Effects of pyraclostrobin on growth, oxidative stress, and gene expression in relation to stress and ATP-binding cassette transporters in Tetrahymena thermophila*
2024-02-27YangLIUJialeZHANGPengXIAOXinLIUYisifuMAJingZHANGBangjunZHANG
Yang LIU, Jiale ZHANG, Peng XIAO, Xin LIU, Yisifu MA, Jing ZHANG,Bangjun ZHANG
1 Henan International Joint Laboratory of Aquatic Ecotoxicology and Health Protection, Xinxiang 453007, China
2 College of Life Sciences, Henan Normal University, Xinxiang 453007, China
3 Journal of Henan Normal University, Xinxiang 453007, China
4 College of Life and Environmental Science, Wenzhou University, Wenzhou 325035, China
5 Department of Ecosystem Studies, School of Environmental Science, University of Shiga Prefecture, Shiga 522-8533, Japan
Abstract Pyraclostrobin (PYR), a widely used fungicide, has negative effects on fish and algae, but its toxicity in protozoa remains unclear.In this study, the effects of PYR on the growth, oxidative stress, and gene expression related to stress and ATP-binding cassette (ABC) transporters in Tetrahymena thermophila were investigated.The result showed that the 96-h IC50 of PYR against T.thermophila was 17.2 mg/L.Moreover, PYR inhibited the growth of T.thermophila in concentration- or time-dependent manner.A morphological study revealed that the shape and size of T.thermophila changed, and damage of cell membrane surface was observed by scanning electron microscopy after 96 h of PYR exposure.The activities of superoxide dismutase (SOD) and catalase (CAT) increased throughout the experiment.In contrast, the glutathione (GSH) content was increased at 24 h and 48 h of exposure and decreased at 96 h.Moreover, a significant increase in malondialdehyde (MDA) level was observed in T.thermophila after 96 h of exposure.Furthermore, PYR upregulated the HSP703, HSP705, GPx2, and ABAC15 gene expression in the 0.1-5-mg/L groups and downregulated the HSP704, HSP90, TGR, and ABCC52 mRNA levels at 96 h of exposure.These results suggest that PYR may exert adverse effects on T.thermophila by inducing oxidative stress and changing the gene expression related to ABC transporters and stress, which may enrich the understanding of the toxicity mechanism of PYR in aquatic organisms and provide reference data for aquatic ecological risk assessments.
Keyword: pyraclostrobin; Tetrahymena thermophila; growth; oxidative stress; gene expression
1 INTRODUCTION
Strobilurin fungicides, which are used worldwide to fight fungal invasion and prevent the outbreak of plant diseases in agriculture, exert their antifungal activity by blocking electron transfer from cytochrome b to c1, inhibiting subsequently the mitochondrial respiration and the generation of cellular adenosine triphosphate (ATP) (Zhang et al., 2020).Strobilurins rank first in the global sales of fungicides, and the application is increasing in the world (Yang, 2014).Pyraclostrobin (PYR), a strobilurin fungicide, was marketed in 2001 and became the second best-selling fungicide in 2014 among strobilurin fungicides (Wang et al., 2021).In the wide application, PYR was monitored in streams of southeastern Australia, in Nebraska’s Rainwater Basin Wetlands in the USA,and in paddy water in China, and the maximum concentration was 0.1, 1.61, and 17.24 μg/L,respectively (Wightwick et al., 2012; Mimbs et al.,2016; Guo et al., 2017), indicating the presence of PYR in many water bodies, and the concentration of PYR in farmland water is relatively high, which could increase the risks in the local aquatic ecosystems.Therefore, studies of the negative impacts of PYR on non-target organisms have drawn more attention in recent years.
Similar to the toxicity of other strobilurin fungicides, PYR also shows high toxicity to aquatic organisms includingDaniorerio(Li et al., 2019;Chen et al., 2022),Oreochromisniloticus(Li et al.,2021a),Xenopustropicalisembryos (Li et al., 2016),Bufocognatus(tadpole) (Hooser et al., 2012),Daphniamagna(Cui et al., 2017),Lymnaeastagnalis(Fidder et al., 2016),Pseudokirchneriellasubcapitata(Ochoa-Acuña et al., 2009),Chlorellavulgaris(Wang et al., 2020),Helisomatrivolvis(Morrison and Belden, 2016), andHyalellaazteca(Morrison et al., 2013).PYR exposure can induce developmental toxicity, cardiotoxicity, hepatotoxicity, and neurotoxicity in zebrafish (Zhang et al., 2017; Li et al., 2019; Mao et al., 2020; Kim et al., 2021; Li et al., 2021b).Moreover, Cui et al.(2017) noted that PYR at 0.15,0.2, and 20 μg/L affected significantly the reproduction,development, and growth ofDaphniamagna.Furthermore, PYR exerted significant respiratory toxicity onChlorellavulgaris(Wang et al., 2020).Due to its broad-spectrum toxicity to aquatic organisms, it is of great significance to understand the impact of PYR on organisms at different trophic levels of aquatic ecosystems, which can help to assess the ecological risk caused by PYR.However,the effects of PYR on microorganisms such as protozoans are still largely unknown.
