Three novel rare TP53 fusion mutations in a patient with multiple primary cancers:a case report
2024-03-03MengyaoLuXuemeiZhangQianChuYuanChenPengZhang
Mengyao Lu,Xuemei Zhang,Qian Chu,Yuan Chen,Peng Zhang
Abstract As survival rates improve and detection technologies advance,the occurrence of multiple primary cancers(MPCs)has been increasing.Approximately 16%of cancer survivors develop a subsequent malignancy,with lung cancer often developing after esophageal cancer due to potential“field cancerization”effects.Despite this observation,the genetic heterogeneity underlying MPCs remains understudied.However,the recent emergence of genetic testing has expanded the scope of investigations into MPCs to investigate signatures underlying cancer predisposition.This report reveals 3 unprecedented TP53 fusion mutations in a Chinese patient afflicted by MPCs,namely,AP1M2-TP53(A1;T11)fusion,TP53-ILF3(T10;I13)fusion,and SLC44A2-TP53(S5;T11)fusion.This patient exhibited an extended period of survival after diagnosis of extensive-stage small cell lung cancer,which occurred 6 years after the diagnosis of esophageal squamous cell cancer.This unique report may provide supplementary data that enhance our understanding of the genetic landscape of MPCs.
Keywords: Multiple primary cancers;TP53 fusion mutation;Esophageal squamous cell cancer;Extensive-stage small cell lung cancer;Immunotherapy;Antiangiogenic therapy
1.Introduction
The prevalence of multiple primary cancers(MPCs)has risen markedly in recent decades,with approximately 16%to 20%of cancer patients now diagnosed with MPCs.[1]Accurate identification of MPCs is vital for determining patient prognosis and treatment approaches.However,differentiating between MPCs and metastasis or recurrence remains a significant issue.Currently,research in this field focuses on identifying susceptibility genes and molecular markers to improve diagnosis accuracy and promote targeted preventive measures.Moreover,the emergence of next-generation sequencing(NGS)has accelerated the discovery of genetic alterations associated with MPCs.
Esophageal cancer ranks as the eighth most prevalent type of cancer and is responsible for the sixth highest mortality globally,with a notably high incidence rate in China.Compared with the general population,individuals treated for esophageal cancer have a 1.5-to 3-fold increased risk of developing subsequent lung cancer.The incidence of lung cancer as a secondary tumor after esophageal cancer treatment varies between 10% and 20%.[2–5]The inherent characteristics of the esophagus and lungs allow for potential“field cancerization” effects,signifying shared exposures to inhaled/ingested carcinogens.[6]However,the detailed genetic landscape enabling the development of MPCs in these organs remains uncertain.
Mutations in the TP53 tumor suppressor gene are prevalent in esophageal (50%–70%)[7–9]and lung cancers (50%–90%).[10]These alterations often lead to genetic instability,[11]increased rates of metastasis,[12]treatment resistance,[13]and adverse outcomes.[14]Xue et al.[15]also identified a predominant TP53 missense mutation in patients with double squamous cell carcinoma of the esophagus and lung.TP53 mutations typically manifest as nonsense or missense mutations,synonymous substitution,in-frame deletions,or insertions.However,as genetic sequencing accuracy and depth continue to advance,the utilization of DNA and RNA NGS for the detection of gene variations is expanding,and an increasing number of rare or atypical TP53 mutations,including TP53 fusion mutations,have been discovered.Notably,the COSMIC database has previously documented an example of TP53–NTRK1 fusion in a benign melanocytic nevus sample.In this case report,we expanded the knowledge of TP53 fusion mutation subtypes by unveiling 3 previously unreported TP53 fusion mutations in an MPC patient who developed second primary extensive-stage small cell lung cancer after esophageal cancer surgery.Notably,this patient experienced a remarkably prolonged survival.
