OsbZIP01 Affects Plant Growth and Development by Regulating OsSD1 in Rice
2024-03-09DongXinliZhouYangZhangYaqiRongFuxiDuJiahongHongZheyuanHUPeisongYusong
Dong Xinli, Zhou Yang, Zhang Yaqi, Rong Fuxi, Du Jiahong, Hong Zheyuan, HU Peisong, Lü Yusong
Research Paper
OsbZIP01 Affects Plant Growth and Development by Regulatingin Rice
Dong Xinli1, 2, #, Zhou Yang1, #, Zhang Yaqi1, Rong Fuxi1, Du Jiahong1, Hong Zheyuan1, HU Peisong1, 2, Lü Yusong1, 2
(310006, China; These authors contributed equally to this work)
s:As the ‘Green Revolution’ gene,(encoding GA20ox2), has been widely applied to improve yield in rice breeding. However, research on its transcriptional regulation is limited. Here, we identified a transcription factor OsbZIP01, which can suppress the expression ofand regulate gibberellin (GA) biosynthesis in rice. Knockout mutants ofexhibited increased plant height, while the over- expression lines showed a semi-dwarf phenotype and diminished germination rate. Furthermore, the semi-dwarf phenotype of-, was caused by the reduced internode length, which was accompanied by a thin stem width. The predominant expression ofwas observed in leaves and sheaths. OsbZIP01 protein was localized in the nucleus and showed transcriptional repression activity. In addition, OsbZIP01 could directly bind to the promoter of thegene, and inhibit its transcription. The semi-dwarf phenotype of-could be rescued by exogenous GA3. Meanwhile, thedouble mutant showed a shorter shoot length compared with the wild type, indicating that OsbZIP01 regulated plant growth mainly through the GA biosynthesis pathway. Collectively, OsbZIP01 negatively regulates GA biosynthesis by restrainingtranscription, thereby affecting plant growth and development.
OsbZIP01;; gibberellin biosynthesis; dwarf and germination
Plant architecture plays a crucial role in crop growth and productivity. Favorable architectural traits confer higher yields of products (Silverstone and Sun, 2000; Gao and Chu, 2020). Semi-dwarfism is one of the most attractive and useful traits in cereal crop breeding as it enhances lodging resistance and increases total biomass from short stature (Liu C et al, 2018; Su et al, 2021). During the ‘Green Revolution’ in rice, the mutation of, encoding OsGA20ox2, results in a semi-dwarf plant architecture through decreased gibberellin (GA) biosynthesis, leading to increased grain yield (Monna et al, 2002; Sasaki et al, 2002; Spielmeyer et al, 2002). Indeed, different alleles ofin rice varieties confer only the semi-dwarf phenotype without adverse pleiotropic effects on yield (Asano et al, 2011; Liu C et al, 2018; Beyene et al, 2022; Zhou et al, 2023). Therefore, it is of great significance to clarify the fine regulation ofin plant height through GA biosynthesis.
Essential GA controls many aspects of plant growth and development, including seed germination, stem and leaf elongation, and floral development. Particularly, GA is one of the major factors determining plant height and germination. The biosynthesis of bioactive GAs involves several parallel enzymatic reactions, including GA20-oxidases (GA20ox) and GA3-oxidases (GA3ox) (Hedden and Phillips, 2000; Gao and Chu, 2020). The first step in GA biosynthesis involves the conversion of geranylgeranyl diphosphate into tetracyclic hydrocarbon intermediate ent-copalyl diphosphate and then into ent-kaurene by ent-copalyl diphosphate synthase (CPS) and ent-kaurene synthase (KS), respectively. Both CPS and KS are members of the terpene synthase family. Subsequently, ent-kaurene is converted into GA12by two cytochrome P450 monooxygenases (P450), ent-kaurene oxidase (KO) and ent-kaurenoic acid oxidase (KAO). Then, GA12, an early GA in the biosynthesis pathway, is converted to bioactive GA4via GA15, GA24, and GA9or to bioactive GA1via GA53, GA44, GA19, and GA20. The flux of bioactive GA intermediates (i.e., GA1and GA4) is regulated by three dioxygenases, GA20-oxidase (GA20ox), GA3-oxidase (GA3ox), and GA2-oxidase (GA2ox). Among them, GA20ox and GA3ox catalyze the formation of active GAs, while GA2ox converts them into inactive forms. In rice, all loss-of-function mutations of GA biosynthesis genes have been identified, and null mutants for these genes, with the exception ofand, show a severe dwarf phenotype (Hedden, 2001; Yamaguchi, 2008). Previous studies have shown that the semi-dwarf phenotype and lodging resistance in plants withalleles are due to shorter lower internodes, which result in a lower center of gravity compared with plants of the same height (Liu F et al, 2018).
Transcription factors (TFs) are essential for plant growth and development by binding to the elements of downstream genes. GA biosynthesis is a complex process involving many enzymes. Some TFs have been identified to participate in plant height development by directly regulating the expression of key enzyme genes (Li et al, 2015; Zhou et al, 2015; Burman et al, 2018). OsNAC2, a negative regulator of plant height, directly interacts with the promoter ofandin rice (Chen et al, 2015). OsYABBY1 and OsYABBY4 are involved in the negative regulation of GA biosynthesis by binding to the promoter regions of() and() genes, respectively (Yang et al, 2016). Overexpression ofandleads to a semi-dwarf phenotype (Yang et al, 2016). OsWOX3A directly binds to the promoter ofin the negative feedback regulation of the GA biosynthesis pathway (Cho et al, 2016). Additionally, OsEATB, an APETALA2 (AP2)/ethylene-responsive element binding factor, can reduce rice plant height by down-regulating the transcription of, whichencodes a GA biosynthetic enzyme (Cho et al, 2016). A recent study found that the ethylene signal core member OsEIL1 can directly bind to the promoter region ofand improve bioactive GA4biosynthesis to enhance flood resistance by internode elongation (Kuroha et al, 2018). The MADS-box TF OsMADS57 directly down-regulates the transcription of GA inactivation genethrough binding to the CArG-box motifs in the promoter region to regulate stem elongation in rice via a GA-mediated regulatory pathway (Lu et al, 2012; Chu et al, 2019; Yin et al, 2019). Thus, the finely-tuned regulation of GA biosynthesis by TFs is essential for plant architecture improvement.