Strobilurin fungicides are proved being able to trigger oxidative stress in aquatic organisms through overproduction of reactive oxygen species (ROS),which subsequently leads to oxidative injury to lipids,proteins, and DNA (Zhang et al., 2020; Fan et al.,2022).Thus, oxidative stress may be an important toxic mechanism of strobilurin fungicides to nontarget organisms.Superoxide dismutase (SOD),catalase (CAT), glutathione peroxidase (GPx), and reduced glutathione (GSH) represent the key components of the antioxidative system of organisms and are widely used as biomarkers to assess the status of oxidative stress (Rahal et al., 2014; Pisoschi and Pop, 2015).Malondialdehyde (MDA), a product of polyunsaturated fatty acid peroxidation, is also a good bioindicator that reflects the degree of oxidative injury (Rahal et al., 2014).Moreover, under various environmental stresses, heat shock proteins (HSPs)are synthesized rapidly to help organisms defend against adverse conditions and maintain key cellular processes including protein folding, repair, and transportation (Feder and Hofmann, 1999).As a group of highly conserved transport proteins, the ATP-binding cassette (ABC) transporter superfamily plays a vital role in cell detoxification defence by acting as an efflux pump to reduce the intracellular concentrations of the toxicants or their metabolic intermediates (Mastrantonio et al., 2017).However,there is little knowledge on the effects of PYR on these parameters inTetrahymena thermophila.
The protozoanTetrahymena, the simplest unicellular animal, plays a crucial role in the aquatic food chain, acting as a grazer of bacterial populations and a prey for metazooplankton (Juganson et al.,2017).Because of its widespread distribution, small size, fast reproduction, and easy culture,Tetrahymenais generally used as a good model animal to assess the risk of environmental pollutants in aquatic ecology (Eisen et al., 2006).Therefore, in this study,T.thermophilawas exposed to PYR and the growth,morphological changes, oxidative stress, and gene expression of HSPs, thioredoxin glutathione reductase(TGR),GPx2, and ABC transporters inT.thermophilawere evaluated, which may provide the information on the potential toxic mechanism and help the risk assessment of PYR in aquatic system.
2 MATERIAL AND METHOD
2.1 Chemical and Tetrahymena culture
PYR (purity ≥98%) was purchased from Shanghai Yuanye Biotechnology Co., Ltd.(Shanghai, China)and dissolved in acetone.All other reagents used in the experiment were of analytical grade.
Tetrahymenathermophila(SB210 strain) obtained from the National Aquatic Biological Resource Center(NABRC, Wuhan, China) was maintained in super proteose peptone (SPP) medium that contained 2%proteose peptone (Bacto Difco, Detroit, USA), 0.1%yeast extract (Oxoid, Basingstoke, Hampshire,England), 0.2% glucose (Sigma, Shanghai, China),and 0.003% ferric citrate (Sigma, Shanghai, China)at 28 °C with shaking at 135 r/min.
2.2 Cell viability assay
Cell viability was measured using Cell Counting Kit-8 (CCK-8) (Beyotime, Shanghai, China) with 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt(WST-8) assay.WST-8 was reduced by dehydrogenase in viable cells to produce a water-soluble orangecolored formazan product and the cell viability was estimated by determining the absorbance of formazan at 450 nm (Cai et al., 2019).Based on our preliminary experiment,T.thermophilawas inoculated into SPP medium to a final density of 2×104cells/mL with different concentrations of PYR (0, 0.1, 1, 5,10, 20, and 50 mg/L) at 28 °C under constant shaking at 135 r/min.After 96 h of exposure, 100 μL ofT.thermophilawas transferred to a 96-well plate(Tissue Culture Plate, Guangzhou, China).Then, 10-μL CCK-8 was added to each well and the plate was cultured in incubator at 30 ℃ for 3 h.Finally, the absorbance at 450 nm was determined with a microplate reader (Varioskan Flash, Thermo Fisher Scientific, USA).The median inhibitory concentration(IC50) was determined using GraphPad Prism 8.3.0(GraphPad, San Diego, CA, USA).
