Genotype-based precision nutrition strategies for the prediction and clinical management of type 2 diabetes mellitus
2024-03-08OmarRamosLopez
Omar Ramos-Lopez
Abstract Globally,type 2 diabetes mellitus (T2DM) is one of the most common metabolic disorders.T2DM physiopathology is influenced by complex interrelationships between genetic,metabolic and lifestyle factors (including diet),which differ between populations and geographic regions.In fact,excessive consumptions of high fat/high sugar foods generally increase the risk of developing T2DM,whereas habitual intakes of plant-based healthy diets usually exert a protective effect.Moreover,genomic studies have allowed the characterization of sequence DNA variants across the human genome,some of which may affect gene expression and protein functions relevant for glucose homeostasis.This comprehensive literature review covers the impact of gene-diet interactions on T2DM susceptibility and disease progression,some of which have demonstrated a value as biomarkers of personal responses to certain nutritional interventions.Also,novel genotype-based dietary strategies have been developed for improving T2DM control in comparison to general lifestyle recommendations.Furthermore,progresses in other omics areas (epigenomics,metagenomics,proteomics,and metabolomics) are improving current understanding of genetic insights in T2DM clinical outcomes.Although more investigation is still needed,the analysis of the genetic make-up may help to decipher new paradigms in the pathophysiology of T2DM as well as offer further opportunities to personalize the screening,prevention,diagnosis,management,and prognosis of T2DM through precision nutrition.
Key Words: Type 2 diabetes mellitus;Nutrigenetics;Single nucleotide polymorphism;Genotype;Diet;Precision nutrition
lNTRODUCTlON
Type 2 diabetes mellitus (T2DM) is a metabolic disease caused by insufficient pancreatic insulin secretion or defective hormone actions in target tissues[1].T2DM is recognized as a major public health concern due to rising global prevalence and negative impact on human wellbeing and life expectancy,being significantly associated with morbidity burden and premature mortality[2].
Several factors have been identified to contribute to the prevalence of T2DM including the genetic background[3].Accordingly,a number of sequence DNA variants across the human genome have been characterized,some of which may affect gene expression and protein functions relevant for maintaining glucose homeostasis[3-5].Largely,single nucleotide polymorphisms (SNPs) have been the most prevalent studied genetic variations in the field of precision medicine,with applications in T2DM prevention and personalized management[6-8].Moreover,genetic risk scores (GRS) have been developed to assess the additive effect of SNPs[9-11].
Of note,the genetic contribution to T2DM status may depend on interactions with environmental issues including diet,which may explain some of the inconsistencies reported among epidemiological studies relating diet to chronic diseases[12].Thus,interrelationships between genetic variants and dietary features (i.e.,intakes of macro and micronutrients,eating behaviors,nutritional patterns,and the consumption of particular foods) may influence T2DM risk or disease complications by affecting critical pathways involved in glucose signaling,insulin secretion,β-cell function,glucolipotoxicity,inflammation and oxidative stress[12-14].Therefore,people with higher genetic predisposition should avoid certain harmful foods or adopt healthy dietary patterns to delay T2DM onset.
In this context,it has been illustrated that the combination of genetic (52 SNPs in 37 genes) and dietary data (food with high sugar content) using machine learning approaches may improve the prediction of T2DM incidence[15].Likewise,high genetic (48 SNPs) and dietary risk scores (based on sugar-sweetened beverages,processed meat,whole grains and coffee) were associated with increased incidence of T2DM[16].
In this document,potential interactions between genetic polymorphisms and dietary factors concerning T2DM susceptibility and disease progression are reviewed,some of which have demonstrated a value as biomarkers of personal responses to nutritional interventions.Also,novel genotype-based dietary strategies for the prevention and clinical management of T2DM are documented.Future directions comprising the integration of genetics with another omics tools are also postulated.These insights may help to explain heterogeneity in predisposition to T2DM and the development of related systemic complications,with relevance in disease stratification and precision nutrition through the study of the human genome.