Basic leucine zipper (bZIP) TFs characteristically harbor a bZIP domain composed of two structural features: a DNA-binding basic region and a Leu zipper dimerization region, which have been shown to regulate diverse plant-specific phenomena, including seed maturation and germination, floral induction and development, and photomorphogenesis (Hasegawa et al, 2021; Bhatnagar et al, 2023). Despite the diverse functions of bZIP TFs in plant growth and development, little is known about their function in regulating plant height in rice. Overexpression ofin transgenic rice reduces the plant height considerably by decreasing the expression of thegene (Burman et al, 2018). However, limited studies have been involved in finely tuning plant architecture through the transcriptional regulation of the ‘Green Revolution’ genein riceOur results indicated that overexpression ofin plants resulted in a semi-dwarf phenotype and reduced germination rate, which could be rescued by the application of exogenous GA3. OsbZIP01 directly bound to thegene promoter, and inhibited its transcription. These results showed that OsbZIP01 affected plant growth by regulating gene expression of GA metabolism, which revealed a molecular mechanism that OsbZIP01 finely controlled plant height in rice.
Results
Characterization of OsbZIP01 gene
To better characterize the function ofin rice, the expression ofwas checked in the CREP database (http://crep.ncpgr.cn/crep-cgi/home.pl), including three main rice varieties, namely Minghui 63 (MH63), Shanyou 63 (SY63), and Zhenshan 97 (ZS97). We found that althoughwas mainly expressed in leaves and sheaths, its transcripts were detectable across all tissues examined (Fig. 1-A). To better investigate the function of OsbZIP01, subcellular localization was performed (Fig. 1-B). The results showed that the OsbZIP01-GFP protein was located in the nucleus of tobacco leaf epidermal cells, whereas the empty vector control displayed fluorescence in various cellular compartments as expected. This suggested that OsbZIP01 may function as a TF. The bZIP TFs possess transactivation domains, enabling them to stimulate the transcription process in the cell (Zong et al, 2016; Ma et al, 2018). To test whether OsbZIP01 also has transcriptional activation activity, a yeast transactivation assay was carried out (Fig. 1-C). The results revealed that while the yeast cells with the positive control grew on SD-Trp medium, the cells with the BK-OsbZIP01 construct did not grow on SD-Trp-Ade-His medium, indicating that OsbZIP01 did not possess a functional transactivation ability.
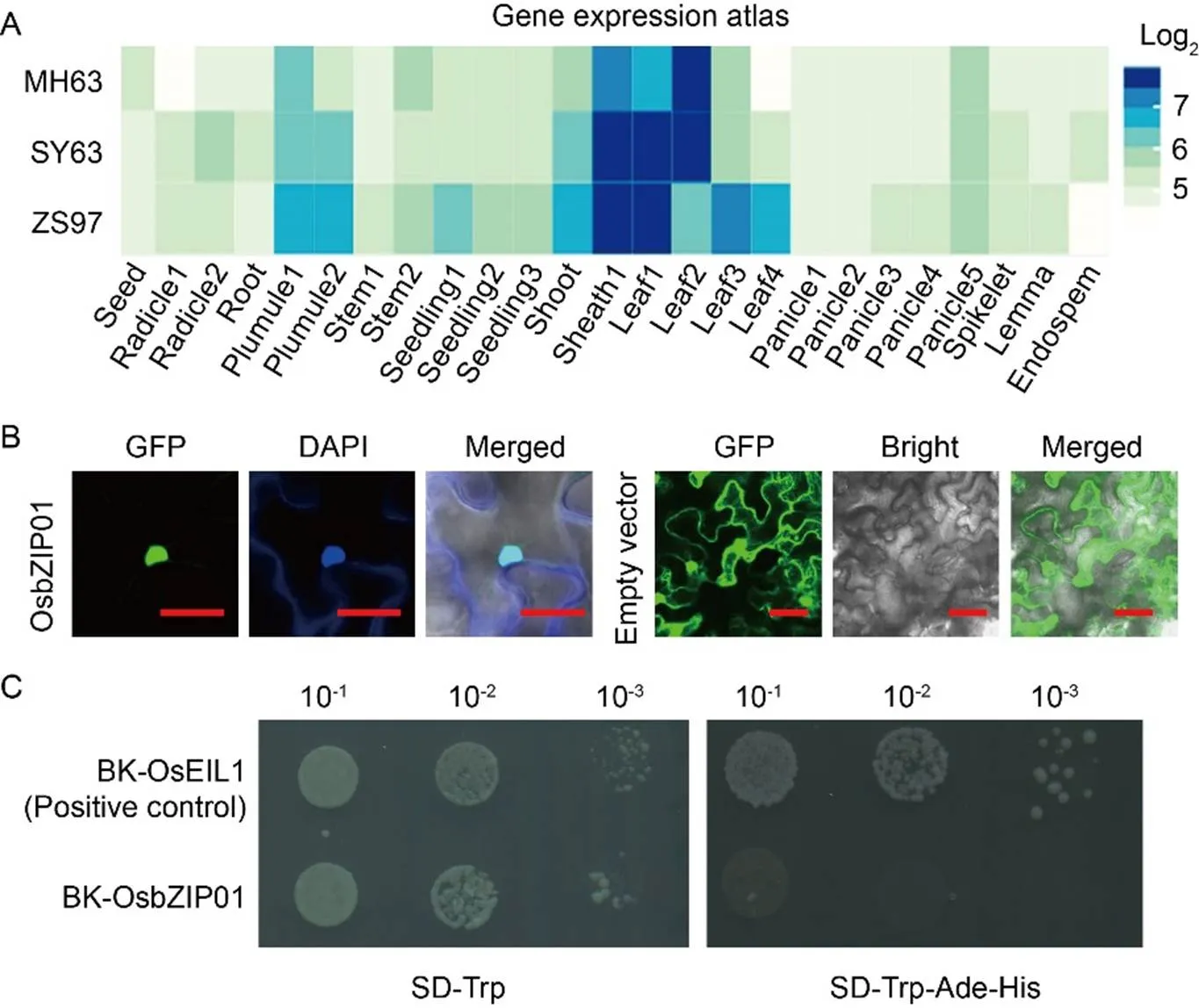
Fig. 1. Expression pattern ofand its protein characterization.