2.3 Cell growth, motility, and morphological observation
The effect of PYR on the growth ofT.thermophilawas observed under light microscopy.Briefly,T.thermophilawas exposed to different concentrations of PYR (0, 0.1, 1, 5, and 10 mg/L)for 24, 48, 72, and 96 h.After sampling, cells were fixed with Lugol’s solution, and the cell number was counted under a light microscope with a hemocytometer (Lainhart et al., 2009).To investigate the changes in motility, shape, and size inT.thermophila, cells were sampled at 96 h of PYR exposure.Then, 100 μL of the test culture from the PYR-treated groups and control group was placed in a plankton-counting chamber, and the motility ofT.thermophilawas recorded with a microscope (Nikon Eclipse Ni, Japan).Video recording was performed for 10 s.Subsequently,T.thermophilawas immobilized with 2.5% glutaraldehyde and observed directly with microscope and the lengthwidth ratio was counted.
2.4 Mitochondrial membrane potential (MMP)assay
The MMP ofT.thermophilaexposed to 0-, 0.1-,1-, 5-, and 10-mg/L PYR for 96 h was estimated using 5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazole carbocyanide iodide (JC-1)detection kit (Beyotime, Shanghai, China).Briefly,5×105cells were collected from each treatment group by centrifugation, incubated with 5-μg/mL JC-1 at 37 ℃ for 20 min, and washed twice with cold phosphate-buffered saline (PBS).The fluorescence was then determined with a microplate reader(Varioskan Flash, Thermo Fisher Scientific, USA)with an excitation/emission wavelengths of 485/535 nm for green monomers and excitation/emission wavelengths of 535/590 nm for red aggregates.MMP was estimated according to the intensity ratio of red to green fluorescence.A decrease in the ratio represents mitochondrial depolarization.
2.5 Scanning electron microscope (SEM) observation
After 96 h of treatment with PYR (0, 5, and 10 mg/L), the samples were fixed with 2.5%glutaraldehyde at 4 °C overnight.The cells were then dehydrated with ethanol (30%, 50%, 70%,90%, and 100%) and tert-butyl alcohol before vacuum freeze-drying at 4 ℃.Finally, the samples were coated with gold and observed by SEM (JSM-7800F, Japan).
2.6 Antioxidant enzyme, GSH, and MDA determinations
Tetrahymenathermophilawas exposed to different concentrations of PYR (0, 0.1, 1, 5, and 10 mg/L)for 24, 48, and 96 h, and the cells were collected by centrifugation, washed three times with precooled PBS (pH 7.4) and resuspended in PBS.Then, the cells were repeatedly frozen and thawed for three times to obtain the homogenate.The homogenate was centrifuged at 4 ℃ at 12 000×gfor 10 min, and the supernatant was used to determine the levels of SOD, CAT, GSH, MDA, and the content of total protein with the kits provided by Beyotime Biotechnology Co., Ltd.(Shanghai, China) as described previously by Liu et al.(2022).Briefly,the SOD enzyme was measured using 2-(2-methoxy-4-ni-trophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H tetrazolium, monosodium salt (WST-8) method.In the reaction, superoxide anion (O·-2) was produced by xanthine oxidase can react with WST-8 to form water-soluble formazan dye, which can be inhibited by SOD.Therefore, the activity of SOD is correlated negatively with the amount of formazan dye, and the SOD activity can be determined with colorimetric analysis.CAT enzyme can degrade H2O2into H2O and O2.Under the catalysis of peroxidase, the H2O2can oxidize the chromogenic substrate to produce a red substance, which can be assayed at 520 nm.Moreover, the levels of GSH, MDA, and the total protein were measured according to the 5, 5′-dithiobis-(2-nitrobenzoic acid) (DTNB), thiobarbituric acid (TBA), and bicinchoninic acid (BCA) method,respectively.
2.7 Real-time quantitative PCR (RT-qPCR)
After treatment with 0-, 0.1-, 1-, 5-, and 10-mg/L PYR for 96 h, total RNA ofT.thermophilawas extracted with RNAiso Plus (TaKaRa, Beijing,China) according to the manufacturer’s instructions,and the concentration and integrity of total RNA was determined with a NanoDrop 2000 (Thermo Scientific, Wilmington, DE, USA) and 1% agarose gel electrophoresis, individually.The total RNA was converted to cDNA with Rescript II RT SuperMix containing gDNA Eraser (Nobelab, Beijing, China).RT-qPCR was performed with SYBR Green qPCR Mix (Monad, Suzhou, China) using LightCycler 96 instrument (Roche, Switzerland) (Ding et al., 2021).Each treatment had three replicates.Primers for HSPs(HSP703,HSP704, andHSP705), ABC transporters(ABCB15andABCC52),GPx2,TGR, and17Sare listed in Table 1.The relative expression of each target mRNA transcript was normalized to17Sexpression and calculated by the Livak and Schmittgen (Livak and Schmittgen, 2001) method.The gene name and primers were previously reported (Yu et al., 2012;Gao et al., 2015; Wu et al., 2021).
