Promotion effects of salt-alkali on ammonia volatilization in a coastal soil
2024-03-07ZhenqiSHIDongliSHEYongchunPANandYongqiuXIA
Zhenqi SHI ,Dongli SHE,* ,Yongchun PAN and Yongqiu XIA
1College of Agricultural Science and Engineering,Hohai University,Nanjing 210098(China)
2Jiangsu Province Engineering Research Center for Agricultural Soil-Water Efficient Utilization,Carbon Sequestration and Emission Reduction,Nanjing 210098(China)
3Key Laboratory of Soil and Sustainable Agriculture,Institute of Soil Science,Chinese Academy of Sciences,Nanjing 210008(China)
ABSTRACT Coastal ecosystems are highly susceptible to salt-related problems due to their formation process and geographical location.As such ecosystems are the most accessible land resources on Earth,clarifying and quantifying the effects of salt-alkali conditions on N concentration and ammonia(NH3)volatilization are pivotal for promoting coastal agricultural productivity.The challenge in establishing this effect is to determine how salt-alkali conditions impact NH3 volatilization through direct or indirect interactions.An incubation experiment using a coastal soil from a paddy farmland,combined with the structural equation modeling(SEM)method,was conducted to reveal the net effects of salt-alkali on NH3 volatilization and the role of environmental and microbial factors in mutual interaction networks.The specific experimental design consisted of four salt treatments(S1,S2,S3,and S4:1‰,3‰,8‰,and 15‰NaCl by mass of soil,respectively),four alkaline treatments(A1,A2,A3,and A4:0.5‰,1‰,3‰,and 8‰NaHCO3 by mass of soil,respectively)and a control without NaCl or NaHCO3 addition(CK),and each treatment had three urea concentrations(N1,N2,and N3:0.05,0.10,and 0.15 g N kg-1 soil,respectively)and three replicates.At the N1,N2,and N3 levels,NH3 volatilization increased by 9.31%-34.98%,3.07%-26.92%,and 2.99%-43.61%as the NaCl concentration increased from 1‰to 15‰,respectively,compared with CK.With an increase in the NaHCO3 concentration from 0.5‰to 8‰,NH3 volatilization increased by 8.36%-56.46%,5.49%-30.10%,and 30.72%-73.18%at the N1,N2,and N3 levels,respectively,compared with CK.According to the SEM method,salinity and alkalinity had positive direct effects on NH3 volatilization,with standardized path coefficients of 0.40 and 0.19,respectively.Considering the total effects(net positive and negative effects)in the SEM results,alkalinity had a greater influence than salinity(total standardized coefficient 0.104 >0.086).Nitrogen concentrations in the incubation system showed a direct positive effect on NH3 volatilization(standardized path coefficient=0.78),with an obvious decrease under elevated salinity and alkalinity levels.Additionally,gene abundances of nitrogen-transforming microbes indirectly increased NH3 volatilization(total indirect standardized coefficient=0.31).Our results indicated that potential NH3 emissions from coastal saline areas could be enhanced more by soil alkalization than by salinization.
Key Words:alkalinity,coastal ecosystem,NH3 emission,N-transforming microbe,salinity,structural equation modeling
INTRODUCTION
Soil salt problems threaten agricultural development worldwide(Rengasamy,2010).The area of saline soil continues to expand rapidly as a result of climate change and agricultural management,especially in coastal areas (Sahabet al.,2021).Global agricultural demands have experienced explosive growth consistent with the burgeoning population(Kopittkeet al.,2019).Reclaiming and improving the productivity of coastal areas are necessary strategies for food security(Sheet al.,2016).
Agricultural ecosystems are considered hotspots of ammonia (NH3) emissions (Shenet al.,2020).Ammonia volatilization caused by the application of fertilizers accounts for 40%-60% of China’s agricultural emissions,reaching its peak in summer (Paulotet al.,2014).As the most common gaseous pollutant from fertilized farmland,NH3has been widely studied.Ammonia volatilization is a main pathway of the nitrogen(N)cycle and is the dominant cause of particulate matter smaller than 2.5 μm(PM2.5)air pollution(Gallowayet al.,2008).Furthermore,NH3in the atmosphere is returned to the soil and then is involved in other N transformation processes(Elliottet al.,2007).