A, Gene expression atlas ofin three representativevarieties of China is from the CREP database (http://crep.ncpgr.cn/crep-cgi/home.pl). The expression value is log2-transformed. MH63, Minghui 63; SY63, Shanyou 63; ZS97, Zhenshan 97.
B, Subcellular localization of OsbZIP01. DAPI, 4′,6-diamidino-2-phenylindole; GFP, Green fluorescent protein. Scale bars are 100 μm.
C,Transactivation assay of OsbZIP01 in yeast cells. BK, pDEST-GBKT7; SD, Synthetic dropout medium; Trp, Tryptophan; Ade, Adenine; His, Histidine.
OsbZIP01 modulates seed germination and plant height development
To investigate the function of, we generated loss-of-function mutants of() in thecv. Shennong 265 (SN265) background using the CRISPR/Cas9 technology. Themutant harbored a 1-bp deletion, while theknockout lines contained a 1-bp insertion in the first exon, leading to a frame shift in the open reading frame (Fig. 2-A). Additionally, we developed transgenic lines overexpressingdriven by thepromoter () in the SN265 background, and the increased expression of the target genes was confirmed by qRT-PCR (Fig. S1).
Compared with wild type (WT, SN265) plants, themutants exhibited a moderate increase (8.0%) in plant height at the heading stage (Fig. 2-B and -D). Conversely, overexpression lines displayed a conspicuous decrease of 20.2% (Fig. 2-C and -D). The results indicated thathad a negative impact on plant height in rice. GA is one of the most important plant growth hormones that plays a fundamental role in regulating plant height in rice (Oikawa et al, 2004). Given this, we hypothesized that the variation in plant height in transgenic plants could be attributed to GA. In addition to its effect on plant height, GA is known to influence seed germination (Zhang et al, 2019). Therefore, we performed seed germination assays on,, and WT seeds (Fig. 2-E). We found that the germination rate ofseeds was significantly higher, while the germination rate of theline was significantly retarded, compared with the WT (Fig. 2-F). These findings substantiated the notion thatmay modulate the GA balance in rice, thereby impacting both plant height and germination.
OsbZIP01 mainly affects cell length of stem
The twooverexpression transgenic lines displayed a semi-dwarf phenotype and were distinctly shorter than WT (Fig. 2-C). Rice plant height is determined by the number and length of internodes (Yang and Hwa 2008; Wang and Li, 2008). To determine the reason for the semi-dwarf phenotype, we compared the number and length of the internodes of WT,mutant, and, and found that they had the same total number of internodes, but all the internodes ofwere significantly shortened (Fig. 3-A and -B), which finally made the height of-lines only 79.8% of that of the WT (Fig. 2-C and -D). In addition, statistical analysis revealed a significant reduction in the length of the internode (counted from the top) incompared with the WT (Fig. 3-B). Thus, our results indicated that the semi-dwarf phenotype of-was caused by the reduced internode length. Besides, the reduction in internode length was accompanied by a reduction in stem width, as the stem of maturetransgenic lines was thinner than that of the WT (Fig. 3-C).
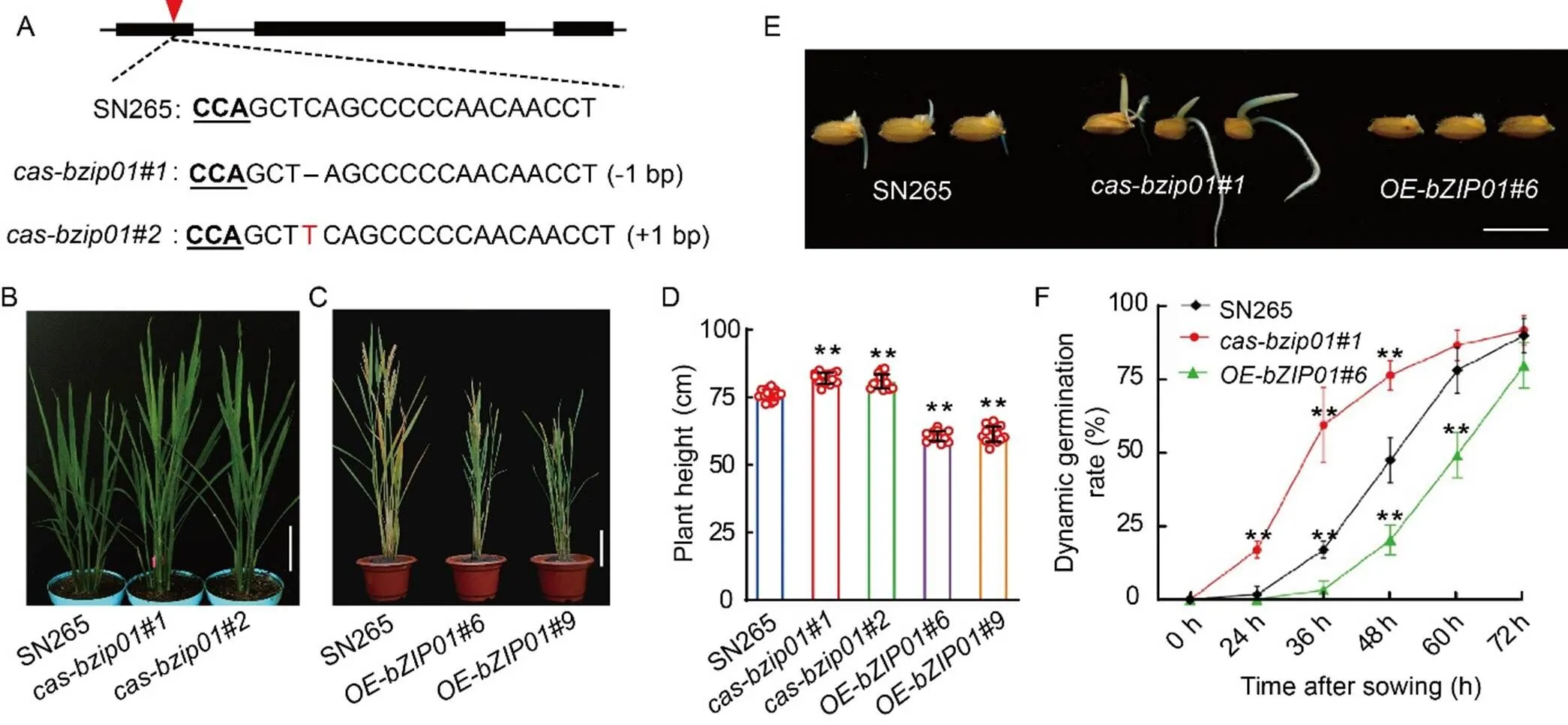
Fig. 2. Phenotypic characterization of Shennong 265 (SN265),, andtransgenic plants.