2.8 Statistical analysis
Data are expressed as the means±standard deviations (SD).The normal distribution and homoscedasticity were assayed by Shapiro-Wilk’s and Levene’s tests.One-way analysis of variance(ANOVA) followed by Tukey’s test or nonparametric tests (Kruskall-Wallis) was used to test the significant differences between the control and PYR-treated groups.Data were analyzed by using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA).The statistical differences were considered as significant whenP<0.05.
3 RESULT
3.1 IC50 of PYR against T.thermophila
After 96 h of exposure to PYR, the cell viability ofT.thermophilawas reduced to 95.9% (treatment with 0.1 mg/L), 87.6% (1 mg/L), 81.6% (5 mg/L),67.4% (10 mg/L), 35.0% (20 mg/L), and 5.4%(50 mg/L) compared to that of the control group(Fig.1).Accordingly, the IC50of PYR toT.thermophilawas determined to be 17.2 mg/L.Subsequently, thePYR concentrations of 0.1, 1, 5, and 10 mg/L were selected for the following investigation.

Table 1 Primer sequences for RT-qPCR
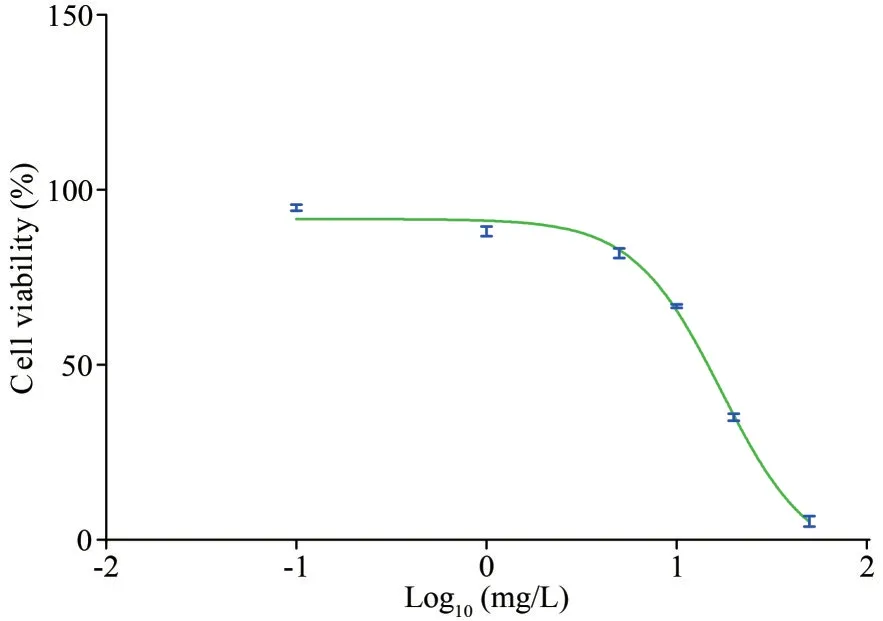
Fig.1 Effect of PYR on cell viability in T.thermophila after 96 h of exposure
3.2 Effect of PYR on the growth of T.thermophila
The growth ofT.thermophilaexposed to PYR is shown in Fig.2.Clearly, PYR affected the growth ofT.thermophilaat all four exposure times, and this impact gradually increased with the extension of exposure time.Moreover, the growth inhibition ofT.thermophilaincreased with increasing of PYR concentration when compared with the control group, indicating that PYR has a negative effect on the growth ofT.thermophila.
