvant chemotherapy (NAC) has an unclear therapeutic effect on advanced gastric cancer (GC).
Research motivation
This work focused on identifying factors related to chemosensitivity to NAC treatment to be able to offer the best treatments for GC patients receiving NAC.
Research objectives
To find factors associated with chemosensitivity to NAC treatment and to provide the optimal therapeutic strategies for GC patients receiving NAC.
Research methods
Predicting factors were identified by least absolute shrinkage and selection operator logistic regression. Additionally, a nomogram model was employed to predict the response to NAC.
Research results
We enrolled 230 patients, consisting of 154 males (67.0%) and 76 females (33.0%). These patients were aged 24-80 years (average, 59.37 ± 10.60). According to the TRG standard, 95 patients were assigned into the obvious response group (grades 0-1) and 135 into the poor response group (grades 2-3), yielding an obvious response rate of 41.3%. As revealed by the least absolute shrinkage and selection operator regression, tumor location (P< 0.001), histological differentiation (P= 0.001), clinical T stage (P= 0.008), and carbohydrate antigen 724 (P= 0.008) were significant risk factors for NAC efficacy. The C-index of the prediction nomogram was 0.806. According to calibration curve analysis, the predicted value was highly consistent with real measurement. Moreover, decision curve analysis revealed the high application value of this nomogram clinically.
Research conclusions
Our nomogram combining tumor location, histological differentiation, clinical T stage, and carbohydrate antigen 724 showed a high performance in predicting NAC response, which can be applied in identifying the best therapeutic strategies for advanced GC patients by gastrointestinal surgeons.
Research perspectives
Candidate predictive factors were identified by the least absolute shrinkage and selection operator logistic regression. The response to NAC was predicted by a nomogram model.
FOOTNOTES
Co-first authors:Bing Liu and Yu-Jie Xu.
Co-corresponding authors:Sheng-Zhong Wang and Guo-Dong Zhao.
Author contributions:Liu B and Xu YJ contributed to paper writing and data analysis; Chu FR revised the manuscript; Sun G contributed to data collection; Wang SZ and Zhao GD contributed to supervision and paper revision; Liu B was in charge of proposing the research ideas, setting the overall research objectives, preparing, creating, and describing the works to be published, and writing the first draft; Xu YJ was responsible for verifying the paper data, writing computer code and supporting algorithms, testing the existing code components, creating models, and proposing improvements of the paper design and for statistical analysis; Wang SZ supervised and led the planning and execution of research activities, the revision of manuscript content, especially the critical commentary and revision, and the polishing of manuscript language; Zhao GD improved the design of the paper, checked the authenticity of the data, verified the overall reusability of the conclusions, experiments, and other contents of the research results, and provided financial support for the publication project. Considering the significant contributions made by Liu B, Xu YJ, Wang SZ, and Zhao GD to this paper, all authors unanimously agreed to designate Liu B and Xu YJ as the co-first authors and Wang SZ and Zhao GD as the co-corresponding authors.
Supported byNatural Science Foundation of Hainan Province, No. 823RC609.
Institutional review board statement:This study was reviewed and approved by the Ethics Committee of The Central South University Xiangya School of Medicine Affiliated Haikou Hospital.
Informed consent statement:All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrollment.
Conflict-of-interest statement:The authors declare that they have no financial relationships to disclose.
Data sharing statement:The data that support the results of this research is available on request from the corresponding author. Considering privacy or ethical restrictions, the data is not publicly available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Sheng-Zhong Wang 0009-0003-1532-9917.
S-Editor:Qu XL
L-Editor:Filipodia
P-Editor:Zheng XM
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Actuality and underlying mechanisms of systemic immuneinflammation index and geriatric nutritional risk index prognostic value in hepatocellular carcinoma
- Mutational landscape of TP53 and CDH1 in gastric cancer
- Phospholipase A2 enzymes PLA2G2A and PLA2G12B as potential diagnostic and prognostic biomarkers in cholangiocarcinoma
- Classification of anatomical morphology of cystic duct and its association with gallstone
- Will partial splenic embolization followed by splenectomy increase intraoperative bleeding?
- Influence of donor age on liver transplantation outcomes: A multivariate analysis and comparative study