A, Schematic diagram of the sgRNA site ofgene in SN265 and two mutants (and) by CRISPR/Cas9 system. The red triangle indicates the sgRNA position.
B, Phenotypes of SN265 andplants (and) at the heading stage. Scale bar is 10 cm.
C, Morphology of SN265and overexpression lines (and) at the maturity stage. Scale bar is 10 cm.
D, Plant heights of SN265 and transgenic plants (,,, and) at the maturity stage.
E, Seed germination phenotypes of SN265,,andplants. Sterile grains were sown on Murashige and Skoog plates, and photographs were takenat 48 h after germination at 30 ºC. Scale bar is 1 cm.
F,Dynamic germination rates of SN265,,andafter sown for 72 h.
In D and F, Data are Mean ± SD of three independent experiments (= 10 for each experiment). ** indicates the significant differences between transgenic plants and wild type (SN265) at the< 0.01 by the Student’s-test.
Internode length is determined by the cell length and number in the intermediate meristem (Wang and Li, 2008; Yang and Hwa 2008). To explore whether the reduction of internode length in-is caused by abnormal cell elongation or/and cell proliferation, we employed paraffin sections to observe the longitudinal sections of cells in the second internode of the WT,,and(Fig. 3-D). Microscopic observation revealed that the average cell length ofwas significantly reduced compared with that of the WT, while thehad a longer cell length than the WT (Fig. 3-E). These results confirmed that the semi-dwarf phenotype ofwas mainly due to the reduction of cell length of the internode.
Semi-dwarf phenotype of OE-bZIP01 can be rescued by GA3
To investigate whether the semi-dwarf phenotype of-is caused by GA deficiency, we examined the response of WT,, andto exogenous GA3. In the absence of GA3, theshowed a higher plant height, whereas theshowed dwarfism compared with the WT (Fig. 4-A). The application of exogenous GA3greatly promoted the growth ofseedlings, which exhibited a slender phenotype, and the shoot length ofandwas fully rescued compared with the WT without GA3application (Fig. 4-B). Furthermore, we measured the endogenous GA derivatives in WT andseedlings using liquid chromatography-tandem mass spectrometry (LC-MS/MS). As shown in Table S1, most GAs were decreased in the OElines, which was associated with the dwarf phenotype. The results implied that the semi-dwarf phenotype of-was mainly caused by GA deficiency.

Fig. 3. Morphology comparison ofandtransgenic plants.
A andB, Comparison of internode lengths between wild type (Shennong 265, SN265),,andat the heading stage. Ⅰ, The first internode from the top; Ⅱ, The second internode from the top; Ⅲ, The third internode from the top; Ⅳ, The fourth internode from the top. Scale bar is 10 cm.
C, Fast green-stained transverse sections of SN265,,andstems, showing differences in the diameter of the second internode from the top. Scale bars are 1 mm.
D, Longitudinal sections of the first internode from the top of SN265,, and. Scale bars are 120 μm.
E, Comparison analysis of parenchyma cell lengths of the first internode from the top of SN265,,and.
In B and F, data are Mean ± SD of three independent experiments (= 10 for each experiment). ** indicates the significant differences between transgenic plants and wild type at the< 0.01 by the Student’s-test.

Fig. 4. Phenotype response of wild type (Shennong 265, SN265),,andto exogenous GA.
A, Seedling phenotype of SN265 and transgenic lines (and), which were treated with exogenous 10 μmol/L GA3for 10 d. GA3(-) represents the absence of GA3, and GA3(+) represents the application of GA3. Scale bar is 5 cm.
B, Shoot lengths of SN265 and transgenic lines (and). Data are Mean ± SD of three independent experiments (= 10 for each experiment). Different lowercase letters indicate the significant differences between transgenic plants and wild type (< 0.01, Student’s-test).
OsbZIP01 directly binds to promoter of SD1
The phenotype of-is caused by GA deficiency, and thegene, encoding OsGA20ox2, is one of the most important genes involved in the GA3biosynthesis pathway. Therefore, we suspected that the expression ofinmight be affected. By qRT-PCR, we found that the expression level ofsignificantly decreased in the, and increased in thelines (Fig. 5-A). In addition, the protein content of SD1 was significantly reduced inand increased in(Fig. 5-B). These results showed that OsbZIP01 negatively regulated the expression of.
To confirm that the OsbZIP01 directly binds to the promoter of, we used an electrophoretic mobility shift assay (EMSA) with GST-OsbZIP01 protein. Indeed, OsbZIP01 bound to the labeled fragment of thepromoter containing the G-box, and the binding of OsbZIP01 protein to the labeled probe was fully competed by the unlabeled probe (Fig. 5-C). Besides, we performed a luciferase (LUC) transient transcriptional activity assay to detect the effectexpected for a negative of OsbZIP01 ontranscription in rice protoplasts (Fig. 5-D and -E). OsEIL1 is an ethylene-responsive TF, which can transcriptionally activate(Kuroha et al, 2018), and was used as a positive control(Fig. 5-E). The results showed that the LUC activity of the::LUC reporter was significantly repressed when OsbZIP01 was introduced(Fig. 5-E), which was consistent with the result that the expression level ofsignificantly decreased in theline (Fig. 5-A). Taken together, we proposed that OsbZIP01 directly bound to thepromoter and repressed its transcription.