3.3 Morphological observation and movement
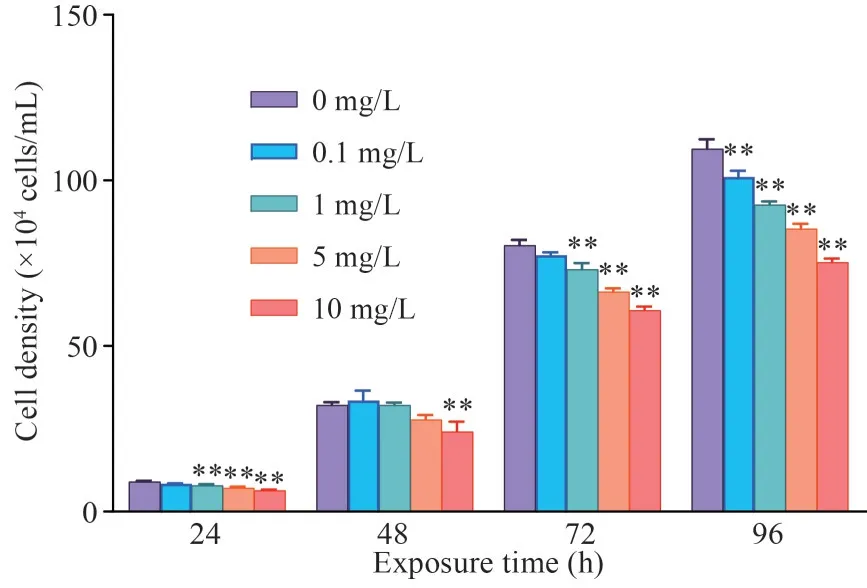
Fig.2 Effect of PYR on cell growth in T.thermophila after 96 h of exposure
As shown in Fig.3a, the cells were transparent and showed a normal pear shape in the control group.However, in the high concentration groups (5 and 10 mg/L), the cells were significantly darker and the shape became more round than that in the control group (Fig.3b-c).Moreover, the lengthwidth ratio of the 0.1-mg/L treatment group was significantly higher than that of the control group.With increasing of PYR concentration, the lengthwidth ratio gradually decreased and was significantly lower than that of the control group when the concentration was higher than 1 mg/L (Fig.3d).
Supplementary Videos S1 and S2 show the movement ofT.thermophilain the control group and 10-mg/L PYR group after 96 h of PYR exposure, respectively.In the control group,T.thermophilaspiraled rapidly and changed its direction with a sharp turn.However, after exposure to 10-mg/L PYR, the shape was changed in some cells.Moreover,the movement speed of some cells slowed down, the direction of movement was uncertain, and some cells even stayed in place and hardly moved.
3.4 Mitochondrial depolarization
In the present study, MMP was measured based on the red-green fluorescence ratio of JC-1.Compared with the control group, MMP was significantly decreased in the groups treated with 1-,5-, and 10-mg/L PYR (Fig.4).
3.5 SEM observation
The surface changes ofT.thermophilawere observed by SEM after 96 h of PYR exposure and are shown in Fig.5.In the control group, the shape ofT.thermophilawas elliptical in shape and surrounded by cilia, and the structure was intact with regular wrinkles (Fig.5a).However, after exposure to 5-mg/L PYR, loss of cilia occurred on the surface ofT.thermophila(Fig.5b).Moreover, 10-mg/L PYR exposure caused shrinking and twisting inT.thermophila(Fig.5c).
3.6 Level of SOD, CAT, GSH, and MDA
The effect of PYR on SOD activity inT.thermophilais shown in Fig.6a.No remarkable change was observed in the 0.1-mg/L PYR-treated group throughout the experiment.However, SOD activity was significantly higher in the 1-5-mg/L groups than in the control group.Similarly, the CAT activity increased significantly in all PYR-treated groups from 24 to 96 h of exposure with the exception of the 0.1-mg/L group at 96 h (Fig.6b).Furthermore, GSH content was markedly increased in all PYR treatment groups after 24 and 48 h of exposure (except 0.1 mg/L at 24 h).However, GSH was decreased significantly after 96 h of exposure in all PYR-treated groups (Fig.6c).Moreover, the MDA content was markedly increased in the treatment groups with PYR concentrations higher than 0.1 mg/L after 96 h of exposure (Fig.6d).

Fig.3 Effects of PYR on cell morphology and length-width ratio in T.thermophila after exposure for 96 h
3.7 Gene expression of HSPs, ABC transporters,GPx2, and TGR
The effects of PYR on gene expression of HSPs inT.thermophilaafter 96 h of exposure are shown in Fig.7a-d.TheHSP703mRNA level was markedly upregulated in the 1- and 5-mg/L groups and downregulated in the 10-mg/L group when compared to the control group (Fig.7a).Moreover, a significant upregulation ofHSP705transcription was also observed in the 0.1- and 5-mg/L treatments (Fig.7c).In contrast, a marked decrease was observed in the gene expression ofHSP704in all treatment groups (Fig.7b)and ofHSP90in the 5- and 10-mg/L groups (Fig.7d).
Compared with the control group, the gene expression ofTGRwas significantly decreased in the 0.1-, 5-, and 10-mg/L treatments (Fig.7e).In addition, significant upregulation ofGPx2mRNA level in the 1- and 5-mg/L groups (Fig.7f) andABCB15mRNA level in all treatment groups (Fig.7g)was observed.However, the transcriptional level ofABCC52was significantly decreased in all treatment groups (Fig.7h).