2.Case report
In August 2016,a 60-year-old man was admitted to the hospital because of a progressively worsening sensation of a foreign object in his throat after eating.Gastroscopy revealed an irregular growth and ulceration in the mucosal layer,positioned approximately 30 to 38 cm from the incisors.Subsequent pathological examination confirmed a diagnosis of esophageal squamous cell carcinoma(Figure 1A).On September 8,2016,the patient underwent curative surgery and was staged as T1bN0M 0 (stage I).Subsequently,he underwent periodic follow-up examinations and remained free of recurrence.The patient had a significant history of smoking and alcohol consumption,but had no family history of cancer.In May 2022,he was readmitted presenting with a persistent headache,with a Performance Status level of 1.Magnetic resonance imaging revealed brain metastases(Figure 1B),and the chest computed tomography showed a 40×39-mm enhancing left lung lesion(Figure 1C).The biopsy of the lung mass and lymph node station 11L revealed small cell lung carcinoma (SCLC;cT4N1M1,stage IV;Figure 1D),and immunohistochemical examination indicated mutant-type P53 protein expression (Figure 1E).Finally,the patient was diagnosed with metachronous extensive-stage SCLC(ES-SCLC)and esophageal squamous cell carcinoma after multidisciplinary team discussions.
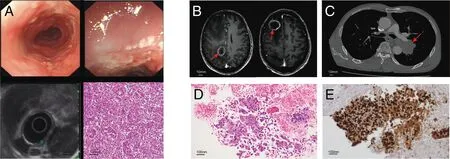
Figure 1.Diagnostic evaluation of the patient with multiple primary cancers.A,Esophagoscope and histopathology for esophageal squamous cell cancer.B,Head MRI at the time of diagnosis.C,Chest CT at the time of diagnosis.D,Histopathology of small cell lung cancer.E,Immunohistochemical staining for P53.CT,computed tomography;MRI,magnetic resonance imaging.
To further understand the genetic spectrum of this case of MPCs,formalin-fixed,paraffin-embedded lung tumor samples were detected by DNA-NGS (769-gene panel;Genecast Biotechnology Co) and RNA-NGS (29-gene panel;Genecast Biotechnology Co).Three unexpected gene fusions were identified: AP1M 2–TP53(A1;T11,fusion frequency 25.79),TP53–ILF3(T10;I13,fusion frequency 18.86),and SLC44A2–TP53 (S5;T11,fusion frequency 26.44).Exon 1 of AP1M 2 (breakpoint: chromosome 19p13.2,10697613) was fused with TP53 exon 11 (breakpoint: chromosome 17p13.1,7 573033;Figure 2A).Exons 1 to 10 of TP53(breakpoint: chromosome 17p13.1,7572863) were fused with ILF3 exons 13 to 20 (breakpoint: chromosome 19p13.2,10 793005;Figure 2B).Finally,exons 5 to 22 of SLC44A2(breakpoint: chromosome 19p13.2,10739654) were fused with TP53 exon 11 (breakpoint: chromosome 17p13.1,7 573033;Figure 2C).Some other single nucleotide variants were detected in this sample,including TP53 c.818G >T(Supplementary Table 1,http://links.lww.com/OTM/A0).

Figure 2.Diagram of fusion mutations in lung tumor.A,AP1M2-TP53 fusion.B,TP53-ILF3 fusion.C,SLC44A2-TP53 fusion.
After a definitive diagnosis,the patient underwent whole-brain radiotherapy(30 Gy in 10 fractions),followed by systemic chemotherapy with etoposide and cisplatin,and atezolizumab immunotherapy based on the phase III IMpower 133 trial.After 4 cycles of systemic therapy,the patient achieved stable disease in August 2022(Figure 3,middle panel).Subsequently,he underwent 30 Gy in 10 fractions of palliative thoracic radiotherapy,followed by maintenance atezolizumab immunotherapy.In November,the patient developed a progression of brain metastases (Figure 3,right panel),and the chemotherapy regimen was changed to irinotecan as a second-line therapy.However,the regimen was promptly stopped because of severe adverse effects including thrombocytopenia,and third-line oral anlotinib was initiated as a replacement therapy.The full treatment timeline is outlined in Figure 4.As of September 1,2023,the patient remains alive,with an overall survival exceeding 15 months since the ES-SCLC diagnosis.

Figure 3.Overview of disease development of the patient.
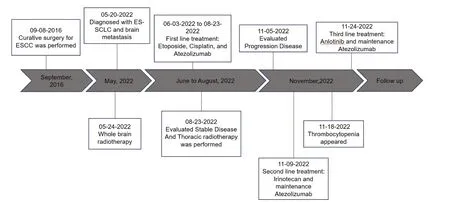
Figure 4.Full treatment timeline of the patient.ESCC,esophageal squamous cell cancer;ES-SCLC,extensive-stage small cell lung cancer.