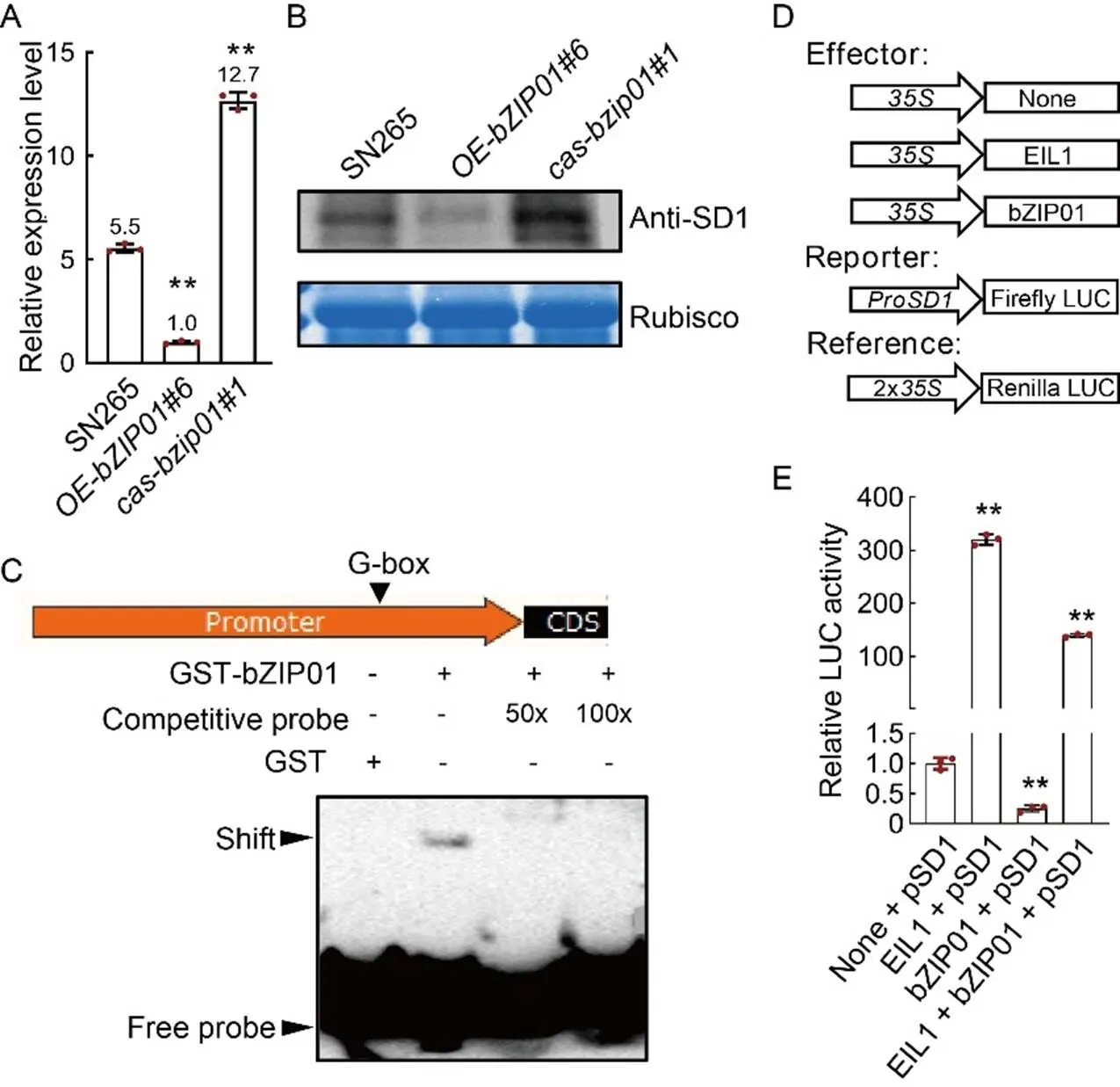
Fig. 5. OsbZIP01 directly binds to promoter region of.
A andB, Relative expression levels of mRNA (A) and protein (B) of SD1 in the stems of wild type (Shennong 265, SN265),,andtransgenic plants.
C, OsbZIP01 bindsto G-box in thepromoter by electrophoretic mobility shift assay. For the assay, the radiolabeled probes were incubated with OsbZIP01 protein. Competitive (unlabeled) probe (50× and 100×), G-box probe were used as indicated. GST, Glutathione S-transferase. CDS, Coding sequence.
D,Schematic diagrams of the effector and reporter constructs used the dual-luciferase reporter assays.
E,Transactivity assay in rice proplasts. EIL1 protein positively regulatesexpression, which is used as the positive control. LUC, Luciferase.
Data in A and E are Mean ± SD of three independent experiments (= 10 for each experiment). ** indicates the significant differences between transgenic plants and wild type at the< 0.01 by the Student’s-test.
OsbZIP01 acts upstream of SD1 to finely regulate plant height
To further investigate the genetic interaction betweenand, we generateddouble mutant plants using the CRISPR/Cas9 technology and genetic crossing in the Zhonghua 11 (ZH11) background. Thedouble mutant displayed a semi-dwarf phenotype, largely behaving like thesingle mutant (Fig. 6). When treated with 10 μmol/L GA3, bothandsingle mutants, anddouble mutant exhibited a slender phenotype, and there was no significant difference in shoot length (Fig. 6-A and -B). These results suggested that OsbZIP01 acted genetically upstream of, as TF that regulates the expression of.

Fig. 6. Genetic relationship ofand.
A andB, Shoot lengths of the single (and) and double () mutant seedlings under Zhonghua 11 (ZH11) background with and without 10 μmol/L GA3treatment [GA3(+) and GA3(-)] for 10 d.
C and D, Phenotypic characterization (C) and shoot lengths (D) of the single (and) and double () mutants at the maturity stage.
In A and C, scale bars are 5 cm. In B and D, data are Mean ± SD of three independent experiments (= 10 for each experiment), and different lowercase letters represent significant differences at the< 0.05 by the Student’s-test.
Discussion
As one of the largest TF families in higher plants, the bZIP TF family plays a crucial role in regulating plant growth, development, aging, and responses to adversity (Nijhawan et al, 2008). In this study, we demonstrated that OsbZIP01 can act as a transcriptional repressor to inhibit the expression of, thereby reducing GA synthesis.knockout mutants exhibited higher plant heights and faster germination rates compared with the WT (Fig. 2-D and -F).is predominantly expressed in leaves and leaf sheaths (Fig. 1-A), its protein is localized in the nucleus (Fig. 1-B), and it did not show transcriptional activation activity (Fig. 1-C). In theoverexpression lines, both the expression level ofand its protein levelwere lower than those in the WT (Fig. 5-A and -B). Furthermore, OsbZIP01 can directly bind to thepromoter and inhibit its transcription (Fig. 5-C and -E). The plant height of thedouble mutant displayed an intermediate phenotype between theandsingle mutants, but was shorter than the WT, indicating that OsbZIP01 partially acted upstream ofand may also control other downstream genes to regulate plant height. In general, OsbZIP01 primarily regulated plant height via the GA synthesis pathway, as the exogenous application of GA3can restore the phenotype inoverexpression lines.