The application of N fertilizer to coastal areas can provide an adequate supply of soil N and can sustainably increase food production;however,the risks of gaseous N loss will increase synchronously.The impacts of soil salinity and alkalinity on N cannot be ignored.Salinity is an extremely important factor that impacts the N cycles dominated by microorganisms(Duan and Xiao,2000;Zhouet al.,2017;Xueet al.,2020).Microbial respiration can be reduced by high soil salinity and hence can impact the mineralization of soil C and N.Rietz and Haynes (2003) demonstrated that increases in soil salinity and alkalinity induced smaller microbial communities,greater stress,and lower metabolic activity.Previous studies have shown that NH3volatilization has high sensitivity to pH andconcentration(Huang Jet al.,2021).It was reported that as the pH increased from 5.0 to 8.9,gaseous NH3emissions increased from 0.005%to 31.1%at 25°C(Kirchmann and Witter,1989).In addition,coastal areas are affected by the combined effects of rainfall infiltration and the strong evaporation of groundwater,and water movement is accompanied by frequent desalination and re-salination processes(Rysgaardet al.,1999;Panet al.,2021b).The pH of the water-soil ecosystem will inevitably change in concert with these changes.However,there are few reports on the specific effects of salt-alkali on NH3volatilization in coastal saline soils(Yaoet al.,2021;Zhaoet al.,2021;Zhanget al.,2022).
For the practical development of coastal agriculture,more comprehensive and deeper knowledge about the effects of variations in salinity and alkalinity on N cycles is essential.Due to the complex multivariable reactions of NH3volatilization,it is difficult to clarify how salinity and alkalinity affect volatilization directly or indirectly.Many researchers have used classical regression and correlation analysis to study multiple causality (Beheraet al.,2013),but these approaches only quantify the complex impact of environmental and microbial factors on NH3volatilization during the evaluation period(Xieet al.,2022).If unobserved specific variables that may affect NH3emissions could be indirectly incorporated into interaction network analysis,the mechanistic understanding of how salt-alkali affects the NH3volatilization process would be more comprehensive.To obtain a more precise evaluation,structural equation modeling(SEM)was used to determine the complex relationships among obvious variables and potential variables.The specific objectives of this study were i)to quantify the effects of salt-alkali on NH3volatilization in coastal ecosystems and ii) to describe how the environmental and microbial factors in such systems influence NH3volatilization rates by interacting with each other.
MATERIALS AND METHODS
Soil samples andexperimental design
Samples of coastal soil were collected from three sediment cores(4 m×4 m plots,0-100 cm soil profiles)in a farmland that is managed as a reclamation paddy area.The farmland is located beside the Yellow Sea in Liuzong Village,Juegang,Rudong City,Nantong City,Jiangsu Province,China(32°19′N,121°14′E).The soil texture is silty loam,with mass fractions of sand particles(0.05-2 mm),silt particles(0.002-0.05 mm),and clay particles(<0.002 mm)of 13.7%,81.3%,and 5.0%,respectively.The basic physical and chemical properties of the soil were as follows:pH 8.0,electrical conductivity(EC)of a 1:5 soil water extract(EC1:5)2.24 mS cm-1,organic carbon(C)3.1 g kg-1,total N 0.41 g kg-1,and cation exchange capacity (CEC)4.99 cmol kg-1.An incubation experiment was conducted from October to November 2020 at the Water-Saving Park of Hohai University in Nanjing,Jiangsu Province,China(31°57′N,118°50′E,144 m above sea level;belonging to a subtropical monsoon climate).We used salinity and alkalinity treatments as two limiting factors to interact with urea concentration.The specific experimental design consisted of four salinity treatments(S1,S2,S3,and S4:1‰,3‰,8‰,and 15‰NaCl by mass of soil,respectively),four alkalinity treatments(A1,A2,A3,and A4:0.5‰,1‰,3‰,and 8‰NaHCO3by mass of soil,respectively),and a control without NaCl or NaHCO3addition(CK)and each treatment had three urea concentrations(N1,N2,and N3:0.05,0.10,and 0.15 g N kg-1soil,respectively)and three replicates.All treatments were arranged randomly among 81 pots(Gaoet al.,2020;Panet al.,2021a)to investigate the effects of different salinity and alkalinity levels on NH3volatilization rates under 3 different N application levels.