3.Discussion
Although public health initiatives to minimize the risk of subsequent cancers have been gaining significant attention,it is important to note that treatment-related and external exposures alone do not comprehensively account for the risk of MPCs.The genetic factors contributing to MPCs,particularly beyond known inherited syndromes,remain relatively unexplored.However,simultaneous sequencing of somatic and germline mutations may prove vital for investigating the interplay between the context of susceptibility genes and distinct tumor combinations.[16]
Herein,we present a rare case of metachronous MPCs,including esophageal cancer followed by extensive-stage small cell lung cancer 6 years later.Small cell lung carcinoma is a highly aggressive type of lung cancer,characterized by rapid growth and early metastasis.The median survival for patients with ES-SCLC is typically around 8 to 10 months.Although countless treatment approaches have been devised and attempted,the prognosis for ES-SCLC remains poor.Upon diagnosis,10%of SCLC patients are found to have brain metastases,typically resulting in a reduced median survival of 3 to 5 months.[17,18]However,the success of the IMpower133 study has ushered in a new era of immunotherapy for ES-SCLC,with overall survival being significantly prolonged.Although PD-L1–based immunotherapy combined with first-line chemotherapy has shown promise in improving the survival of ES-SCLC patients,there remains a lack of data concerning the safety and survival benefits associated with thoracic palliative radiotherapy.Both the ongoing TREASURE study[19]and the research of Li et al.[20]focused on further improving the response to upfront chemoimmunotherapy by applying consolidative thoracic radiotherapy before disease progression in the first-line setting of ES-SCLC.Although the CREST study demonstrated that thoracic radiotherapy contributes to improved survival outcomes by enhancing local control,there remains an important gap in the available data regarding patients with brain metastases.Furthermore,the median overall survival of patients with brain metastases in the IMpower133 study was less than 10 months,which was inferior to that observed in the intent-to-treat population.However,our patient,who presented with symptomatic brain metastases,survived for more than 15 months.This was achieved through a combination of 3 different lines of systemic treatment and meticulous local radiation treatment.Such a long-term survival observed in our case cannot be explained comprehensively according to the existing clinical data.Therefore,we speculate that potential unique molecular mechanisms related to this particular case of MPCs may play a role in this positive outcome.
Through genetic sequencing of SCLC tissue from this case,we report the first case of 3 distinct fusion partners evolving TP53,all of which are closely located on chromosome 19p13.2.Notably,none of these fusion partners(AP1M 2,ILF3,and SLC44A2)have been previously documented in fusion events.Both the TP53–NTRK1 fusion,previously reported in COSMIC,and the AP1M 2–TP53 and SLC44A2–TP53 fusions in our report share a breakpoint in exon 11 of TP53.The complicated TP53 heterogeneity observed in this case reaffirms the pivotal role of TP53 mutations in the co-occurrence of esophageal and lung cancer,while also expanding our understanding of the involvement of TP53 in MPCs.
AP1M 2 is a component of AP complexes involved in intracellular protein trafficking and sorting[21]and has been proposed a potential marker for endometrial cancer;[22]breast cancer,[23]and chromophobe renal cell carcinoma.[24]The formation of AP1M 2–TP53 fusion protein may lead to altered cellular trafficking processes,affecting the localization and function of P53.The subsequent dysregulation of P53 could compromise its tumor suppressor activities,potentially contributing to uncontrolled cell growth and development of multiple cancers.Future work is needed to investigate the conditions of protein expression,explore the effects on P53 pathway regulation,and correlate the presence of fusion with patient outcomes.
ILF3 is an RNA-binding protein that plays important roles in several cellular processes,including RNA metabolism and regulation of the immune response.[25]Dysregulation of ILF3 has been implicated in abnormal cell proliferation and transformation in a variety of cancer types.[26]In lung cancer specifically,ILF3 has been shown to influence the EGFR-mediated cellular pathway.[27]This TP53–ILF3 fusion transcript was generated by fusion of TP53 exons 1 to 10 and ILF3 exons 13 to 20,encompassing the crucial transcriptional activation domain and DNA-binding domain of TP53.We hypothesized that the joining of ILF3 may have activated this aberrant P53 protein as a transcription factor.Future investigation of this fusion protein's impact on TP53 expression and downstream transcription networks could help to elucidate the oncogenic mechanisms underlying lung and esophageal cancers.