4 DISCUSSION
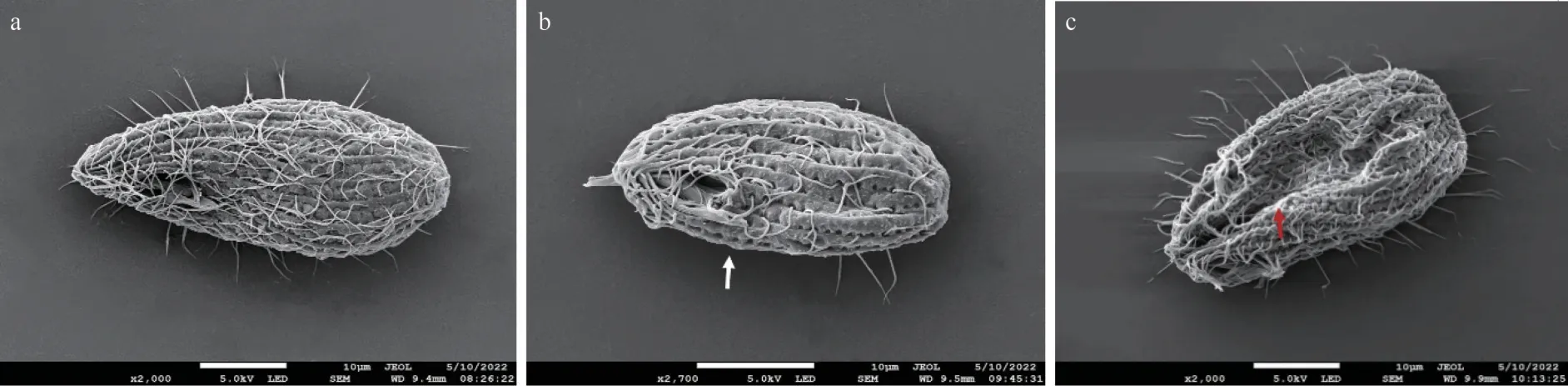
Fig.5 The SEM images of T.thermophila after 96 h of PYR exposure

Fig.6 Effects of PYR on the levels of SOD (a), CAT (b), GSH (c), and MDA (d) in T.thermophila
Previous studies have shown that PYR is highly toxic to aquatic organisms.For example, Liu et al.(2018) found that the 96-h EC50values of PYR onChlorellavulgarisandC.pyrenoidsawere 0.190 and 11.538 mg/L, respectively.The 96-h LC50was 0.022 mg/L againstHyalellaazteca(Morrison et al.,2013), 0.056 mg/L against zebrafish (Zhang et al.,2017), and 70.7 mg/L against juvenileLymnaea stagnalis(Fidder et al., 2016).The 72-h IC50of PYR againstPseudokirchneriellasubcapitatawas 1.4 mg/L(Ochoa-Acuña et al., 2009).The 24-h LC50againstDaphniasimiliswas 0.011 mg/L (Li, 2019).In the present study, the 96-h IC50of PYR toT.thermophilawas 17.2 mg/L, which indicated thatT.thermophilaexhibited more tolerance to PYR than most of the abovementioned organisms.Therefore,T.thermophilamay not be a good biomarker for monitoring the PYR pollution in aquatic environments.However, understanding the toxicity of PYR inT.thermophilais helpful to evaluate its health risk assessment for aquatic ecosystems.

Fig.7 Changes genes expression in T.thermophila after PYR exposure for 96 h
Behavioral tests are considered as a very useful tool in ecotoxicological research and have become an important indicator in toxicity evaluation for unicellular organisms (Tahedl and Häder, 2001;Carrasco-Pujante et al., 2021).Previous studies also showed that the motility behavior was changed whenTetrahymenawas under the stress of toxic substances(Maurya et al., 2019; Maurya and Pandey, 2020).In the present study,T.thermophilaspiraled rapidly in a straight-line direction and changed its direction through a sharp turn in the control group.However,in the 10-mg/L PYR-treated group, some animal shapes were changed and moved slowly or even stayed in place and rotated around themselves.The length-width ratio was used to further investigate the change in shape ofT.thermophilaunder PYR stress.In this study, the length-width ratio ofT.thermophiladecreased gradually with increasing of PYR concentration.Therefore, exposure to PYR can disturb the motility behavior and change the shape ofT.thermophila.
