海底热液硫化物中金属、非金属和稀有气体同位素组成的关系及其地质意义
2024-02-27曾志刚


摘要:海底熱液硫化物的同位素组成不仅可以示踪其来源,也记录了流体及其沉淀过程。本文分析了全球海底热液硫化物的金属(铅、铼、锇、铁、铜、锌)、非金属(硫)及其流体包裹体中的稀有气体同位素组成,探讨了硫化物中金属、非金属和稀有气体同位素组成之间的关系。结果表明:海底热液硫化物中的硫同位素组成与锇、铁同位素组成之间,铁同位素组成与铅和氦同位素组成之间,存在负相关性;其锇同位素组成与铁同位素组成之间,氙同位素组成与铅、锇同位素组成之间,则存在正相关性。在岩浆去气注入流体阶段形成的硫化物,具δ34SVCDT值较低(约0‰),3He/4He(>8 Ra)、40Ar/36Ar(>300)和129Xe/132Xe(>0.99)值较高的特点。在流体-岩石相互作用阶段,随着岩石中含铅矿物的不断溶解,即流体-岩石相互作用程度的增加,流体中沉淀的黄铁矿、黄铜矿和闪锌矿的铅质量分数增加,伴随206Pb/204Pb值轻微的减小。在流体-海水混合阶段,海水影响的加剧可使硫化物中的锇质量分数(约0×10-9)急剧降低,δ57Fe值(<—1.6‰)、187Os/188Os值(>1)明显增大;随着流体-海水混合作用的增强,硫化物中黄铁矿的δ34SVCDT值将随着其流体包裹体中3He/4He、40Ar/36Ar、129Xe/132Xe值轻微降低而升高,而其3He/4He值随着其130Xe/132Xe值的降低而降低。以上表明,通过综合分析海底硫化物中金属、非金属和稀有气体的同位素组成和其质量分数,并讨论它们之间的关系,可以揭示岩浆去气、流体-岩石相互作用和流体-海水混合对海底热液循环的影响,进而了解硫化物沉淀过程中流体-岩石相互作用和流体-海水混合的程度。
关键词:金属、非金属和稀有气体同位素;同位素组成之间的关系;海底热液硫化物
doi:10.13278/j.cnki.jjuese.20230310 中图分类号:P736.4;P597 文献标志码:A
收稿日期:2023-10-20
作者简介:曾志刚(1968—),男,研究员,博士生导师,主要从事海底热液地质学方面的研究,E-mail:zgzeng@qdio.ac.cn
基金项目:国家自然科学基金项目(42330409,42221005,91958213);中国科学院战略性先导科技专项子课题(XDB42020402);国家重点基础研究发展计划(973计划)项目(2013CB429700);泰山学者工程(ts201511061)
Supported by the National Natural Science Foundation of China (42330409, 42221005, 91958213), the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB42020402), the National Basic Research and Development Program of China (2013CB429700) and the Taishan Scholars Program (ts201511061)
The Relationship Between Isotopic Compositions of Metals, Non-Metal, and Rare Gases in Seafloor Hydrothermal Sulfides and Its Geological Significances
Zeng Zhigang1, 2, 3
1. Institute of Oceanology/Key Laboratory of Marine Geology and Environment, Chinese Academy of Sciences, Qingdao 266071, Shandong,China 2. Laboratory for Marine Mineral Resources, Laoshan Laboratory, Qingdao 266061, Shandong,China 3. College of Marine Sciences, University of Chinese Academy of Sciences, Qingdao 266400, Shandong,China
Abstract: The isotopic composition of seafloor hydrothermal sulfides can not only trace their sources but also record the fluids and their precipitation processes. This article analyzes the isotopic compositions of metals (lead, rhenium, osmium, iron, copper, zinc), non-metal (sulfur), and rare gases in fluid inclusions of global seafloor hydrothermal sulfides, and explores the relationship between the isotopic compositions of metals, non-metal, and rare gases in sulfides. The results indicate that there is a negative correlation between sulfur isotopic composition and osmium, iron isotopic compositions, as well as between iron, lead, and helium isotopic compositions in seafloor hydrothermal sulfides. There is a positive correlation between osmium isotopic composition and iron isotopic composition, and between xenon isotopic composition and lead, osmium isotopic compositions. During the stage of magma degassing and material injecting fluid, sulfides are formed with the characteristics of low δ34SVCDTvalues (about 0‰) and high3He/4He (>8 Ra),40Ar/36Ar (>300), and129Xe/132Xe (>0.99) ratios. In the stage of fluid-rock interaction, as lead-containing minerals in the rock continue to dissolve, i.e., the degree of fluid-rock interaction increases, the lead content of pyrite, chalcopyrite, and sphalerite precipitated in the fluid increases, accompanied by a slight decrease in the206Pb/204Pb ratios. In the fluid-seawater mixing stage, with the increase of seawater influence degree, the Os content (about 0×10-9) in sulfides can sharply decreased, and the δ57Fe value (<-1.6‰), the187Os/188Os ratio (>1) significantly increases; With the enhancement of fluid-seawater mixing degree, the δ34SVCDTvalues of pyrite in sulfides will increase with a slight decrease in the3He/4He,40Ar/36Ar, and129Xe/132Xe ratios in its fluid inclusions, while their3He/4He ratios will decrease with a decrease in its130Xe/132Xe ratios. The above indicates that by comprehensively analyzing the isotopic composition and content of metals, non-metal, and rare gases, and discussing their relationships, the effects of magma degassing, fluid-rock interaction, and fluid-seawater mixing on seafloor hydrothermal circulation can be revealed, and the degree of fluid-rock interaction and fluid-seawater mixing during sulfide precipitation can be understood.