GENETlC ΒACKGROUND,DlETARY lNTAKE,AND T2DM RlSK
A relevant precision nutrition approach in T2DM risk prediction/prevention include the analysis of associations between genetic polymorphisms and T2DM that are modulated by dietary features.Indeed,a number of nutrigenetic studies have identified significant gene-diet interactions related to T2DM predisposition (Table 1).These include single SNPs mapped to genes involved in pivotal physiological processes such as energy breakdown,nutrient utilization,insulin signaling,circadian rhythm,cell cycle regulation,pancreatic function,hypothalamic food intake control,neuronal synapse,signal transduction,and taste perception,which interact with nutritional factors to influence T2DM risk (Table 1).Among them,the consumption of particular foods (vegetables,whole grains,coffee,olive oils,alcoholic beverages,and dairy products),macronutrients (carbohydrates,fatty acids,protein,fiber) and micronutrients (iron,folate) intakes,adherence to dietary patterns,and eating time schedules (Table 1).
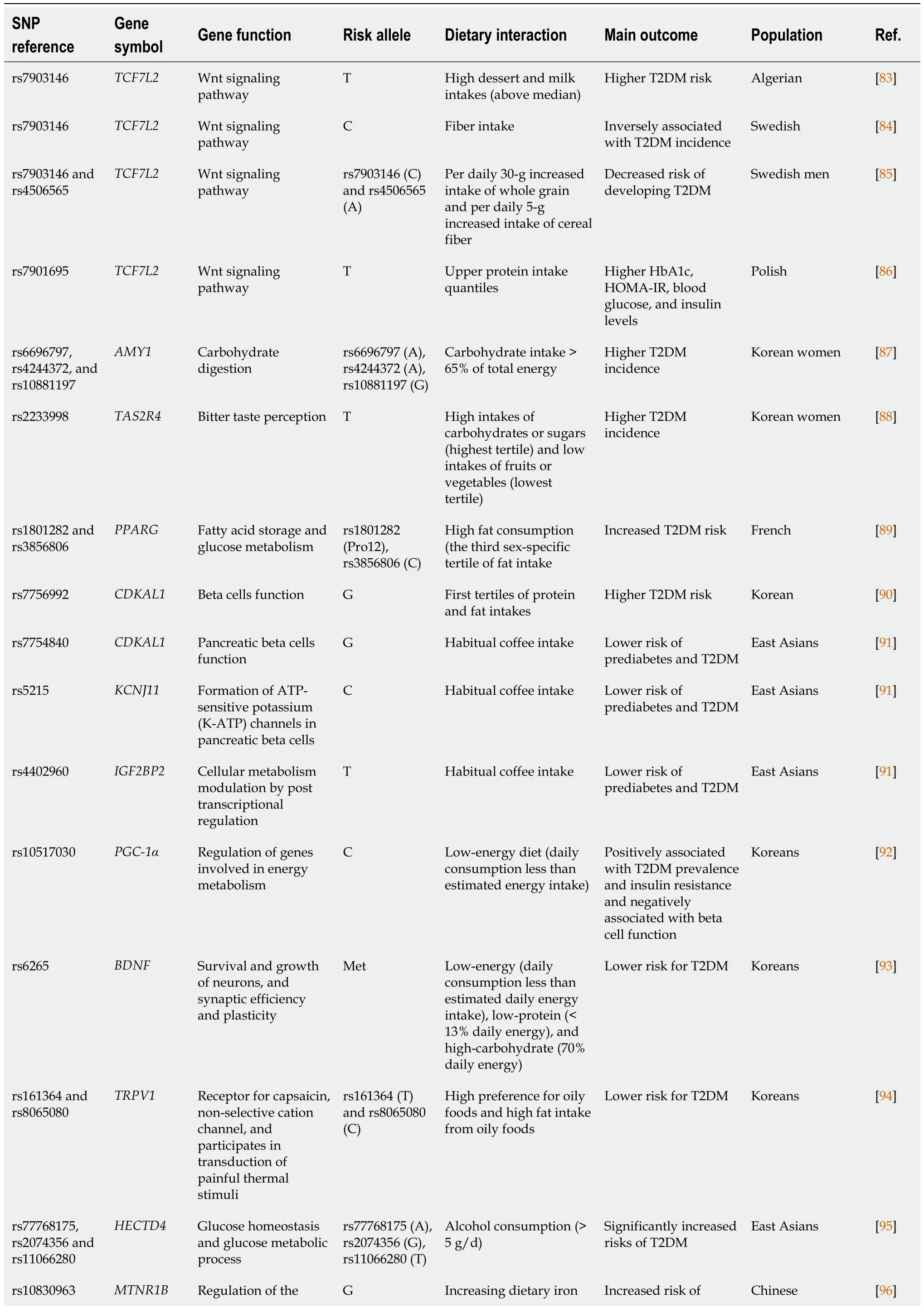
Table 1 Gene-diet interactions concerning the risk of developing type 2 diabetes mellitus and individual responses to nutritional interventions
In addition,GRS have been constructed to evaluate the cumulative effects of SNPs on T2DM susceptibility,where dietary factors are implicated.For instance,and obesity GRS positively interacted with dietary intake of cholesterol to affect insulin resistance in overweight/obese Spanish individuals[17].Of note,Brazilian subjects with high GRS for metabolic disease and total fat intakes had increased blood glucose and insulin-related traits than those with low GRS[18].Conversely,lower serum levels of glycated hemoglobin were found in Ghanaian adults with low total fat intake (≤36.5 g/d) despite carrying more than two risk alleles of vitamin D-related genetic variants[19].Also,associations between a GRS related to insufficient glucose-stimulated insulin secretion and T2DM risk was accentuated in Asian individuals with high energy and calcium intakes[20].Moreover,Korean subjects carrying polygenic variants linked to oxidative stress had increased risk of T2DM,which was lowered the by the intakes of dietary antioxidants[21].Besides,the genetic predisposition to T2DM was exacerbated with higher intakes of dietary branched-chain amino acids in Chinese[22].