The experiment was completed in three steps.i) Soil samples were air-dried in a greenhouse and sieved to 5 mm in the laboratory and then spread out in a 0.5-mm layer on plastic film.Four concentrations of NaCl solutions and 4 concentrations of NaHCO3solutions were sprayed onto the corresponding soil samples according to the experimental design.The specific spraying process used a mist spray to spray each area once at the same height,and the soil was remixed and spread out in each round;spraying and remixing/spreading were repeated until the solution ran out.ii)Seven-kilogram soil samples were placed into PVC incubators(thickness 5 mm,internal size 340 mm×270 mm× 130 mm).A 4-cm water layer was maintained during the whole 1-month incubation period.Glucose(1.20 g)was added to each incubator for preculture for 2 weeks.Urea solutions were subsequently applied to the water layer.iii)Water and soil samples were taken 24 h after fertilization to measure the environmental and microbial factors.The NH3volatilization rate was measured continuously for 7 d.
NH3 volatilization
A static semi-open chamber method was used to determine the NH3emissions from each incubator(Martineset al.,2010;Sheet al.,2018).The collection device was a PVC tube with an inner diameter of 15 cm and a height of 20 cm,equipped with a stainless-steel grid to control the height of the sponge(Fig.1).The device was inserted into the soil until the chamber was 4 cm above(from the water layer to the grid) the water.Two sponges were infiltrated with 15 mL of phosphor-glycerol solution containing 50 mL L-1phosphoric acid and 40 mL L-1glycerin.One piece was placed on the grid as an NH3absorbent.The other was placed on the top of the tube to isolate the lower sponge from the NH3deposited from the atmosphere,and the lower sponge was replaced with a freshly prepared sponge daily at 10 a.m.(the used sponge was transported to the laboratory immediately).The absorbed sponge was completely immersed in a plastic bottle filled with 300 mL of 2.0 mol L-1KCl solution,and the bottle was sealed and then shaken for l h on a gas-bath reciprocating shaker at 200 r min-1.The concentrations ofin the extracts were determined by a flow injection analyzer(TRACCS2000,Skalar Analytical,Breda,the Netherlands).

Fig.1 Device used for NH3 collection in this study.
The NH3volatilization rate(RNH3,μmol m-2h-1)was calculated as follows:
whereMis the mass of NH3adsorbed per day(mg),Ais the cross-sectional area of the collection device(m2),andDis the time of each collection(h).
Environmental andmicrobial factors
The environmental factors were divided into“overlying water”and“sediment”groups and were determined in each treatment 24 h after fertilization (Panet al.,2021b).The overlying water samples extracted from each pot(100 mL)were transported to the laboratory and refrigerated at 5°C.The samples were frozen until analysis after being filtered through 0.7-μm Whatman GF/Ffilters.Two sediment aliquots from each pot(20 g of well-mixed samples from the 0-5 cm soil layer)were sampled.One of the soil samples was air-dried and ground for cation leaching(soil/water ratio of 1:5,weight/volume;50 mL of 2 mol L-1KCl),and the other was used for microbial determination and frozen to extract the total genomic deoxyribonucleic acid(DNA)using an UltraClean®soil DNA isolation kit(MoBio Laboratory,Carlsbad,USA).
A DDS-307 conductivity meter and a PHSJ-4F pH meter(Shanghai Precision Scientific Instrument Co.Ltd.,Shanghai,China)were used to determine the EC(or EC1:5)and pH,respectively,of the overlying water and sediment leaching solution.Theandconcentrations of the filtered overlying water samples and soil-extracted suspensions were measured by a flow injection analyzer(Skalar Analytical,Breda,the Netherlands).A NanoDrop spectrophotometer(Thermo Scientific,Waltham,USA)was used to determine the abundance and purity of the DNA.