Key words: metal, non-metal, and rare gas isotopes;the relationship between isotopic compositions;seafloor hydrothermal sulfide
0 引言
我们曾对海底热液产物中的硫化物[1-7]、硫酸盐[8-9]、流体[10]、热液柱[11-12]、含金属沉积物[13]、蚀变产物[14-18]以及喷口生物[19]进行了研究,揭示了海底热液硫化物中的金属(铅(Pb)、铼(Re)、锇(Os)、铁(Fe)、铜(Cu)、锌(Zn))[3, 6, 8]、非金属(硫(S))[8]和稀有气体(氦(He)、氖(Ne)、氩(Ar)、氪(Kr)、氙(Xe))[1, 5]同位素组成特征及其来源,明确了海底热液区及其邻域的岩浆[20-24]、沉积、构造及其板块俯冲背景,促进了对海底热液系统的构造演化、成矿作用、流体-岩石相互作用、流体-海水混合、沉积过程以及生物的响应、记录、作用和适应的深入认识[25]。
海底热液硫化物的S和Pb同位素组成是探索海底热液过程、流体-岩石相互作用和岩浆活动的有效示踪剂[26-31],可用于揭示热液产物的起源[29-34],特别是硫化物中S和Pb的来源[2, 8, 35-41]。硫化物中的S具有海水、火成岩、岩浆、沉积物中细菌成因硫化物以及地幔与海水来源S的混合等多来源的特征[42-46],且在有沉积物覆盖的洋中脊,负的δ34S值通常与低S质量分数的硫化物一起出现,其可能是细菌还原硫酸盐的结果[47-48]。与海底热液硫化物中S的来源类似,其Pb也具有多来源的特征:1)热液区的火成岩围岩[2, 8, 26, 49];2)弧后盆地(BAB)[34, 38]和洋中脊(MOR)[40]的沉积物;3)俯冲板块来源的Pb[26, 41]。在沉积物缺乏的洋中脊、弧后盆地和岛弧上,热液硫化物中的Pb是在热液循环过程中从火成岩中迁移出来的[2, 8, 26, 28, 41, 49-50];在有沉积物覆盖的洋中脊和弧后盆地上,其海底热液硫化物中的Pb则来源于火山物质(包括洋中脊玄武岩(MORB))中Pb和沉积物中Pb的混合[26, 34, 38]。不仅如此,全球海底热液硫化物中的Pb同位素组成比其围岩-火山岩的更均匀,表明火山岩中Pb同位素组成在热液循环过程中已被均一化,源自海水和沉积物中Pb的贡献非常低[2, 8, 26, 30, 49-54]。
与海底热液硫化物中的S和Pb一致,硫化物的Re和Os同位素组成也提供了其来源和成矿条件的信息[55-58]。目前,已结合硫化物中主量元素的含量,分析了海水Os的贡献[56, 59]以及地壳来源Os与海水来源Os的混合[56, 58],揭示了Re-Os的富集条件,并评价了从热液流体到海底热液硫化物沉淀的Os通量[3]。
不仅如此,Fe、Cu和Zn同位素组成也已被广泛用于示踪流体的路径以及海底热液系统、火山岩和沉积物的同位素分馏及其物质来源[6, 60-65]。包括:通过分析海底热液系统中硫化物的Fe、Cu和Zn同位素组成特征[6, 60-61, 63, 66-70]及其來源,揭示了流体-岩石相互作用以及流体和海水之间的混合对硫化物中Fe、Cu和Zn同位素组成的影响[71-79],明确了低温热液蚀变导致的蚀变洋壳中Cu和Zn同位素分馏有限,而洋中脊岩石在经历高温热液蚀变过程中却发生了显著的Cu和Zn同位素分馏[71-73]。
与研究海底热液硫化物中的金属和非金属同位素组成同等重要,开展海底热液硫化物中稀有气体的研究是揭示海底热液系统演化的关键[1, 79-81]。此类研究可用于分析流体的时间变异性[80-86],了解喷口流体与洋中脊玄武岩的稀有气体同位素组成特征[84-89],并可将对氦/热比值的了解扩展到对地质记录的揭示[1, 88-94]。迄今为止,已研究了海水和岩浆稀有气体对硫化物的贡献,探索了流体-岩石相互作用和流体-海水混合对硫化物中稀有气体同位素组成的影响,并评估了热液流体向海底热液硫化物堆积体的氦和热通量[5]。
上述可见,我们已对全球海底热液硫化物中金属、非金属和稀有气体同位素组成的特征及其来源有了初步认识,示踪了海底热液活动及其成矿的条件和过程,明确了岩浆去气、流体-岩石和/或沉积物相互作用、流体-海水混合以及生物活动对海底热液系统及其多金属硫化物的影响及制约。尽管如此,目前国内外依然局限于将海底热液硫化物中的金属、非金属和稀有气体同位素分开来研究,有关将海底热液硫化物中的多个同位素组成结合起来讨论分析依然鲜见。因此,基于海底热液活动及其热液硫化物形成过程中金属、非金属和稀有气体同位素并非独立、分别行动、互不相关的事实,本文将在已有对海底热液硫化物中金属、非金属和稀有气体同位素研究的基础上,进一步将硫化物中金属(Pb、Re、Os、Fe、Cu、Zn)、非金属(S)和稀有气体(He、Ne、Ar、Kr、Xe)同位素组成的分析结果结合起来,探讨海底热液硫化物形成过程中金属、非金属和稀有气体同位素之间的内在关系,综合揭示岩浆去气、流体-岩石相互作用和流体-海水混合期间金属、非金属和稀有气体同位素的行为。