Regarding specific foods,it was reported that middle-aged Korean adults with high GRS affecting insulin signaling presented more instances of insulin resistance when combined with high coffee (≥ 10 cups/wk) or caffeine (≥ 220 mg/d) intakes[23].Likewise,alcohol consumption significantly increased the risk of T2DM especially in Chinese men with low genetic predisposition to insulin secretion deterioration[24].In the same way,the association between the consumption of sugar-sweetened beverages and serum glucose abnormalities was stronger in Chileans with high T2DM genetic susceptibility[25].Conversely,augmented genetic risk for T2DM was ameliorated by increasing the consumption of fruits in Chinese population[26].In line with this finding,lower plant protein intake (< 39 g/d) was identified as a factor contributing to increase the risk of T2DM in genetically predisposed Asian Indians[27].
Furthermore,a high GRS for impaired insulin secretion increased the risk of T2DM by consuming a low-carbohydrate Western dietary pattern in Korean adults[28].In Asians,higher fasting serum glucose concentrations were found in participants with high T2DM-linked GRS who adopted a Western dietary pattern[29].On the contrary,it was reported that Koreans with high GRS for insulin resistance may be benefited by consuming a plant-based diet with high amounts of fruits,vitamin C,and flavonoids[30].
These studies show evidence concerning interactions between genetic variants and T2DM risk depending on dietary intakes,which may be useful for the design of nutritional therapies aimed to control the burden of T2DM,although more research is needed in populations with different genetic ancestries including Hispanics and Africans.
GENE-DlET lNTERACTlONS AFFECTlNG METAΒOLlC STATUS lN T2DM PATlENTS
Once T2DM has established,several physiopathological processes affecting glucose/lipid metabolism homeostasis,immune function,adipokine secretion,and gut microbiota dysbiosis play a critical role in the development of vascular injuries including diabetic heart disease and stroke[31].Thus,it is important to monitor the metabolic status in T2DM in order to prevent or delay the progression of complications associated with this disease.