Statistical analysis
The data series consisted of NH3volatilization rates,“overlying water”(,EC,pH,and sodium adsorption ratio(SAR),“sediment”(,EC1:5,pH,and SAR),and“N-transforming microbes”(nirS,nirK,andnosZgene abundances).Prior to the analyses,the variable values were normalized by log transformation.First,Pearson’s correlation analysis was conducted to determine the linear relationships between the NH3volatilization rate and each independent variable.Then,two-way analysis of variance(ANOVA)with Duncan’s multiple comparison test was performed to investigate the net effects of experimental treatments on the NH3volatilization rate.Before the SEM analysis,influential variables in the incubation system were divided into 4 groups,salinity(EC and SAR),alkalinity(pH),N concentrations,and N-transforming microbes(nirS,nirK,andnosZgene abundances),for principal component analysis according to significant relationships between similar variables in the bivariate correlation matrix(Table SI).All statistical analyses were conducted using SPSS 25.0(IBM SPSS Statistics,Chicago,USA).
Structural equation modeling analysis was performed to evaluate the complex relationships between potential variables among multivariate data series by considering intermediate variables(Grace,2006).The SEM was chosen to assess the relationships between environmental factors and the NH3volatilization rate in detail (Xiaet al.,2018).This model can directly simulate NH3volatilization rates,partition the direct and indirect effects of variables on NH3volatilization,and correspondingly assess the strengths of complicated interactions(Sheet al.,2018).The SEM was performed in SPSS AMOS 24.0 software(IBM SPSS Statistics,Chicago,USA).
RESULTS
Variations in NH3 volatilization rates andassociatedfactors
The NH3volatilization rates ranged from 177.21 to 1 586.04 μmol m-2h-1in the incubation experiment(Fig.2).Under each treatment,the daily cumulative NH3volatilization showed a trend of first increasing and then decreasing(Fig.3).The cumulative NH3volatilization increased with increasing NaCl and NaCO3concentrations at the same urea level.Compared with CK,the cumulative NH3volatilization increased by 9.31%-34.98%,3.07%-26.92%and 2.99%-43.61%as the salt concentration increased from 1‰to 15‰at N1,N2,and N3 levels,respectively.With an increase in NaCO3concentration from 0.5‰ to 8‰,NH3volatilization significantly increased by 8.36%-56.46%,5.49%-30.10%,and 30.72%-73.18%at the three respective urea levels(Table I).

Fig.2 NH3 volatilization rates in the soil salinity and alkalinity treatments at the three urea levels(N1,N2,and N3:0.05,0.10,and 0.15 g N kg-1 soil,respectively).CK=control without NaCl or NaHCO3 addition;S1,S2,S3,and S4=1‰,3‰,8‰,and 15‰NaCl by mass of soil,respectively;A1,A2,A3,and A4=0.5‰,1‰,3‰,and 8‰NaHCO3 by mass of soil,respectively.Data are means with standard deviations(n=3).Means with different letters are not significantly different at P <0.05 among different treatments at each N level.

Fig.3 Cumulative NH3-N volatilization in the soil salinity and alkalinity treatments after urea application at the three N levels(N1,N2,and N3:0.05,0.10 and 0.15 g N kg-1 soil,respectively)during the incubation period(7 d).CK=control without NaCl or NaHCO3 addition;S1,S2,S3,and S4=1‰,3‰,8‰,and 15‰NaCl by mass of soil,respectively;A1,A2,A3,and A4=0.5‰,1‰,3‰,and 8‰NaHCO3 by mass of soil,respectively.Data are means with standard deviations(n=3).