1 地质概况
全球的海底热液硫化物主要分布在洋中脊、弧后盆地、岛弧(IA)和热点(hot spot)地质环境的热液区中。海底热液区的围岩既可以是火成岩,也可以是沉积物(例如,东太平洋Juan de Fuca洋脊上的Middle Valley热液区),且均受上覆海水的影响。其中,来自东太平洋海隆(EPR)13°N、11°N和1°S—2°S附近热液区,中印度洋脊(CIR)Edmond热液区,大西洋洋中脊(MAR)13°S附近热液区和西南印度洋脊(SWIR)A区热液区的硫化物,其围岩主要为洋中脊玄武岩 [2-5, 95]。同时,中印度洋脊的Kairei热液区虽位于玄武岩上,但其热液循环过程中的流体却经历了与附近超镁铁质岩石的相互作用[96]。在大西洋洋中脊Logatchev热液区中,热液硫化物堆积体与超镁铁质岩石和镁铁质岩石有关,包括蛇纹石化方辉橄榄岩、蛇纹石化纯橄榄岩、辉长岩和含橄榄石玄武岩[3-5, 97]。在北斐济海盆(NFB)的Sonne 99热液区,基底岩石主要为玄武岩,且其形成受到了两种不同来源岩浆的影响[31, 98-100]。此外,硫化物堆积体中既有通过烟囱体流出的高温(>300℃)流体[101],也有通过丘状体流出的低温(<200℃的)流体[97, 100, 102-104]。
2 样品与数据来源
1998年,德国科学家在使用“太阳”号科考船实施“HYFIFLUX II”项目的SO 134航次过程中,用电视抓斗从北斐济海盆的Sonne 99热液区采集了海底热液硫化物样品。随后,2005年、2007年、2008年、2009年和2010年,中国科学家使用“大洋一号”科考船执行DY105-17、DY115-19、DY115-20和DY115-21航次期间,用电视抓斗分别在快速扩张东太平洋海隆13°N、1°S—2°S附近热液区,中速扩张中印度洋脊的Kairei和Edmond热液区,慢速扩张大西洋洋中脊Logatchev热液区,超慢速扩张西南印度洋脊A区中采集了硫化物样品[3-5, 8](表1),其主要由黄铁矿±白铁矿、黄铜矿、闪锌矿、硬石膏、重晶石、蛋白石,以及少量方铅矿和无定形二氧化硅组成[3-6, 8],且所研究的洋中脊和弧后盆地硫化物样品分别由高温(>300℃)、中温(300~200℃)和低温(<200℃的)流体形成[101, 102-107]。
本文所分析的海底热液硫化物样品中的金属(铅、铼、锇、铁、铜、锌)[3, 6, 8]、非金属(硫)[8]和稀有气体[5]同位素组成及其含量数据分别来自作者已发表的论文。
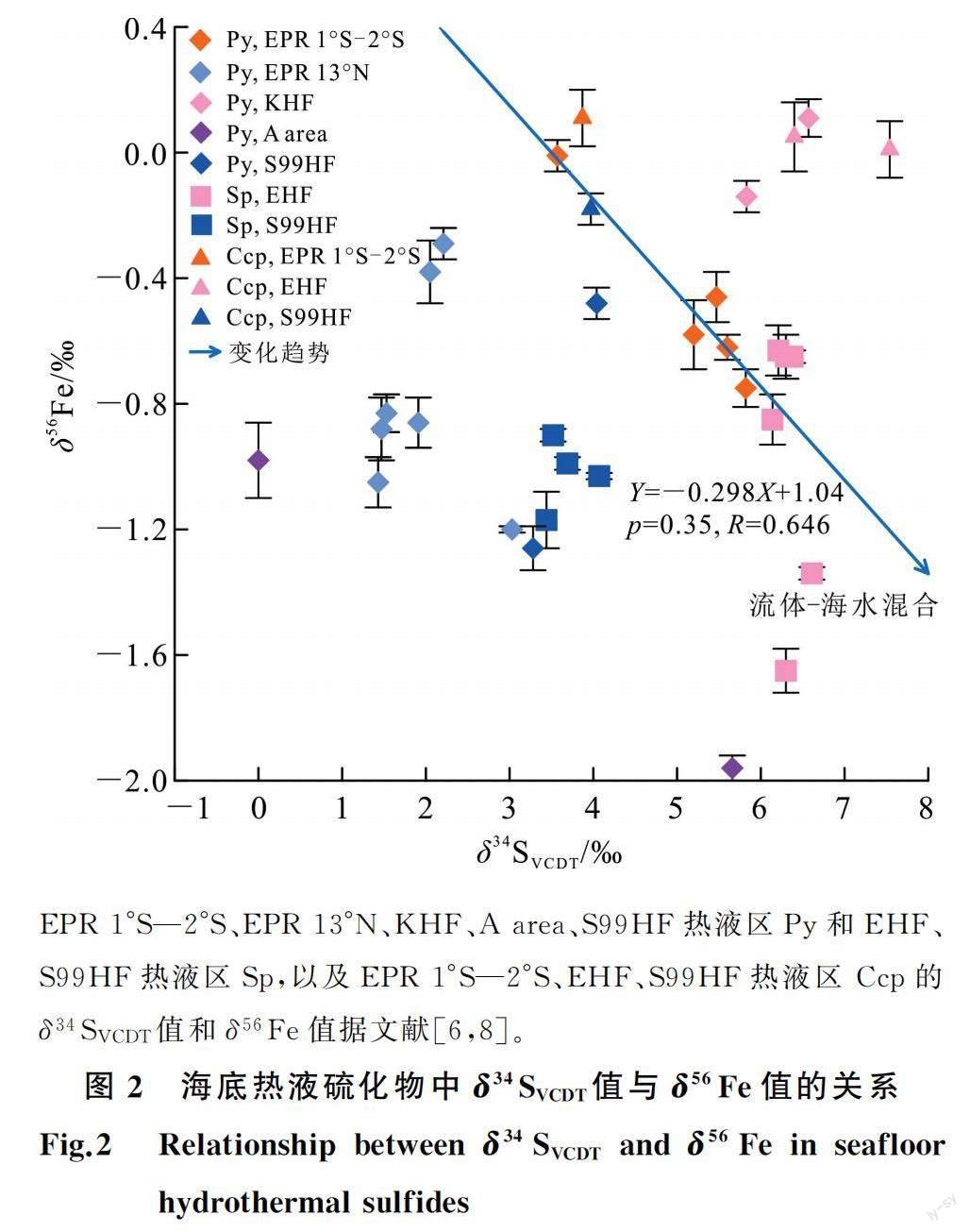
3 硫化物中金属、非金属与稀有气体同位素之间的关系海底热液硫化物的δ34SVCDT值与3He/4He值(图1a)、40Ar/36Ar值(图1b)、129Xe/132Xe值(图1c)、187Os/188Os值(图1d)、δ56Fe值(图2)之间,海底热液硫化物的Pb质量分数与206Pb/204Pb值(图3a)、Os质量分数与187Os/188Os值(图3b)之间,海底热液硫化物的δ56Fe值与207Pb/204Pb值(图3c)、δ57Fe值与3He/4He值(图3d)之间,均存在负相关关系。
海底熱液硫化物的187Os/188Os值与δ57Fe值(图4)之间,20Ne与132Xe(图5a)之间,3He/4He值与130Xe/132Xe值(图5b)之间,136Xe/132Xe值与187Os/188Os值(图5c)、207Pb/204Pb值(图5d)之间,则存在正相关关系。
4 讨论
4.1 岩浆去气
本文分析的海底热液硫化物的δ34S值,其范围为0.0 ‰~9.6‰,平均值为4.7‰(样品数量:60),与全球海底热液硫化物样品的δ34S值范围一致(1‰~ 9‰;样品数量:1 841)[8]。全球海底热液硫化物、硫酸盐和蛋白石中流体包裹体的He变化范围较大(4He值为 0.017 4×10-8~22.1×10-8cm3STP/g)[5],硫化物中流体包裹体的3He/4He值范围为0.29~13.30 Ra [1, 79-86],大多数硫化物样品中流体包裹体的4He值显著高于硫酸盐矿物(硬石膏和重晶石)和蛋白石样品[5],其大多数3He/4He值与大气值相比明显少放射性成因的He,而与喷口流体的3He/4He值(5.3~8.3 Ra)[87-92]和洋中脊玄武岩(6 ~11 Ra)[93]几乎一致,这表明海底热液区硫化物中流体包裹体的氦主要来源于热液系统下方的岩浆源[83-85],且硫化物的流体包裹体可靠地记录了原始热液流体的氦同位素组成特征[85]。此外,海底热液硫化物中流体包裹体的Ne、Ar、Kr与Xe的质量分数也是可变的,并且与He一样,可能在一定程度上受到流体包裹体密度或破碎效率变化的影响[5]。