Most bZIP TFs can activate the expression of downstream genes. For instance, OsbZIP75 and OsbZIP78 enhance the expression ofby binding to their promoters (Im et al, 2019; Wang et al, 2020). OsbZIP86 can directly bind to the promoter ofand positively regulate its expression (Gao et al, 2022). OsbZIP81 and OsbZIP84 also have transactivation activities (Liu F et al, 2018). However, in this study, we discovered that OsbZIP01 had no transcriptional activation activity. The function of OsbZIP01 is similar to OsbZIP12, which can also directly bind to the promoter region ofto repress its transcription and regulate GA biosynthesis (Tang et al, 2021). OsABF1 interacts with the OsEMF2b-PRC2 complex and mediates H3K27me3 on the promoter of(Tang et al, 2021). It is possible that OsbZIP01 also possesses similar regulatory mechanisms. Although we have not conducted in-depth research in this area, it is worth further exploration.
As the ‘Green Revolution’ gene,, encodes the essential enzyme in GA biosynthesis,and its alleles have been widely selected in modern breeding practices by reducing plant height with stronger lodging resistance and panicle size (Su et al, 2021). In this study, we identified a new component that acts upstream to regulate the transcription of. Overexpression ofshowed a dwarf architecture and stronger stems, which can be a new resource in rice breeding. Meanwhile, the, another allele of, was found involved in the seed dormancy development (Ye et al, 2015). We also found that the loss-of-function ofhad an effect on the germination rate. Another elitewas reported to be responsible for submergence-induced internode elongation in rice (Kuroha et al, 2018). Hereafter, how the upstream OsbZIP01 regulates the rice submergence tolerance remains intriguing. Recently,was reported to promote root development in rice through repressing auxin signaling (Hasegawa et al, 2021). Taken together,plays multiple roles in the development and growth of rice, not only through the GA pathway. Further research is needed to explore additional details and evidence in this regard.
Methods
Rice materials and growth conditions
Theandtransgenic plants were obtained in thecv. Shennong 265 (SN265) background. Bothandsingle mutants, and double mutant() were in the background of thecv. Zhonghua 11 (ZH11). Theandmutants under different backgrounds were generated using the CRISPR/Cas9 technology, described by Ma et al (2015). Thedouble mutant was obtained by crossing theandmutants. All the transgenic plants of the T2generation were sown in paddy fields in Hangzhou, Zhejiang Province and Lingshui, Hainan Province, China.
For the seed germination assay, rice seeds were disinfected in 75% alcohol for 1 min, then treated with 5% NaClO solution for 30 min, followed by three rinses with sterile water. The seeds were germinated in water for 3 d at 37 ºC.
For GA3treatments, pre-germinated rice seeds were sown on a 0.6% agar medium containing 10 µmol/L GA3. The plants were housed in a growth chamber under a 14 h light (30 ºC)/10 h dark (25 ºC) photoperiod, with a light intensity of 150 µmol/(m2∙s)(white light) and 60% relative humidity for 10 d.
Total RNA extraction and qRT-PCR analysis
The stems of 7-day-old seedlings of WT and transgenic plants were harvested and immediately frozen in liquid nitrogen. Total RNA was extracted using the Trizol Reagent (Ambion, TX, USA). cDNA synthesis was carried out using the First Strand cDNA synthesis Kit (Toyobo, Osaka, Japan). qRT-PCR was performed on a QuantStudio 3 Real-Time PCR instrument with ReverTra Ace qPCR RT Master Mix (Toyobo, Osaka, Japan) according to the manufacturer’s instructions.()was used as an internal control. The relative expression levels were calculated using the 2-ΔΔCtmethod, with three biological repeats of each qRT-PCR. The primer sequences are shown in Table S2.
Paraffin section assay
At the heading stage, the second internodes from the top of WT and transgenic plants were collected and immersed in FAA solution (consisting of 70% ethanol, 90 mL; 38% formaldehyde, 5 mL; and glacial acetic acid, 5 mL) for 24 h, followed by dehydration through a series of alcohol baths with increasing concentrations. The dehydrated tissues were then embedded in a mold filled with molten paraffin wax and sectioned. The sections were stained with Fast Green and subjected to cell size measurements using ImageJ software (National Institutes of Health, Maryland, USA).
Cellular localization assay
The segment encodingwas amplified via PCR and subsequently incorporated into the pCAMBIA1305.1-GFP vector. Following this,EHA105 carrying the indicated construct was transformed into the abaxial surface of 4-week-oldleaves. Subsequently, the plants were cultivated for 2 d before examination. The green fluorescent protein signals were visualized using a confocal laser-scanning microscope (LSM 700, Zeiss, Oberkochen, Germany).
Transactivation assay
The completecoding sequence was integrated into the pDEST-GBKT7 vector and then introduced into the AH109 yeast strain. The OsEIL1 construct was used as a positive control. Subsequently, serially diluted transformed colonies were planted onto SD-Trp-Ade-His synthetic dropout selection media, and incubated at 30 ºC for a duration of 3 to 5 d.
Western blot analysis
The stems of WT and transgenic plants were flash-frozen in liquid nitrogen and subsequently ground, with the addition of protein extraction buffer (25 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% Nonidet P-40, 5% glycerol, 1 mmol/L PMSF, and protease inhibitor cocktail), and centrifuged at 14 000 r/min, 4 ºC for 5 min. The supernatant was then subjected to centrifugation once more for 5 min. Protein extracts of equivalent concentrations were subsequently loaded onto SDS-PAGE (sodium dodecylsulphate polyacrylamide gel electrophoresis) gels, and then utilized for western blot analysis. The antibody of SD1 was obtained by GenScript Biotechnology Co., Ltd (NJ, USA) using the peptide synthesis (Peptide sequences: PQPHQPPPMDSTAG) with 1:2 000 ratio dilution. Rubisco was used as a loading control.