Accordingly,some studies have analyzed the effect of gene-diet interactions on glycemic,lipid,and inflammatory features in T2DM patients,with relevance in clinical disease management.In this regard,studies in Mexican population have evidenced relevant gene-nutrient interactions concerning glycemic control and lipid profile in T2DM.For example,positive correlations were found between calcium intake and glycated hemoglobin and potassium intake and triglyceride-glucose index only in carriers of the 408 Val risk allele of theSLC22A1/OCT1Met408Val polymorphism[32].Also,higher blood concentrations of total cholesterol,non-high-density lipoprotein cholesterol,and low-density lipoprotein cholesterol were found in carriers of theAPOEε2 allele with low consumption of monounsaturated fatty acids (MUFA),whereas carriers of the apolipoprotein E (APOE) ε4 allele with high dietary ω-6:ω-3 polyunsaturated fatty acids (PUFA) ratio presented higher glycated hemoglobin levels[33].Likewise,A1 allele carriers of theDRD2/ANKK1TaqIA polymorphism were protected from serum triglyceride increases by maltose intake,but A2A2 homozygotes were susceptible to triglyceride rises through excessive consumptions of total fat,MUFA,and dietary cholesterol[34].
In Iranians with T2DM,Met allele carriers of the brain-derived neurotrophic factor (BDNF) Val66Mat polymorphism with high scores of dietary indices showed lower blood levels of triglycerides ((healthy eating index and diet quality index),total cholesterol,and interleukin-18 (phytochemical index) than Val/Val homozygotes[35].Meanwhile,C-allele carriers of theAPOA2-265 T>C polymorphism had highest means of body mass index,waist circumference,blood cholesterol and serum ghrelin and leptin levels when dietary acid load (either potential renal acid load or net endogenous acid production) values were high[36].Of note,higher inflammatory and antioxidant markers including C-reactive protein,total antioxidant capacity,superoxide dismutase,and 8-isoprostaneF2alpha were found in B2B2 homozygotes of theCETPTaqB1 polymorphism when they consumed diets with high dietary insulin index[37].Similarly,risk-allele carriers (CG,GG) of the peroxisome proliferator-activated receptor (PPAR)-γPro12Ala polymorphism who consumed a diet with high dietary insulin load and insulin indexes were more likely to be obese and have increased inflammatory markers (i.e.,interleukin-18,isoprostaneF2α,and pentraxin-3) compared to individuals with the CC genotype[38].Moreover,worse plasma lipid profile was found in participants carrying the AA/AG genotype of theApoBEcoRI polymorphism when increasing the percentage of energy derived from dietary fat,carbohydrates,protein,saturated fatty acids (SFA),and cholesterol in comparison to GG homozygotes[39].In the same way,Del-allele carries of theApoBIns/Del genetic variant who consumed high amounts of MUFA (≥ 12% E) and carbohydrates (≥ 54% E) had higher blood levels of triglycerides and low density lipoprotein-cholesterol,while low carbohydrate (< 54% E) intakes were associated with raised serum concentrations of leptin and ghrelin in T2DM patients with this same genetic profile compared to Ins/Ins homozygotes[40].In addition,an increased risk of obesity was found in carriers of the Del allele ofApoBgene when combined with a low consumption of dietary ω-3 PUFA (< 0.6% E) in T2DM subjects[41].Taken together,these results could be useful to prevent cardiometabolic risk factors and later complications in T2DM patientsviamanipulation of dietary intakes of selected nutrients mainly in genetically susceptible individuals.However,more investigation is needed in other populations with diverse ancestries and exposed to different environments in order to regionalize antidiabetic nutritional treatments.
GENETlC POLYMORPHlSMS AS ΒlOMARKERS OF GLYCEMlC RESPONSES TO DlETARY ADVlCE
Dietary strategies aimed to achieve or improve glucose homeostasis not always have a positive impact in all individuals,which can be due to genetic factors.In this sense,some trials have evaluated the value of SNPs as potential biomarkers of glycemic outcomes in response to different nutritional interventions.For instance,the variant rs3071 of theSCDgene modified blood glucose response to dietary oils varying in MUFA content in adults with obesity,where CC genotype carriers showed an increase in blood glucose levels with a high SFA/low MUFA control oil,but reductions in this outcome with both high MUFA oil diets[42].Within the multicenter NUGENOB study,the T allele of the protein phosphatase Mg(2+)/Mn(2+)-dependent 1K (PPM1K) rs1440581 genetic variant was associated with higher reductions of serum insulin and homeostasis model assessment (HOMA)-B after a high-fat (40%-45% E) diet,whereas an opposite effect was found in the low-fat (20%-25% E) diet group[43].Also,obese individuals who were homozygous for the T-risk allele of the transcription factor 7 like 2 (TCF7L2) rs7903146 polymorphism and consumed a high-fat (40%-45% E) diet,underwent smaller reductions in HOMA-estimated insulin resistance (HOMA-IR)[44].