硫化物、硫酸盐和蛋白石中流体包裹体的20Ne、40Ar、84Kr和132Xe值分别为(0.029~5.6)×10-8、(28~3 500)×10-8、(26~2 900)×10-12和(1.7~180)×10-12cm3STP/g[5],且硫化物、硫酸盐和蛋白石中流体包裹体的Ne、Ar和Xe同位素比值范围较窄(20Ne/22Ne值为 9.7~10.2;38Ar/36Ar值为 0.187 7~0.191 2;129Xe/132Xe值为 0.979~0.993)[5],与矿物的类型无关[5]。同时,硫化物中流体包裹体的Ne、Ar、Kr和Xe元素质量分数及其比值与大气值无法区分或接近大气比值,表明其来源于周围的海水[1, 79, 83, 85-86],且硫化物中流体包裹体存在的40Ar过剩(可高达2.6%,即40Ar/36Ar值高达303)特征,则可能是地幔物质进入热液系统的结果[94]。
在岩浆去气注入流体的阶段形成的硫化物,其δ34SVCDT值较低(约0‰),3He/4He(>8 Ra)、40Ar/36Ar(>300)和129Xe/132Xe(>0.99)值较高(图1a,b,c),且岩浆去气和/或细菌活动可导致硫化物的δ34S值低于0‰(例如,Lau海盆的Hine-Hina热液区(全扩张速率为60 mm/a)[42]和Guaymas海盆热液系统(全扩张速率为45 mm/a)[50]中的海底热液硫化物)。不仅如此,全球海底热液硫化物具高He含量的特征[1, 5, 79, 85-86],也是由于循环热液从岩浆中提取地幔挥发物所致[108],且在单个海底热液系统中,He含量在短时间尺度上随岩浆构造过程[109]或热液系统流体通道的变化而变化[110]。而且,全球海底热液硫化物的3He/4He值范围很广(0.29~13.3 Ra)[5]。其中,来自北斐济海盆的海底热液硫化物,其流体包裹体中3He/4He值>7 Ra(113.1GTV电视抓斗样品的3He/4He值为10.4±1.0 Ra)[5],该值与目前从北斐济海盆热液区中喷出的热液流体的3He/4He值几乎无法区分(9.04~10.0 Ra)[105],表明北斐济海盆中的硫化物是在高温(>200℃)流体条件下形成的,仅被周围的海水轻微稀释[105]。而且高溫流体条件下形成的硫化物,其流体包裹体中缺少放射成因的He,则表明热液循环受到了活动岩浆的影响,而不是仅受<1 Ma洋壳的影响[111-114]。与大气3He/22Ne值(4.36×10-6)和3He/36Ar值(2.33×10-7)[115]相比,海底热液硫化物的高3He/22Ne值((4.1~260)×10-5)和3He/36Ar值((1.5~90)×10-6)[5]也表明He来源于岩浆挥发物的直接去气[1, 81, 109]。尽管如此,在Juan de Fuca洋脊的Middle Valley热液区,硫化物中流体包裹体的3He/4He值(5.8~7.1 Ra)低于典型洋中脊喷口流体的3He/4He值(约8 Ra)[88-89],更接近有沉积物覆盖的Guaymas海盆的喷口流体(7 Ra),表明放射成因He的贡献来源于沉积物中的孔隙流体或北Juan de Fuca洋脊下方的岩浆系统[80]。同时,岩浆活动的流体路径变化也可能导致热液流体化学性质及其Pb同位素组成的变化[116-119]。在东马努斯海盆中热液流体的Cu和Zn同位素组成表明,重Cu(δ65Cu= 0.3‰±0.2‰)和Zn(δ66Zn=-0.04‰~0.94‰)同位素的系统富集,可被解释为热液系统中亚浅层沉淀/再溶解过程的结果,而不是富含金属的岩浆流体在一定深度蒸发/冷凝的结果[79]。
4.2 流体-岩石相互作用
在超快速和快速扩张的洋中脊中,海底热液硫化物中的大多数玄武岩来源S与海水来源S之比在70%~100%之间,在超慢速、慢速和中速扩张洋中脊中,硫化物中玄武岩来源S与海水来源S之比的变化范围为40%~100%,表明海底热液硫化物的S同位素组成受流体-玄武岩相互作用和流体-海水混合程度的双重控制[8],且较大(>50%)的玄武岩来源S与海水来源S之比则表明海底热液硫化物中的大部分S来自玄武岩[8]。
全球海底热液硫化物的Pb同位素比值范围分别为17.541±0.004~19.268±0.001(206Pb/204Pb)、15.451±0.001~15.684±0.001(207Pb/204Pb)和37.557±0.008~38.988±0.002(208Pb/204Pb)(样品数量:21)[8]。在流体-岩石相互作用阶段,随着岩石中含Pb矿物的不断溶解,即流体-岩石相互作用程度的增加,致使流体中沉淀的黄铁矿、黄铜矿和闪锌矿,其Pb质量分数的增加伴随着206Pb/204Pb值轻微的减小(图3a)。全球海底热液硫化物中Pb同位素组成非常均匀,范围很窄,分别落在围岩-玄武岩的Pb同位素组成范围内,表明硫化物中的Pb主要来源于玄武岩[8]。此外,海底热液系统中硫化物的S和Pb同位素组成变化,不仅受S和Pb源的控制,也受到流体过程,包括流体-岩石相互作用、流体-海水混合和海底下热液滞留时间等过程的综合影响[8]。