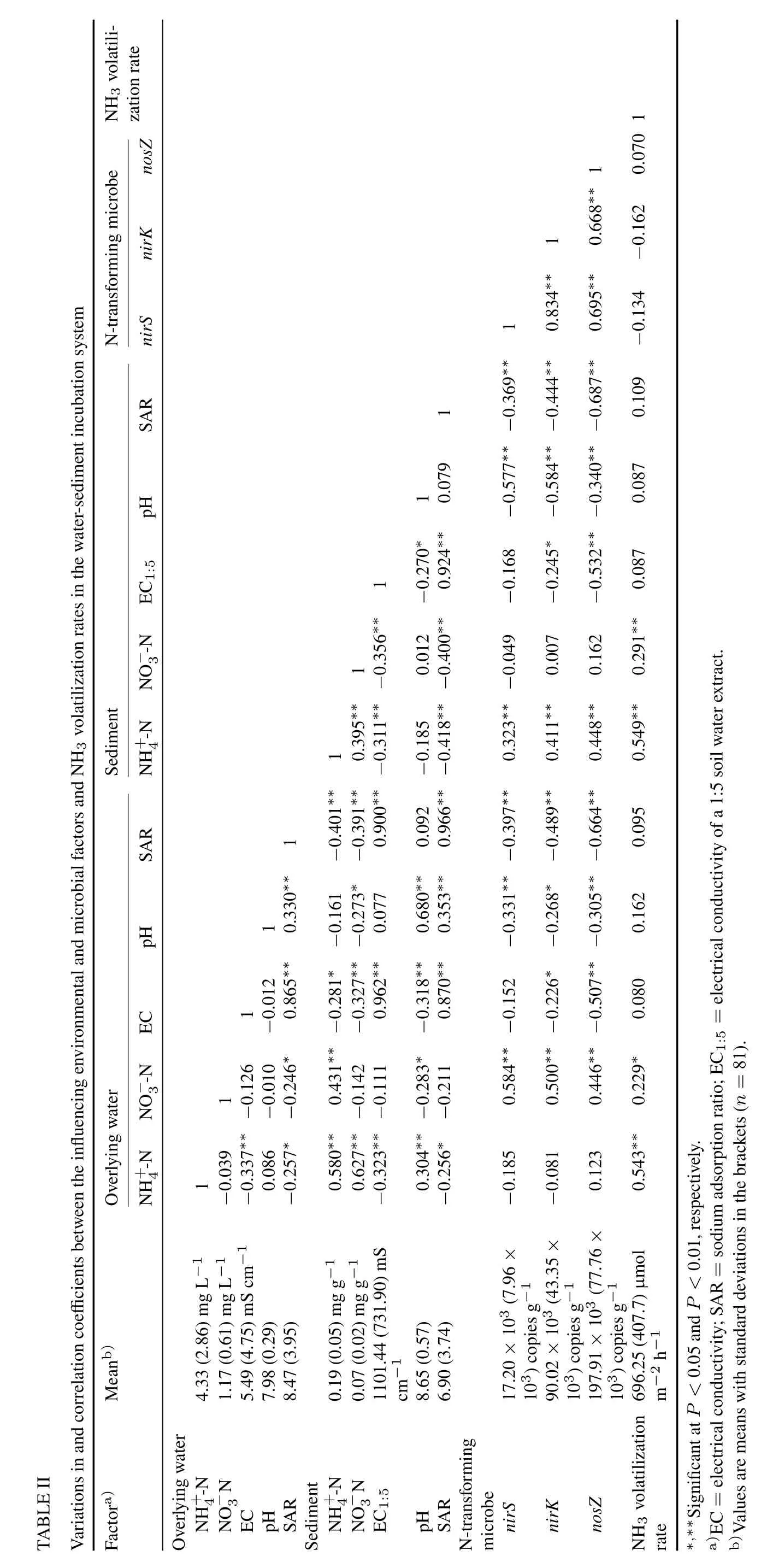
By Pearson’s correlation analysis,bivariate correlations between environmental and microbial factors and NH3volatilization rates are shown in Table II.The results showed that both theandconcentrations in the overlying water or the sediment were significantly positively correlated with the NH3volatilization rate (P <0.05).However,the correlation matrix did not exhibit a significant linear correlation between NH3volatilization rate and EC(or EC1:5),pH,or SAR in either the overlying water or the sediment.The promotion effects of salinity and alkalinity on NH3volatilization could be obscured by Pearson’s correlation analysis.