It was reported that PYR could inhibit the activity of mitochondrial complex III and disrupt the energy production in aquatic organisms, leading to mitochondrial dysfunction (Zhang et al., 2020).MMP is a widely used parameter to reflect the health of mitochondrial function (Díaz et al., 2016).Previous studies have observed that PYR can decrease the MMP in 3T3-L1 cells (Luz et al., 2018)and zebrafish gills (Huang et al., 2021).Consistent with these results, the present results also demonstrated that PYR caused remarked mitochondrial depolarization inT.thermophila, indicating that mitochondria may be a target of PYR.Moreover,mitochondria are closely related to the ROS production in cells, and mitochondrial perturbations such as inhibition of complex III can markedly induce ROS production (Fridovich, 2004).Therefore,oxidative stress has been reported to be involved in PYR-induced toxicity (Ott et al., 2007; Bleier et al.,2015; Wang et al., 2021).Lipid peroxidation is an important consequence of oxidative injury to cell membranes caused by ROS overproduction (Su et al.,2019).As a main end-product, MDA is widely used to assess ROS-induced damage.The increase in MDA content in the present study indicated that PYR caused membrane lipid peroxidation inT.thermophila,which was in consistent with those results reported in zebrafish embryos and livers and tilapia gills(Zhang et al., 2017; Li et al., 2021b).Huang et al.(2016) found that cell membrane damage caused by the triazole fungicides myclobutanil and cyproconazole could be observed in SEM images.The SEM results in the present study also confirmed that PYR exposure could lead to cell membrane injury, which may be due to the lipid peroxidation triggered by PYR (Huang et al., 2016).
The antioxidant enzyme system is a major part of the body defense system to maintain the balance between the production of ROS and elimination(Guo et al., 2021; Liu et al., 2022).The SOD-CAT system is the most important defense mechanism against oxidative stress.SOD converts superoxide free radicals (O·-2) into H2O2, while CAT further converts H2O2into O2and H2O for detoxification(Blokhina et al., 2003).In this study, SOD and CAT activities inT.thermophilawere generally induced after exposure to 1-10-mg/L PYR, which is the adaptive response of the antioxidant defense system to neutralize ROS during detoxification.GPx is also a crucial enzyme in eliminating H2O2and transforming peroxides to nontoxic alcohols and O2by catalyzing GSH into GSSH (Pan et al., 2022; Veedu et al.,2022).In our experiment, the transcription level ofGPx2was increased in the PYR-treated groups (1 and 5 mg/L) compared to the control, suggesting that GPx activity may also play a protective role against oxidative damage.However, no significant change was observed in the level ofGPxmRNA between the 10-mg/L PYR and control groups,indicating that the GPx enzyme may not be involved in the protection offered by the antioxidant system against PYR toxicity, which may cause the accumulation of ROS and aggravate PYR injury inT.thermophila.GSH is the second line of defense to eliminate ROS and reduce the stress caused by excessive ROS (Guha et al., 2011).As an antioxidant,GSH can prevent damage to important cellular components caused by free radicals and peroxides(Couto et al., 2013; Huang et al., 2016; Zhang et al.,2021).In this study, significant increase in GSH contents after 24 and 96 h of exposure to PYR was observed.The higher GSH level may be due to the upregulation of GSH synthesis enzymes (Choi et al.,1997), which can be affected by oxidative stress(Shi et al., 1994).Moreover, higher GSH levels can help cells against oxidative stress (Yu et al., 2009).However, GSH was reduced in a concentrationdependent manner inT.thermophilaafter 96 h of PYR exposure.This is due to the huge consumption of GSH byT.thermophilain response to PYR stress, which can lead to cells being more sensitive to ROS attack, as confirmed by the significant increase in the MDA content in PYR-treatedT.thermophila.A similar phenomenon was also demonstrated in zebrafish embryos and the hepatopancreas of common carp (Li et al., 2018;Zhao et al., 2022).Moreover, TGR can catalyze the reduction of both thioredoxin and glutathione disulfides (GSSG), thereby increasing the antioxidant capacity (Williams et al., 2013).The present study found that the transcriptional level ofTGRwas significantly decreased in the 0.1-, 5-, and 10-mg/L PYR treatment groups and showed no remarkably change in the 1-mg/L group after 96 h of exposure,which may weaken the conversion of GSSG to GSH.