全球海底热液硫化物的Os和Re质量分数变化范围分别为(1.70~79.90)×10-12,和(0.10~73.60)×10-9[3],而其187Os/188Os值在0.645~1.209之间变化[3],其187Os/188Os值与矿物类型(如黄铁矿、黄铜矿、闪锌矿)之间没有关系[3],且海底热液硫化物的187Os/188Os值明显比MORB更具放射性,大多数硫化物的187Os/188Os值与现代海水的值(约1.06)一致,或略低于现代海水的放射性成因值[3, 108]。同时,海底热液硫化物的187Re/188Os值变化范围很大(64~100 334)[3],且硫化物中黄铁矿和Fe-Cu硫化物矿物的187Re/188Os值通常高于闪锌矿或富含Zn的硫化物矿物[3]。
Os和Re在玄武岩-流体相互作用过程中属易迁移的元素[56]。因此,在流体-岩石相互作用过程中,可导致Os和Re在流体中富集,并致使其沉淀的海底热液硫化物具较高的Os和Re质量分数[3]。随着流体-玄武岩相互作用程度的增加,海水硫酸盐还原产生的H2S增加,流体与岩石的187Os/188Os值趋与一致,致使从流体中沉淀的海底热液硫化物δ34SVCDT值逐渐增加,187Os/188Os值逐渐降低(图1d)。例如,在大西洋洋中脊Logatchev热液区,其硫化物的187Os/188Os值(0.645±0.066,MAR05-TVG1-10-2和0.730±0.066,MAR05-TVG1-21)与周围海水相比,其放射性成因的Os较少,这表明经过流体-岩石相互作用,从流体中沉淀的硫化物受到了源自海水放射性成因Os和源自MORB和/或超镁铁质岩石蚀变释放的非放射性成因Os共同的影响[3, 56, 58]。
海底热液硫化物的δ56Fe和δ57Fe值分别在-1.96‰~0.11‰和-2.89‰~0.19‰之间,且海底热液硫化物的δ56Fe、δ57Fe值与矿物类型(黄铁矿、黄铜矿和闪锌矿)之间也没有明显关系[6],其大多数显著低于其围岩-玄武岩的δ56Fe值(0.06‰~0.18‰)[6, 120],与热液流体的δ56Fe值相似(-1.85‰~-0.14‰)[66-67, 121]。这不仅表明热液流体为海底热液硫化物提供了Fe,也为深海提供了轻Fe同位素[66-68, 121]。而且其围岩-玄武岩中的54Fe更有可能在流体-玄武岩相互作用过程中进入流体中,即围岩-玄武岩和流体之间的相互作用可导致轻Fe同位素优先从热液蚀变玄武岩中浸出,而较重的Fe同位素则留在蚀变的洋壳中[61, 122-123],这意味着含有蚀变岩(具有较重的Fe同位素组成)的板块俯冲成分可对弧后盆地和岛弧岩浆源产生影响,从而导致较重的Fe同位素在弧后盆地或岛弧火山岩中优先富集[6]。不仅如此,海底热液硫化物中黄铜矿的δ56Fe值范围有限,在-0.18‰~0.11‰之间,表明流体-岩石相互作用过程中,黄铜矿中的Fe来源于热液与玄武岩之间的相互作用,且不仅在围岩-玄武岩(0.06‰ ~0.18‰)和喷口流体(-1.85‰~-0.14‰)相互作用期间存在轻微的Fe同位素分馏(达0.3‰)[6],还有在黄铜矿沉淀过程中也发生了轻微的Fe同位素分馏[67],进而在高温流体条件下56Fe和57Fe更有可能进入黄铜矿中[124],致使高温黄铜矿具δ56Fe和δ57Fe值較高的特征[6]。此外,海底热硫化物中黄铜矿的δ56Fe值(0.11‰±0.09‰)与围岩-玄武岩的δ56Fe值(0.06‰~0.18‰)接近,表明Fe主要从围岩-玄武岩中浸出,在高温流体条件下进入黄铜矿中,这意味着在高温玄武岩-流体相互作用过程中没有发生显著的Fe同位素分馏[6]。
另一方面,海底热液硫化物中闪锌矿的δ66Zn值和δ68Zn值范围分别为-0.39‰~-0.03‰和-0.77‰~-0.03%[6],显著低于围岩-洋中脊玄武岩(δ66Zn值为0.25‰~ 0.51‰)[6]和热液流体(δ66Zn值为0.00‰~1.33‰)[64],但大多在海水范围内(δ66Zn值为-0.33‰~0.96‰)[6, 72, 125]。同时,已知平衡同位素分馏是温度的函数,在较低的温度下会发生明显的同位素分馏[126],且实验研究表明,在30~50℃的温度范围内,Zn同位素的变化有限[127]。中印度洋脊Edmond热液区和北斐济海盆中的流体温度分别为273~382℃[100]和285~291℃[105-106, 128],表明在中等和/或低温流体条件下的流体-玄武岩相互作用过程中,Zn在闪锌矿中的沉淀将66Zn和68Zn带出了围岩-玄武岩 [6],导致在玄武岩-流体相互作用过程中热液蚀变玄武岩优先富集较轻的Zn同位素,这意味着含有蚀变岩的板块俯冲成分对岩浆源的影响将导致弧后盆地和岛弧火山岩优先富集较轻的Zn同位素。