The factors that affected NH3volatilization were also correlated with each other (Table II).The variations in N concentrations (and) in the incubation system were complex due to the impacts of salinity and alkalinity.Theandconcentrations in both the overlying water and the sediment showed significant negative correlations with EC and SAR in the overlying water(P <0.05).With increasing pH,the concentration ofin the water-sediment system showed an increasing trend,while the concentration ofshowed a decreasing trend(Table II).Nitrogen-transforming microbes(nirS,nirK,andnosZgene abundances) showed significant positive correlations within the overlying water and within the sediment(P <0.01).Gene abundances(exceptnirS)were significantly negatively correlated with EC and pH in the water-sediment system.Nitrogen-transforming microbes interacted with N in the system under salinity and alkalinity limitations.The negative correlations between gene abundances and theconcentration indicated that nitrification was strictly inhibited only at higher salinities and alkalinities.

TABLE IPercentage loss of N input via NH3 volatilization from the soil salinity and alkalinity treatments at the three urea levels(N1,N2,and N3:0.05,0.10,and 0.15 g N kg-1 soil,respectively)
Performance of the SEM of NH3 volatilization rate
The results of the PCA indicated that the grouping of variables was reasonable.PC 1 of the salinity group explained 91.87%of the variation in EC and SAR in the water-sediment system.PC 1 of the alkalinity group represented 83.00%of the variation in pH.The concentrations ofandin the environment were in the N group,whose PC1 and PC2 accounted for 78.96% of the variation in N concentration.PC 1 of thenirS,nirK,andnosZgene abundances explained 82.24% of the variation in the Ntransforming microbe group(Table SI,see Supplementary Material for Table SI).
The weak relationships between NH3volatilization and associated factors may have been masked by collinearity in the bivariate regression.The SEM method can be used to trace the complicated relationships between all factors.The SEM represented 52%of the variance in the NH3volatilization rate(minimum chi-square(χ2)=2.696,P=0.260,R2=0.52)(Fig.4).The results showed that salinity exerted a significant and positive direct effect (standardized path coefficient=0.40)on the NH3volatilization rate.In addition,salinity had negative effects on N-transforming microbes and N concentration(standardized path coefficients=-0.44 and-0.22,respectively),with a negative indirect effect on the NH3volatilization rate.Alkalinity had a positive direct effect(standardized path coefficient=0.19)and a negative indirect effect(total indirect standardized coefficient=-0.089)on the NH3volatilization rate(Fig.5).Nitrogen concentrations had greater direct positive contributions(standardized path coefficient=0.78)than salinity and alkalinity to the NH3volatilization rate,which was consistent with the two-way ANOVA results(Table SII).Nitrogen-transforming microbes had a positive indirect effect on the NH3volatilization rate(total indirect standardized coefficient=0.31(i.e.,0.40×0.78)).
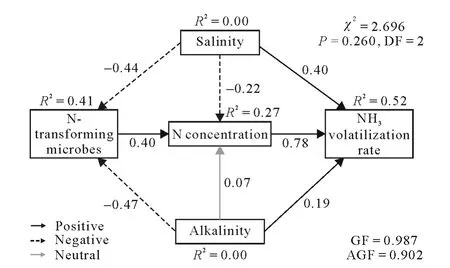
Fig.4 Structural equation model estimating the multivariate effects of variables on the NH3 volatilization rate in the incubation system.The influential variables in the incubation system are shown in Table SI.Standardized path coefficients are shown on lines and represent partial regression coefficients. χ2=minimum chi-square;DF=degree of freedom;GF=goodness of fit;AGF=adjusted goodness of fit.

Fig.5 Total standardized effects of salinity and alkalinity on the NH3 volatilization rate derived from the structural equation model.