Findings from the POUNDS lost trial revealed greater decreases in fasting glucose,serum insulin,and HOMA-IR in Tallele participants of the glucose-dependent insulinotropic polypeptide receptor (GIPR) rs2287019 variant who were assigned to low-fat (20%-25% E) diets[45].In addition,subjects with the risk-conferring CC genotype of the insulin receptor substrate-1 (IRS1) rs2943641 SNP had greater decreases in insulin and HOMA-IR than those without this genetic profile in the highest-carbohydrate (65% E) dietary group[46].Whereas,the T allele of deficient activity of 7-dehydrocholesterol reductase (DHCR7) rs12785878 polymorphism was associated with higher decreases in serum insulin and HOMA-IR only in high-protein (25% E) diets[47].Similarly,greater drops in fasting insulin levels were related to thePCSK7rs236918 G allele in high-dietary carbohydrate (65% E) intakes,especially in white Americans[48].Of note,carriers of the risk allele (A) of the Fat mass and obesity associated (FTO) rs1558902 variant benefited more in improving insulin sensitivity by consuming high-fat (40%-45% E) diets rather than low-fat (20%-25% E) regimens[49].
In a Spanish cohort with obesity,improvements in serum insulin levels and HOMA-IR were associated with theADRB3Trp64Trp genotype after hypocaloric diet with high protein (34% E) content[50].Besides,AA genotype carries of theBDNFrs10767664 variant underwent reductions in insulin resistance markers when consumption of MUFA (67.5%) was high[51].Likewise,TNFA-308GG homozygotes had a better glycemic response after high (22.7%) dietary intakes of PUFA[52].In the same say,UCP355CC genotype carriers benefited more (more decreases in blood glucose,serum insulin,and HOMA-IR) when consumed a high-protein (34% E) diet[53].Interestingly,it was suggested that the T allele of theADIPOQrs1501299 SNP was related to a lack of response of fasting glucose/insulin and HOMA-IR secondary to a Mediterranean-style diet in Spanish obese individuals[54].Insulin resistance was ameliorated after the consumption of this same dietary pattern in T allele carries of theRETNrs10401670 gene polymorphism[55].Comparable results were reported concerning insulin resistance reductions in CC genotype carries of the melatonin receptor 1B (MTNR1B) rs10830963 variant but not in GC+GG groups after following a hypocaloric diet with Mediterranean pattern[56].
Some studies have evaluated the cumulative effect of multiple SNPs (by calculating GRS) instead of single variants.In this context,participants with high genetic risk of glucose abnormalities showed increased fasting glucose after consuming a high-fat diet (40%-45% E),which was not observed in subjects assigned to the low-fat (20%-25% E) group[57].A lower GRS for diabetes was associated with higher reductions in fasting insulin,glycated hemoglobin,and HOMA-IR,and a lesser increase in HOMA-B only when the consumption of dietary protein (15% E) was low[58].In the meantime,insulin resistance improvements were limited to individuals with a higher GRS of habitual coffee consumption following a low-fat (20%-25% E) dietary intervention[59].
The influence of the genetic background on metabolic outcomes after dietary treatments have also been assessed in T2DM patients.For example,a dietary intervention based on increased intakes of whole grains,vegetables,and legumes was able to prevent an age-related increase in blood triglyceride concentrations in Koreans with impaired fasting glucose or new-onset of T2DM carrying the TT genotype of theAPOA5-1131 T>C SNP[60].Accordingly,low glycemic index diets induced significant decreases of serum lipids,fasting blood glucose,and glycated albumin only in Chinese women with T2DM who wereFABP2Ala54 homozygotes[61].Furthermore,carriers of theFTOrs9939609 risk allele (A) underwent a better response in improving body mass index and diastolic blood pressure in response to supplementation with epigallocatechin-3-gallate (300 mg/d) in Iranian patients with T2DM[62].