在中印度洋脊的Edmond热液区,很多海底热液硫化物的3He/4He值(1 Ra<3He/4He<7 Ra)与洋中脊玄武岩和大气的相比,具有较宽的变化范围[5],且He与较重惰性气体的含量比值与空气或空气饱和海水的值相似[5],表明在流体-岩石相互作用过程中,海底热液硫化物受到了海水中放射性成因He和热液系统下地幔释放的非放射性成因He的双重影响,其流体包裹体中He是海水和地幔He的混合物[5]。随着流体-玄武岩相互作用程度的增加,流体的δ57Fe值减小,流体中3He/4He值增加,致使从流体中沉淀的海底热液硫化物3He/4He值逐渐增加,δ57Fe值逐渐降低(图3d),且在流体-海水混合的影响下,可使流体的3He/4He值趋于大气值(1)(图3d)。此外,随着流体-玄武岩相互作用程度的增加,不排除流体-海水的混合,流体的δ56Fe值减小,重稀有气体同位素值增加,流体中海水硫酸盐还原产生的H2S增加,海水来源的Pb同位素值轻微增加,致使从流体中沉淀的海底热液硫化物δ34S值(图2,图1c)、207Pb/204Pb(图3c)值逐渐增加和129Xe/132Xe值(图1c)、δ56Fe值逐渐降低(图2,图3c)。
4.3 流体-海水混合
海底热液硫化物经历了多阶段的热液活动过程[8]。在烟囱体壁或丘状体内缓慢的流体-海水混合过程中,海水中硫酸盐的还原,会产生富含34S的硫化物[45],致使硫化物的S同位素组成偏重[8],且流体在海底热液系统中的停留时间越长,流体-海水混合过程中将导致海水中硫酸盐被还原的程度更大[129]。因此,海底热液硫化物样品中δ34SVCDT值的变化(0.0‰~9.6‰)可归因于海底热液系统中海水硫酸盐还原产生的H2S的贡献增加[8]。此外,随着流体-海水混合程度的增加,沉淀硫化物的δ34SVCDT值逐渐增加(接近21‰)[8],且玄武岩S与海水S之比较小(<50%),则表明海底热液硫化物中的大部分S来自海水[8]。
海底热液硫化物中的Os质量分数及其同位素组成也受流体和海水混合程度的控制[3]。在海底热液系统中,流体-海水混合过程既可以发生在喷口位置,也可以发生在海底下[130-131]。而且硫化物样品的大多数187Os/188Os值落在狭窄的范围内(0.968~1.209)[3],接近或在现代海水187Os/188Os值的范围内(约1.06)[110],且明显比洋中脊玄武岩更具放射性成因的Os[3],这表明全球海底热液硫化物中的Os主要来源于海水[3],且硫化物较高的187Os/188Os值[3],也反映了在海水和热液流体混合过程中结合到硫化物中的海水Os组分比例相对较大,这也为海底热液系统中存在流体-海水混合过程提供了证据[58]。此外,Re在氧化态的海水中高度可溶(大量海水与热液混合可导致产生更氧化的流体),致使从氧化态流体中沉淀的硫化物Re质量分数较低,而在还原态热液流体中Re的流动性较低,可使其从还原态流体中沉淀的硫化物具Re富集的特征[56],且伴随氧化态的海水与还原态的热液混合程度的增加,可导致沉淀的硫化物具较低的187Re/188Os值[3]。
不僅如此,流体-海水混合程度的增加,致使流体的温度由高到低的变化,此过程可使Fe同位素产生明显的分馏(高达2‰)[6]。海底热液硫化物的δ56Fe值略低于海水的δ56Fe值(-0.88‰~0.10‰)[132],并与热液流体的δ56Fe值相似(-1.85‰~-0.14‰)[6, 61, 66–67, 121],表明流体可能是硫化物中轻Fe同位素的来源,且在海水和热液混合期间54Fe更有可能优先进入黄铁矿相,致使低温黄铁矿的δ56Fe和δ57Fe值降低[6]。此外,硫化物中Fe同位素组成的巨大变化可能受到矿物沉淀速率的影响[6],且在烟囱体环境中热液流体与海水的混合,可导致黄铁矿的快速沉淀并产生显著的Fe同位素动力学分馏[67]。还有在MAR的Lucky Strike热液区中,硫化物的轻Fe同位素组成(低至3.24‰)可以通过流体-海水混合期间硫化物沉淀过程中的Fe同位素平衡分馏来解释,这也为北大西洋热液系统中Fe同位素的非生物分馏提供了证据[63]。而且在EPR 9°N—10°N的热液区中,流体和硫化物中黄铁矿的Fe同位素组成不平衡,可以通过流体-海水混合期间黄铁矿沉淀过程中与流体的Fe同位素交换或洋中脊FeS快速形成黄铁矿的过程来解释[61, 67],且随着海水混入流体比例的增加,流体的δ56Fe值降低,其Os同位素组成与海水趋于一致,致使沉淀的硫化物具有较高的187Os/188Os值和δ57Fe值(图4)。