DISCUSSION
The drivers of NH3 volatilization
In total,the NH3emissions from all treatments accounted for 5.26%-15.05%of the N inputs(Table I),which confirmed the finding that NH3volatilization accounted for 9.5%-14.5%of the total N inputs in a coastal area(Sunet al.,2013;Liet al.,2020).The peak volatilization rates were observed on Days 2 and 3(Fig.3),consistent with the results that the highest NH3volatilization rates were found on Day 3 after fertilization(Tiet al.,2021).The NH3volatilization losses from the salinity and alkalinity treatments were significantly greater than those from CK under the same urea addition(Fig.2,Tables SII and SIII,see Supplementary Material for Tables SII and SIII).Similar results indicate that the NH3volatilization from soils is affected by increases in three types of salt (Mcclung and Frankenberger,1985).Covaliet al.(2021) found that NH3volatilization decreased by approximately 50%in acid-neutralized soil treatments,and our results also indicated that pH was a key facilitator of NH3emissions (Liet al.,2022).The promotion effect of salt-alkali on NH3volatilization was significant(P <0.05).
The two-way ANOVA results indicated that salinity treatment,alkalinity treatment,and urea concentration had significant(P <0.001)effects on NH3volatilization rates(Table SII).The NH3volatilization rates increased significantly with increasing NaCl or NaHCO3concentration.Under the same NaCl or NaHCO3concentration,the N concentration increased with the increasing urea application level,which also led to a significant increase in the NH3volatilization rate (P <0.05).At the same urea application level,the volatilization rates under 15‰NaCl and 8‰NaHCO3were the highest among the salinity and alkalinity treatments,respectively.In addition,all NH3volatilization rates at the N3 level were the highest in the salinity or alkalinity treatments(Figs.2 and 3).
The main factors affecting NH3volatilization rates were salinity,alkalinity,and N concentrations in the incubation system.The SEM results suggested that the network relating NH3volatilization with influencing factors was complex and interactive.The relationship between N-transforming microbes(nirS,nirK,andnosZgene abundances)and NH3volatilization was obscured by Pearson’s correlation analysis.The SEM revealed this influence and identified these factors by the latent parameter“N-transforming microbe”.Salinity and alkalinity indirectly influenced the NH3volatilization rates by directly affecting N-transforming microbes and N concentrations,thus.The NH3volatilization process is a physical process that is mainly affected by pH and N processes(Beheraet al.,2013;Huang Jet al.,2021),and the indirect effect of microorganisms is rarely considered.Therefore,we could separate the whole net effects of impact factors into direct and indirect effects through the SEM method to further understand the impact mechanism of NH3volatilization in coastal ecosystems.
Trade-offbetween the mechanisms promoting andlimiting NH3 volatilization
The direct effects of salinity and alkalinity were positive,and the indirect effects were negative(Fig.5).We will explain the direct and indirect effects of salinity and alkalinity on NH3volatilization through abiotic and biotic aspects.The links in the SEM model suggested that salinity played a more complicated role than alkalinity in the NH3transformation process (Figs.4 and 5).The positive effect on the NH3volatilization process caused by changes in salinity was attributed to the chemical action of salt ions,which influenced the adsorption and release ofin sediment and changed the availability of(Heet al.,2015).High salinity weakens the adsorption capacity of sediment forand promotes the release ofinto water (Westonet al.,2010).
At the N2 or N3 levels,the cumulative NH3volatilization in the alkalinity treatments had a higher threshold than that in the salinity treatments(Fig.3).The positive(direct)effect of alkalinity on NH3volatilization is directly related to the pH andconcentration of the sediment and overlying water.After urea was applied,theand pH in the field water showed a short-term increase and then gradually decreased with time (Tiet al.,2021).An increase in the amount of N will increase the pH andconcentration in field water,thereby promoting NH3volatilization loss(Liet al.,2020).However,a high pH promotesleaching into the overlying water from the sediment(Rochetteet al.,2013).The NH3volatilization rate will increase due to the impact of pH on the chemical balance:+OH-⇆NH3+H2O.