Overall,current evidence suggests a role of selected genetic polymorphisms in modulating the individual metabolic responses to some dietary treatments.However,available studies have been performed mainly in Europeans/Caucasians,with particular genetic backgrounds;therefore,additional studies in different populations are required including Latin Americans,Africans,and Asians.Also,the analysis of the effects of supplementation with antioxidant micronutrients and bioactive compounds with anti-inflammatory properties is warranted.
GENOTYPE-ΒASED DlETARY lNTERVENTlONS AND GLYCEMlC OUTCOMES
The knowledge about the implication of genetic variants and dietary factors in the onset and progression of T2DM has motivated the interest for the design and implementation of genotype-based intervention strategies for improving glycemic/metabolic outcomes compared to traditional nutritional prescriptions.For instance,it was evidenced that a personalized low-glycemic index nutrigenetic diet (utilizing 28 SNPs with evidence of gene-diet/lifestyle interactions) induced higher fasting glucose reductions than a Ketogenic diet in overweight/obese individuals[63].Likewise,healthier effects in HOMA-IR and insulin serum levels were observed in MTHFR 677T allele carriers consuming a GENOMEX diet comprising of diet-related adaptive gene polymorphisms highly prevalent in Mexicans[64].However,no differences were detected regarding glucose homeostasis outcomes at 24 wk of follow-up between a nutrigenetic-guided diet (using genetic information of a proprietary algorithm) and a standard balanced diet in obese or overweight American veterans[65].
In T2DM patients,a case study based on the N-of-1 approach revealed better glycemic control when adhered to a genetically-guided Mediterranean diet (high-quality foods rich in fiber and antioxidants that have been proven to exert beneficial glycaemia effects) considering genetic variants guiding the personalized selection of macronutrients for the nutritional management of T2DM[66].Similarly,greater improvements in fasting plasma glucose and glycosylated hemoglobin concentrations were found in patients with pre-diabetes or T2DM following a personalized nutritional plan (taking in consideration SNPs associated with individual responses to macronutrient intakes) compared to conventional medical nutrition therapy[67].
Furthermore,some studies have evaluated the utility of genetic disclosure as a tool for T2DM prevention and disease control.For example,participants who received diabetes genetic risk counseling together with general education about modifiable risk factors and personal stimulus to adopt diabetes lifestyle prevention behaviors reported high levels of support,perceived personal control and satisfaction with the genetic counseling sessions[68].Nevertheless,diabetes genetic risk testing and counseling did not necessarily improved disease prevention behaviors such as self-reported motivation or prevention program adherence among overweight individuals at increased phenotypic risk for T2DM[69].Moreover,comparison analyzes did not revealed significant differences between genetic testing results and traditional risk counseling concerning behavior changes to reduce the risk of T2DM in non-diabetic overweight/obese veterans[70].Given inconsistences in available evidence,more research is needed to translate this knowledge into clinical care in T2DM.Further investigation should contemplate information that could interfere with the results including the prevalence and metabolic effects of selected SNPs,cultural level of populations,compatibility of dietary plans with genotypic characteristics,and the quality of nutritional/lifestyle advice.
FUTURE DlRECTlONS
In addition to genetics,progresses in other omics areas are improving current understanding of the biological/molecular mechanisms involved in T2DM pathogenesis and clinical outcomes[71].Similar to the influence of the genetic background,it has been evidenced that epigenetic modifications may alter transcriptional activity resulting in different T2DM traits and phenotypes;certainly,different genes responsible for the interindividual variability in responses to antidiabetic treatments (including dietary advice) are subjected to epigenetic regulation[72].More importantly,interactions among polymorphisms in key metabolic genes (i.e.,TCF7L2),related methylation status,and environmental factors have been suggested as a possible etiologic pattern for T2DM[73].Besides,SNPs in microRNA (miRNA) genes may change the structure of miRNAs and their target gene expressions to influence T2DM risk[74].
Also,metagenomic and metabolomic methodologies have emerged to investigate the interrelationships between the gut microbiota dysbiosis and their related metabolites (affecting critical metabolic pathways in the host such as immunity and nutrient metabolism) in the development of T2DM[75].Of note,characterization of gut microbiota of individuals carrying the risk alleles of thePPARGC1A(rs8192678) andPPARD(rs2267668) variants revealed some taxa (with overrepresentation of ABC sugar transporters) putatively associated with insulin resistance and T2DM[76].Correspondingly,theMMP27rs7129790 polymorphism was strongly associated with high gut abundance of Proteobacteria in Mexican Americans with a high prevalence of obesity and T2DM[77].