海底热液硫化物中黄铜矿的δ65Cu值在-0.88‰~-0.16‰之间[6],该范围低于洋中脊玄武岩(δ65Cu值为-0.10‰~0.73‰)[6, 65, 133]和热液流体(δ65Cu值為0.1‰~0.5‰)[79]。不仅如此,具有较低δ65Cu值的黄铜矿,其和热液流体的Cu同位素不平衡,且黄铜矿和热液流体之间发生了显著的Cu同位素分馏(高达0.7‰)[6],表明在流体-海水混合期间的黄铜矿形成过程中,63Cu优先从热液流体中去除,并进入到黄铜矿中,而较重的Cu同位素更有可能保留在沉淀黄铜矿的高温流体中[6, 60, 65],这意味着在流体-海水混合和硫化物沉淀过程中,具有较重Cu同位素组成的热液流体可能为热液柱、海水和含金属沉积物提供重Cu同位素[6]。此外,在流体-海水混合期间,富Cu硫化物的沉淀对热液烟囱体的δ65Cu值没有明显的控制,且初始沉淀的含Cu硫化物氧化可能是热液系统中Cu同位素分馏的主要原因(高达3‰)[63, 70, 76-77],具重δ65Cu值的洋中脊硫化物可以是高温流体对已沉淀硫化物进行改造的结果;而具负δ65Cu值的硫化物则是经历了再结晶过程的结果[63, 69],在弧后盆地(东马努斯海盆、北斐济海盆和Lau海盆)和西太平洋岛弧环境(汤加岛弧),海底热液硫化物的δ65Cu值变化可能与热液喷口区附近的蚀变和氧化还原反应过程中的Cu同位素分馏有关[63, 78]。在海底热液系统中,温度效应[69]、多种Zn源的混合[134]和矿物沉淀过程中的动力学Raleigh分馏[135-136]是控制成矿过程中Zn同位素变化及其分馏的潜在原因。由于流体-海水混合和闪锌矿沉淀过程中的Zn同位素交换,闪锌矿和热液流体之间发生了显著的Zn同位素分馏[6]。然而,在海水和热液流体的混合过程中,热液流体中的64Zn更有可能进入闪锌矿,导致闪锌矿沉淀后的热液流体优先富集较重的Zn同位素,而海底热液硫化物及闪锌矿则具低δ66Zn值的特征[6]。同时,分析洋中脊喷口流体和烟囱体硫化物的Zn同位素组成表明,热液流体的δ66Zn值存在较大变化,且流体-海水混合期间闪锌矿沉淀是导致流体δ66Zn值变化的主要因素[64, 69, 77],这表明在流体-海水混合和硫化物沉淀过程中,具有较重Zn同位素组成的流体也可成为热液柱、海水和含金属沉积物中重Zn同位素的来源。
海底热液硫化物(巴布亚新几内亚PACMANUS热液区黄铁矿和硬石膏)中流体包裹体的3He/4He值降低(0.29~6.91 Ra)[5],也被解释为是低温流体和海水混合引起的结果[86]。海底热液硫化物中流体包裹体的大多数Ne、Ar和Xe同位素比值与现代大气的值一致[115, 137],或者只是略低或略高[5],它们的比值与洋中脊和洋岛玄武岩地幔端元的比值显著不同,这不仅证实了全球海底热液硫化物中流体包裹体的Ne、Ar和Xe主要来源于周围的海水[5],也为硫化物沉淀过程中海水与热液流体混合提供了证据[80]。此外,海底热液区的蛋白石在低温(<200℃)条件下形成[138-139],会泄漏氦气[140],大多数海底热液硫化物中流体包裹体的He值明显高于蛋白石中的He值,且热液流体中的4He值(10-6~10-5cm3 STP/g)[141]显著高于海水中的4He值(约3.8×10-8cm3 STP/g),在热液流体-海水混合过程中致使沉淀蛋白石的低温流体,其He更容易被周围的海水稀释[82],且在低温环境下He的损失影响了蛋白石中初始热液3He/4He值的保持。因此,海底热液硫化物和蛋白石中较低的He值和/或3He/4He值很可能与流体-海水混合过程有关,且在流体-海水混合过程中,伴随黄铁矿的沉淀,其流体包裹体中的20Ne值随着132Xe值的增加而增加(图5a)以及136Xe/132Xe值随着207Pb/204Pb值的增加而增加(图5d)。
以上表明,在流体-海水混合阶段随着海水影响的增加可使硫化物中的Os质量分数(约0×10-12)急剧降低,187Os/188Os值(>1)、δ57Fe值(<-1.6‰)和136Xe/132Xe值明显增大(图3b,图4,图5c);随着流体-海水混合作用的增强,硫化物中黄铁矿的δ34SVCDT值将随着其流体包裹体中3He/4He、40Ar/36Ar值轻微降低而升高(图1a,b),而其3He/4He值随着130Xe/132Xe值的降低而降低(图5b)。
5 结论
1)海底热液硫化物中的金属、非金属及其流体包裹体中的稀有气体同位素组成之间存在相关性。其中,海底热液硫化物中的S与Os、Fe同位素之间,Fe与Pb、He同位素之间,存在负相关性;其Xe与Pb同位素、Os同位素之间则存在正相关性。
2)岩浆活动對海底热液系统产生了影响。受岩浆去气作用影响的海底热液流体,其形成的硫化物具低δ34SVCDT值,高3He/4He、40Ar/36Ar和129Xe/132Xe值的特征。
3)流体-岩石相互作用为海底热液活动做出了物质贡献。随着流体-岩石相互作用程度的增加,岩石中含Pb矿物不断的溶解,致使流体中沉淀的黄铁矿、黄铜矿和闪锌矿,其Pb质量分数的增加伴随着Pb同位素比值的减小。
4)海底热液硫化物的同位素记录了流体-海水混合的信息。随着流体-海水混合作用的增强可使硫化物中的Os质量分数急剧降低,δ57Fe值、187Os/188Os值明显增大,且黄铁矿的δ34SVCDT值将随着其流体包裹体中稀有气体同位素比值的降低而升高,而其3He/4He值随着其130Xe/132Xe值的降低而降低。