Previous studies have indicated that HSPs can be rapidly synthesized in response to oxidative stress and are regarded as a major biomarker for assessing environmental stress (Kalmar and Greensmith, 2009).HSPs work as molecular chaperones to protect cells from a variety of stresses by degrading of unstable proteins, refolding misfolded proteins, and preventing protein aggregation (Ikwegbue et al., 2017).It was reported that strobilurin fungicides can induce the expression of HSPs.Zhu et al.(2015) reported that trifloxystrobin exposure induces significant stimulation ofHSP70mRNA expression in rare minnow larvae at 72 and 144 postfertilization (hpf).An increase inHSP70expression was also found in grass carp(Ctenopharyngodonidella) juveniles after 48 h of exposure to trifloxystrobin, azoxystrobin, and kresoximmethyl (Liu et al., 2013).In the present study, PYR exposure generally increased the mRNA level ofHSP703in the 1- and 5-mg/L groups andHSP705in the 0.1-5-mg/L groups, which may be due to the oxidative effect of PYR and the upregulation ofHSP703andHSP705mRNA levels as a cellular defense.However, high concentration PYR (10 mg/L)inhibited the gene expression ofHSP703andHSP705.This may be due to the destruction of the heat shock response in injured cells (Zhao et al.,2019).Moreover, the transcription levels ofHSP704andHSP90were downregulated after PYR exposure,suggesting that different HSPs have different responses whenT.thermophilaencounters to PYR stress and that HSP703 and HSP705 may have a stronger capacity than HSP704 and HSP90 to protectT.thermophilaagainst oxidative damage.
Increasing evidence has demonstrated that aquatic organisms develop some specific biological mechanisms to prevent the binding of xenobiotics to target molecules in vivo, or eliminate the cytotoxic effects of xenobiotics after binding to target molecules or target organs, protecting the organisms from xenobiotic-induced injury (Bard, 2000).The multixenobiotic resistance (MXR) mechanism is a universal cellular defense mechanism for aquatic organisms to resist endogenous and exogenous toxicants,which is mediated by the ABC transporter superfamily,including P-glycoprotein (P-gp) and multidrug resistance-associated proteins (MRPs) (Bard, 2000;Dean et al., 2001; Gao et al., 2015; Wu et al., 2021).The ABCB15 ofT.thermophilashares a 32.3%amino acid with human P-gp and ABCC52 is also an MRP-like gene (Xiong et al., 2010).However,the effect of PYR on ABC transporters in aquatic organisms has not been reported.A previous study has shown that ABCB15 can increase the tolerance ofT.thermophilato dichlorodiphenyltrichloroethane(DDT) stress by efficiently transporting the toxicant to the extracellular space (Ning et al., 2015).Our results showed that PYR significantly upregulated the transcriptional level ofABCB15in comparison with the control, indicating thatABCB15may play an important role in exporting PYR inT.thermophila.Moreover, theABCB15mRNA level in the 5- and 10-mg/L groups was lower than that in the 1-mg/L group, which may lead to a decreased export ability of PYR fromT.thermophilaand increase the accumulation of PYR inT.thermophila.However, theABCC52expression level was generally downregulated in the PYR-treated groups,indicating thatABCC52may not be involved in the pumping PYR out ofT.thermophilacells.
5 CONCLUSION
The present study shows that PYR exposure could inhibit the growth, damage the outer membrane, and cause changes in behavior and shape ofT.thermophila, which might be due to oxidative stress, as PYR increased the SOD and CAT activities significantly, decreased the GSH contents,and increased the MDA.PYR disrupted the expression of theHSP703,HSP704,HSP705, andHSP90genes, indicating that PYR could destroy the protein homeostasis.The changes inGPx2andTGRmRNA also confirmed the occurrence of oxidative stress.Moreover, the increase inABCB15suggested thatABCB15might be involved in the detoxification of PYR inT.thermophila.For the case in which PYR is used in agriculture and pollutes the aquatic environments, the data provided in the present study may help to assess the potential ecological risks of PYR in aquatic systems.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We are grateful to Prof.Wei MIAO (Institute of Hydrobiology, the Chinese Academy of Sciences,Wuhan) and Dr.Jing ZHANG (Institute of Hydrobiology, the Chinese Academy of Sciences,Wuhan) for their help and the valuable comments and suggestions that greatly improved the manuscript.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Contrasts of bimodal tropical instability waves (TIWs)-induced wind stress perturbations in the Pacific Ocean among observations, ocean models, and coupled climate models*
- Variability of the Pacific subtropical cells under global warming in CMIP6 models*
- Identification of thermal front dynamics in the northern Malacca Strait using ROMS 3D-model*
- Magmatic-tectonic response of the South China Craton to the Paleo-Pacific subduction during the Triassic: a new viewpoint based on Well NK-1*
- An improved positioning model of deep-seafloor datum point at large incidence angle*
- Microplastics in sediment of the Three Gorges Reservoir:abundance and characteristics under different environmental conditions*