Moreover,high-throughput proteomics assays have allowed the discovery and representation of potential protein-T2DM links,providing novel intervention targets in this disease[78].Interestingly,a set of circulating proteins causally associated with T2DM were identified using two-sample Mendelian randomization approaches,which is a validated method to examine the causal effect of variation in genes of known function on disease[79].Also,Mendelian randomization analyses did not uncover significant causal effects between proteins (i.e.,retinal dehydrogenase 1,galectin-4,cathepsin D,and lipoprotein lipase) and diabetes,suggesting that identified proteins are expected to be biomarkers for T2DM,rather than demonstrating causal pathways[80].
Additionally,coupling genomic data (i.e.,GRS) with conventional phenotypical information (i.e.,age,sex,body composition,medication use,and vital signs) is being useful for enhancing individual T2DM risk stratification and disease prediction[81,82].Advances in next-generation sequencing technologies and the use of machine learning and other artificial intelligence methods became fundamental to analyze these T2DM-associated multiomics datasets.
CONCLUSlON
Current evidence support the impact of genetic variation on the risk of developing blood glucose/insulin alterations and subsequent T2DM as well as its implication in affecting the lipid,inflammatory,and carbohydrate status in T2DM patients through interactions with dietary factors.These include SNPs and other structural variants mapped to metabolically active genes such asTCF7L2,amylase 1,TAS2R4,PPARG,CDKAL1,KCNJ11,insulin-like growth factor 2 binding protein 2,proliferator-activated receptor-gamma coactivator-1alpha,BDNF,transient receptor potential vanilloid-1 channel,HECT domain E3 ubiquitin protein ligase 4,MTNR1B,IRS1,GIPR,S100A9,PSMD3,KCNMB3,Caveolin-2,NOTCH2,zinc finger BED-type containing 3,GLP1R,FTO,melanocortin 4 receptor,SLC22A1/OCT1,APOE,DRD2/ANKK1,APOA2,CETP,PPAR-γ,andApoB,which have been analyzed using single and cumulative approaches.Moreover,some genetic polymorphisms have been identified as putative biomarkers of individual responses to energyrestricted nutritional prescriptions aimed to glucose control including those located inSCD,PPM1K,FTO,TCF7L2,GIPR,IRS1,DHCR7,PCSK7,ADRB3,BDNF,TNFA,UCP3,ADIPOQ,RETN,MTNR1B,APOA5,andFABP2genes.Furthermore,some genotype-based dietary strategies have been developed for improving T2DM control in comparison to general lifestyle recommendations for all people.However,more research is needed in order to expand and confirm these findings in other populations less explored such as Latin Americans and Africans considering some sources of variability (i.e.,allele frequency,quantitative trait locus,and gender influence) incorporating the assessment of the role of food bioactive compounds and micronutrients in prospective dietary interventions.In any case,the analysis of the genetic make-up may help to decipher new paradigms in the pathophysiology of T2DM as well as offer further opportunities to personalize the screening,prevention,diagnosis,management,and prognosis of T2DM.
FOOTNOTES
Author contributions:Ramos-Lopez O contributed to the writing and revision of this manuscript.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Mexico
ORClD number:Omar Ramos-Lopez 0000-0002-2505-1555.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Chen YX
杂志排行
World Journal of Diabetes的其它文章
- Elucidating the cardioprotective mechanisms of sodium-glucose cotransporter-2 inhibitors beyond glycemic control
- Emerging and multifaceted potential contributions of polyphenols in the management of type 2 diabetes mellitus
- ldentification of hub genes associated with Helicobacter pylori infection and type 2 diabetes mellitus: A pilot bioinformatics study
- Experience of humanistic nursing in hemodialysis nursing for patients with diabetic kidney disease
- Analysis of the influencing factors and clinical related characteristics of pulmonary tuberculosis in patients with type 2 diabetes mellitus
- Vitamin D,selenium,and antidiabetic drugs in the treatment of type 2 diabetes mellitus with Hashimoto's thyroiditis