Dual-luciferase reporter assay
Protocol of protoplast isolation for transient gene expression in rice was adopted from Zhang et al (2011). The effector, reporter, and reference plasmids were co-transferred into rice protoplasts at a ratio of 6:6:1 and cultured overnight. After lysis and centrifugation, 50 μL of the supernatant was used for the measurement of luciferase activity. The activities of firefly luciferase (fLUC) and Renilla luciferase (rLUC) were quantified using the Dual Luciferase Reporter Assay System (Promega, WI, USA). The relative activity was expressed as fLUC/rLUC, and each sample was analyzed with three biological replicates. OsEIL1 was used as a positive control.
Electrophoretic mobility shift assay (EMSA)
PCR-amplified DNA sequences ofwas individually cloned intopGEX-4P-1 vector and transformed into Rosetta (DE3) competent cells (Sangon Biotech, Shanghai, China) forprotein expression. Bacterial cells were cultured in 250 mL of fresh Luria-Bertani broth at 18 ºC for 16 h in the presence of 0.5 mmol/L of isopropyl-β-d-thiogalactopyranoside. The GST-tag proteins were subsequently purified using Glutathione S-transferase resins (Sangon Biotech, Shanghai, China) according to manufacturer’ instructions. Proteins were stored at -80 ºC after being flash-frozen in liquid nitrogen.
The single-stranded probe labeled with biotin at the 5′-end for EMSA was synthesized by Zhejiang SUNYA Company (Hangzhou, China), and annealed to form a double-stranded probe. The specific experimental procedure was conducted according to the instructions of the Chemiluminescent EMSA Kit (GS009) from Beyotime Company (Shanghai, China).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 32101763), China National Postdoctoral Program for Innovative Talents (Grant No. BX2021266), and China Postdoctoral Science Foundation (Grant No. 2021M692853).
SUPPLEMENTAL DATA
The following materials are available in the online version ofthis article at http://www.sciencedirect.com/journal/rice-science;http://www.ricescience.org.
Fig. S1. Expression assay of two individual overexpression lines.
Fig. S2. Stem widths quantification analysis of wild type and transgenic plants.
Table S1. Decreased gibberellin abundance intransgenic plants.
Table S2. Primers used in this study.
Asano K, Yamasaki M, Takuno S, Miura K, Katagiri S, Ito T, Doi K, Wu J Z, Ebana K, Matsumoto T, Innan H, Kitano H, Ashikari M, Matsuoka M. 2011. Artificial selection for a green revolution gene duringrice domestication., 108(27): 11034–11039.
Beyene G, Chauhan R D, Villmer J, Husic N, Wang N, Gebre E, Girma D, Chanyalew S, Assefa K, Tabor G, Gehan M, McGrone M, Yang M Z, Lenderts B, Schwartz C, Gao H R, Gordon- Kamm W, Taylor N J, MacKenzie D J. 2022. CRISPR/Cas9- mediated tetra-allelic mutation of the ‘Green Revolution’() gene confers lodging resistance in tef ()., 20(9): 1716–1729.
Bhatnagar A, Burman N, Sharma E, Tyagi A, Khurana P, Khurana J P. 2023. Two splice forms of OsbZIP1, a homolog of AtHY5, function to regulate skotomorphogenesis and photomorphogenesis in rice., 193(1): 426–447.
Burman N, Bhatnagar A, Khurana J P. 2018. OsbZIP48, a HY5 transcription factor ortholog, exerts pleiotropic effects in light- regulated development., 176(2): 1262–1285.
Chen X, Lu S C, Wang Y F, Zhang X, Lv B, Luo L Q, Xi D D, Shen J B, Ma H, Ming F. 2015.encoding a NAC transcription factor that affects plant height through mediating the gibberellic acid pathway in rice., 82(2): 302–314.
Cho S H, Kang K, Lee S H, Lee I J, Paek N C. 2016. OsWOX3A is involved in negative feedback regulation of the gibberellic acid biosynthetic pathway in rice ()., 67(6): 1677–1687.
Chu Y L, Xu N, Wu Q, Yu B, Li X X, Chen R R, Huang J L. 2019. Rice transcription factor OsMADS57 regulates plant height by modulating gibberellin catabolism., 12(1): 38.
Gao S P, Chu C C. 2020. Gibberellin metabolism and signaling: Targets for improving agronomic performance of crops., 61(11): 1902–1911.
Gao W W, Li M K, Yang S G, Gao C Z, Su Y, Zeng X, Jiao Z L, Xu W J, Zhang M Y, Xia K F. 2022. miR2105 and the kinase OsSAPK10 co-regulate OsbZIP86 to mediate drought-induced ABA biosynthesis in rice., 189(2): 889–905.
Hasegawa T, Lucob-Agustin N, Yasufuku K, Kojima T, Nishiuchi S, Ogawa A, Takahashi-Nosaka M, Kano-Nakata M, Inari-Ikeda M, Sato M, Tsuji H, Wainaina C M, Yamauchi A, Inukai Y. 2021. Mutation of, which encodes a member of the basic leucine zipper transcription factor family, promotes root development in rice through repressing auxin signaling., 306: 110861.
Hedden P. 2001. Gibberellin metabolism and its regulation., 20(4): 317–318.
Hedden P, Phillips A L. 2000. Gibberellin metabolism: New insights revealed by the genes., 5(12): 523–530.
Im J H, Lee S G, Lee E, Park S R, Ahn I, Hwang D J. 2019. OsbZIP75 positively regulates plant defense against the bacterial leaf blight pathogenpv.., 13(6): 645–651.
Kuroha T, Nagai K, Gamuyao R, Wang D R, Furuta T, Nakamori M, Kitaoka T, Adachi K, Minami A, Mori Y, Mashiguchi K, Seto Y, Yamaguchi S, Kojima M, Sakakibara H, Wu J Z, Ebana K, Mitsuda N, Ohme-Takagi M, Yanagisawa S, Yamasaki M, Yokoyama R, Nishitani K, Mochizuki T, Tamiya G, McCouch S R, Ashikari M. 2018. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding., 361: 181–186.
Li J T, Zhao Y, Chu H W, Wang L K, Fu Y R, Liu P, Upadhyaya N, Chen C L, Mou T M, Feng Y Q, Kumar P, Xu J. 2015. SHOEBOX modulates root meristem size in rice through dose-dependent effects of gibberellins on cell elongation and proliferation., 11(8): e1005464.
Liu C, Zheng S, Gui J S, Fu C J, Yu H S, Song D L, Shen J H, Qin P, Liu X M, Han B, Yang Y Z, Li L G. 2018.encodes a gibberellin 2-oxidase and contributes to lodging resistance in rice., 11(2): 288–299.