SLC44A2 is a solute carrier involved in choline transport and is an important molecule for phospholipid and neurotransmitter synthesis.[28]The gene coding for this molecule is situated upstream of the ILF3 gene.However,current research primarily focuses on its involvement in thrombosis[29]and carrying blood group antigens.[30]Although knowledge regarding the relevance of SLC44A2 to cancer is limited,its proximity to ILF3 is noteworthy.We speculate that the involvement of SLC44A2 could influence choline transport,thereby affecting cellular lipid metabolism.This alteration may contribute to modifications in cell membrane composition and signaling,potentially facilitating the progression of malignancy.
The presence of these 3 closely located fusion proteins suggests that a single chromosomal rearrangement event may have occurred.This illuminates the mechanisms underlying the involvement of genomic instability in development of MPCs.
TP53,a pivotal tumor suppressor gene,is the most frequently mutated gene in human cancers.Beyond the loss of its tumor-suppressing capabilities,mutant P53 often acquires novel oncogenic functions.Emerging research indicates a strong association between mutant TP53 and advanced malignancies and poor prognosis,making this protein an attractive target for the development of innovative cancer therapies.TP53 mutations have previously been linked to high genomic instability,[11]which has proven to be a significant risk factor in the MPC population.Inactivated P53 can lead to a more immunosuppressive tumor microenvironment and increased expression of factors such as PD-L1.These carriers have consistently exhibited improved responses to immune checkpoint inhibitors[31,32]and antiangiogenic therapy.[33,34]
After reviewing this report,we have shared our case experiences in the utilization of immunotherapy and antiangiogenic agents in an MPC patient carrying TP53 fusions.Nonetheless,because of the infrequency of these fusion mutations and the restricted specificity of TP53 as a biomarker,the surveillance of lung cancer with similar mutations in esophageal cancer survivors remains controversial.
The limitations of this report include the absence of RNA validation and functional fusion protein expression data,as well as the reliance on data from a single patient.Future studies should validate fusion allele frequencies to eliminate potential artifacts,confirm the presence of fusions through polymerase chain reaction,and quantify fusion RNA/protein levels.In addition,tracking fusion status over time and correlating it with clinical outcomes could provide valuable insights.Moreover,investigations with larger and more diverse cohorts should be conducted to comprehensively evaluate the prevalence of these fusion proteins and to assess their functional impact in MPC populations.Further research should focus on risk stratification to identify patient subgroups at the highest risk of developing secondary cancers.The knowledge gained regarding patients with cancer predisposition through this study will hopefully allow for the development of specific management and surveillance measures.
Acknowledgments
We acknowledge Genecast Biotechnology Co,Ltd for gene analysis and technical support.
Financial support and sponsorship
This research was supported by the National Natural Science Foundation of China(grant numbers 81974483 and 82072589)and the Chinese Society of Clinical Oncology–Hengrui Cancer Research Fund(Y-HR2020QN-0946).
Conflicts of interest statement
The authors have no conflicts of interests.
Author contributions
P.Z.conceived the topic for this case report;M.Y.L and X.M.Z.prepared the main manuscript text and figures;Q.C.and Y.C.supervised the manuscript revision;and X.M.Z.was responsible for corrections.All authors made substantial and intellectual contributions to this investigation.
Data availability statement
Not applicable.
Ethical approval
Cancer tissues and paired normal samples were obtained from the Tongji Hospital affiliated to Huazhong University of Science and Technology.Written informed consent was obtained from the patient related to this case study.The study was approved by the Ethics Committee of Tongji Hospital(TJ-IRB-20220547).
杂志排行
Oncology and Translational Medicine的其它文章
- Wan-Yee Joseph Lau:the promoter of modernization and internationalization of Chinese surgery
- In vivo study of the effect of anlotinib on the stemness of the lenvatinib-resistant hepatocellular carcinoma cells and the underlying mechanisms
- Utilizing bioinformatics for integrated analysis of multiple genes in the diagnosis and pathogenesis of metastatic pheochromocytoma and paraganglioma
- Role of tumor necrosis factor alpha-induced protein 6(TNFAIP6)in tumors:a pan-cancer analysis
- Clinical and pathological characteristics and expression of related molecules in patients with airway disseminated lung adenocarcinoma