In the SEM,salinity and alkalinity both had direct negative effects on N-transforming microbes.The negative indirect effects resulted from the influence on microbial abundance and N concentration.Many scholars attribute the influence of salt on N cycles to the poor tolerance of microorganisms involved in the denitrification-nitrification process to salt stress(Piaoet al.,2012;Gaoet al.,2016).Salinity directly affects the activity of nitrifying and denitrifying bacteria through physiological mechanisms(Parket al.,2021).For example,a high-sodium environment can easily lead to cell wall separation,thereby inhibiting cell activity(Rysgaardet al.,1999).The inhibition of microorganisms could cause variations in N concentration (Oren,1999;Mosquera-Corralet al.,2005).It takes longer for urease to completely decompose urea in high-salinity soil than in non-salinized soil,which would ultimately lead to a decrease in(Yaoet al.,2021),and hence,the inhibition of nitrifying microorganisms would decrease theconcentration.The limitedconcentration increased salinity by inducing a shift from denitrification to dissimilatoryreduction to(Giblinet al.,2010).Furthermore,the peak NH3volatilization rates occurred on Days 2 and 3(mostly Day 3),so peaks inconcentration appeared before the NH3volatilization peaked.However,the water and sediment sampling times were fixed.So the concentrations ofandin this study could not be used to characterize dynamic changes during the whole incubation period,also explaining why theconcentration was negatively correlated with EC and SAR.
Variations in alkalinity may lead to microbes being restricted by the availableand alkalinity of the environment (Liet al.,2017;Panet al.,2021b),eventually exerting a negative influence on microbial activities.There is a balance between nitrification and denitrification processes,and the addition of salt and alkali will disrupt the balance and inhibit both the processes (Figs.4,S1,and S2,see Supplementary Material for Figs.S1 and S2).Liet al.(2020)found that salinity could induce concomitant increases in soil NH3volatilization and nitrous oxide(NO2)emissions,which means that low salinity (1 dS m-1<EC<5 dS m-1)only slightly inhibited ammonia-oxidizing bacteria but strongly inhibited nitrite oxidation bacteria(Santoset al.,2018).Nitrogen was emitted in the form of NH3and NO2,which could result in a decrease in theandconcentrations in the overlying water.In the current case,the average soil EC was lower than 5 dS m-1,which could cause a reduction in the amount ofgenerated during nitrification(Moussaet al.,2006).Although the first stage of nitrification(conversion to)would not be strictly prevented (Zhaoet al.,2021),the inhibition of nitrifying microorganisms would indirectly increase the concentration of(Sigurdarsonet al.,2018).This could also provide an explanation for the SEM results of this study,where N-transforming microbes had an indirect positive effect on NH3volatilization(total indirect standardized coefficient=0.31(i.e.,0.40×0.78)).
Nitrogen concentration in the incubation system had the most important positive effect on NH3volatilization(Fig.4).Both salinity and alkalinity also significantly promoted NH3volatilization,but alkalinity contributed more than salinity to NH3volatilization after accounting for the trade-offbetween promotion and limitation effects(Fig.5).
CONCLUSIONS
The NH3volatilization rate increased significantly with increasing salinity or alkalinity.The cumulative NH3volatilization losses observed in the alkalinity treatments were higher than those in the salinity treatments at all urea application levels.As the salinity increased,the concentrations ofin both water and sediment showed a downward trend.According to the results of the SEM,the direct promotion effects of salinity and alkalinity on NH3volatilization were 0.40 and 0.19,respectively.From the result of total standardized effects,alkalinity had a greater influence than salinity.Both salinity and alkalinity affected NH3volatilization by influencing N transformation(N concentration and N-transforming microbes).Additionally,alkalinity also induced pH changes to directly impact NH3volatilization.Nitrogen concentrations in the incubation system affected volatilization by directly providing more.The promotion effect of the coastal environment(salt-alkali conditions)on NH3volatilization was considerable when estimating the N loss in coastal agriculture.
ACKNOWLEDGEMENT
This study was financially supported by the National Natural Science Foundation of China(No.42177393)and the Water Conservancy Science and Technology Project of Jiangsu Province,China(No.2021054).
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Developing the new soil science-Advice for early-career soil scientists
- Biophotoelectrochemistry:An emerging frontier for channeling photoelectric effect into darkness zone of soils and sediments
- Balancing machine learning and artificial intelligence in soil science with human perspective and experience
- Soils in extraterrestrial space:Need for studies under microgravity
- Role of biochar in raising blue carbon stock capacity of salt marshes
- Long-term fertilizer nitrogen management-Soil health conundrum