Liu F, Wang P D, Zhang X B, Li X F, Yan X H, Fu D H, Wu G. 2018. The genetic and molecular basis of crop height based on a rice model., 247(1): 1–26.
Lu S J, Wei H, Wang Y, Wang H M, Yang R F, Zhang X B, Tu J M. 2012. Overexpression of a transcription factor OsMADS15 modifies plant architecture and flowering time in rice (L.)., 30(6): 1461–1469.
Ma H Z, Liu C, Li ZX, Ran Q J, Xie G N, Wang B M, Fang S, Chu J F, Zhang J R. 2018. ZmbZIP4 contributes to stress resistance in maize by regulating ABA synthesis and root development., 178(2): 753–770.
Monna L, Kitazawa N, Yoshino R, Suzuki J, Masuda H, Maehara Y, Tanji M S, Sato M, Nasu S, Minobe Y. 2002. Positional cloning of rice semidwarfing gene,: Rice ‘green revolution gene’ encodes a mutant enzyme involved in gibberellin synthesis., 9(1): 11–17.
Nijhawan A, Jain M, Tyagi A K, Khurana J P. 2008. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice., 146(2): 323–324.
Oikawa T, Koshioka M, Kojima K, Yoshida H, Kawata M. 2004. A role of, encoding an isoform of gibberellin 20-oxidase, for regulation of plant stature in rice., 55: 687–700.
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush G S, Kitano H, Matsuoka M. 2002. Green revolution: A mutant gibberellin-synthesis gene in rice., 416: 701–702.
Silverstone A L, Sun T P. 2000. Gibberellins and the green revolution., 5(1): 1–2.
Spielmeyer W, Ellis M H, Chandler P M. 2002.(), green revolution rice, contains a defective gibberellin 20-oxidase gene., 99(13): 9043–9048.
Su S, Hong J, Chen X F, Zhang C Q, Chen M J, Luo Z J, Chang S W, Bai S X, Liang W Q, Liu Q Q, Zhang D B. 2021. Gibberellins orchestrate panicle architecture mediated by DELLA-KNOX signalling in rice., 19(11): 2304–2318.
Tang L Q, Xu H Y, Wang Y F, Wang H M, Li Z Y, Liu X X, Shu Y Z, Li G, Liu W N, Ying J Z, Tong X H, Yao J L, Xiao W F, Tang S Q, Ni S, Zhang J. 2021. OsABF1 represses gibberellin biosynthesis to regulate plant height and seed germination in rice (L.)., 22(22): 12220.
Wang Q, Lin Q B, Wu T, Duan E C, Huang Y S, Yang C Y, Mou C L, Lan J, Zhou C L, Xie K, Liu X, Zhang X, Guo X P, Wang J, Jiang L, Wan J M. 2020.regulates seed dormancy through the abscisic acid pathway in rice., 298: 110570.
Wang Y, Li J. 2008. Molecular basis of plant architecture., 59: 253–279.
Yamaguchi S. 2008. Gibberellin metabolism and its regulation., 59: 225–251.
Yang C, Ma Y M, Li J X. 2016. The ricegene regulates plant growth and development through modulating the gibberellin pathway., 67(18): 5545–5556.
Yang X C, Hwa C M. 2008. Genetic modification of plant architecture and variety improvement in rice., 101(5): 396–404.
Ye H, Feng J H, Zhang L H, Zhang J F, Mispan M S, Cao Z Q, Beighley D H, Yang J C, Gu X Y. 2015. Map-based cloning ofidentified a gibberellin synthesis gene regulating the development of endosperm-imposed dormancy in rice., 169(3): 2152–2165.
Yin X M, Liu X, Xu B X, Lu P Y, Dong T, Yang D, Ye T T, Feng Y Q, Wu Y. 2019. OsMADS18, a membrane-bound MADS-box transcription factor, modulates plant architecture and the abscisic acid response in rice., 70(15): 3895–3909.
Zhang W, Cochet F, Ponnaiah M, Lebreton S, Matheron L, Pionneau C, Boudsocq M, Resentini F, Huguet S, Blázquez M Á, Bailly C, Puyaubert J, Baudouin E. 2019. The MPK8-TCP14 pathway promotes seed germination in Arabidopsis., 100(4): 677–692.
Zhang Y, Su J B, Duan S, Ao Y, Dai J R, Liu J, Wang P, Li Y G, Liu B, Feng D R, Wang J F, Wang H B. 2011. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes., 7(1): 30.
Zhou J P, Liu G Q, Zhao Y X, Zhang R, Tang X, Li L, Jia X Y, Guo Y C, Wu Y C, Han Y S, Bao Y, He Y, Han Q Q, Yang H, Zheng X L, Qi Y P, Zhang T, Zhang Y. 2023. An efficient CRISPR- Cas12a promoter editing system for crop improvement., 9(4): 588–604.
Zhou W, Malabanan P B, Abrigo E. 2015.regulates GA signaling by interacting with DELLA-like genes and GA oxidase genes in rice., 201(1): 97–107.
Zong W, Tang N, Yang J, Peng L, Ma S Q, Xu Y, Li G L, Xiong L Z. 2016. Feedback regulation of ABA signaling and biosynthesis by a bZIP transcription factor targets drought-resistance-related genes., 171(4): 2810–2825.
7 July 2023;
27 November 2023
Lü Yusong (lvyusong0907@163.com)
Copyright © 2024, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
https://doi.org/10.1016/j.rsci.2023.11.007
(Managing Editor: Wang Caihong)
杂志排行
Rice Science的其它文章
- Rice Variety Classification Based on Optimized Near-Infrared Spectral Classification Model
- Effects of Nitrogen-Regulating Gene AreA on Growth,Pathogenicity,and Fumonisin Synthesis of Fusarium proliferatum
- Rice Husk at a Glance:From Agro-Industrial to Modern Applications
- Smart Farming for Sustainable Rice Production:An Insight into Application,Challenge, and Future Prospect
- A β-Carotene Ketolase Gene NfcrtO from Subaerial Cyanobacteria Confers Drought Tolerance in Rice
- Potential Secretory Transporters and Biosynthetic Precursors of Biological Nitrification Inhibitor 1,9-Decanediol in Rice as Revealed by Transcriptome and Metabolome Analyses