5)综合分析海底热液硫化物中多同位素组成及其含量的相互关系,不仅可以揭示海底热液硫化物中金属、非金属和稀有气体同位素的来源,还可以明确硫化物沉淀过程中流体-岩石相互作用和流体-海水混合的程度,进而了解岩浆去气、流体-岩石相互作用和流体-海水混合对海底热液活动及其成矿的影响。
致谢:DY105-17、DY115-19、DY115-20和DY115-21航次期间“大洋一号”所有船队员为样品采集作出了贡献并提供了帮助,在此表示感谢!
参考文献(References):
[1]Zeng Z G, Qin Y S, Zhai S K. He, Ne and Ar Isotope Compositions of Fluid Inclusions in Hydrothermal Sulfides from the TAG Hydrothermal Field Mid-Atlantic Ridge[J]. Science in China:Earth Sciences, 2001, 44(3): 221-228.
[2]Zeng Z G, Chen D G, Yin X B, et al. Elemental and Isotopic Compositions of the Hydrothermal Sulfide on the East Pacific Rise near 13°N[J]. Science China:Earth Sciences, 2010, 53(2): 253-266.
[3]Zeng Z G, Chen S, Selby D,et al. Rhenium-Osmium Abundance and Isotopic Compositions of Massive Sulfides from Modern Deep-Sea Hydrothermal Systems: Implications for Vent Associated Ore Forming Processes[J]. Earth and Planetary Science Letters, 2014, 396: 223-234.
[4]Zeng Z G, Ma Y, Yin X B,et al. Factors Affecting the Rare Earth Element Compositions in Massive Sulfides from Deep-Sea Hydrothermal Systems[J]. Geochemistry, Geophysics, Geosystems, 2015, 16(8): 2679-2693.
[5]Zeng Z G, Niedermann S, Chen S,et al. Noble Gases in Sulfide Deposits of Modern Deep-Sea Hydrothermal Systems: Implications for Heat Fluxes and Hydrothermal Fluid Processes[J]. Chemical Geology, 2015, 409: 1-11.
[6]Zeng Z G, Li X H, Chen S,et al. Iron, Copper, and Zinc Isotopic Fractionation in Seafloor Basalts and Hydrothermal Sulfides[J]. Marine Geology, 2021, 436: 106491.
[7]Zeng Z G, Chen Z X, Qi H Y,et al. Chemical and Isotopic Composition of Sulfide Minerals from the Noho Hydrothermal Field in the Okinawa Trough[J]. Journal of Marine Science and Engineering, 2022, 10(5): 678.
[8]Zeng Z G, Ma Y, Chen S,et al. Sulfur and Lead Isotopic Compositions of Massive Sulfides from Deep-Sea Hydrothermal Systems: Implications for Ore Genesis and Fluid Circulation[J]. Ore Geology Reviews, 2017, 87: 155-171.
[9]Zeng Z G, Chen Z X, Qi H Y. Two Processes of Anglesite Formation and a Model of Secondary Supergene Enrichment of Bi and Ag in Seafloor Hydrothermal Sulfide Deposits[J]. Journal of Marine Science and Engineering, 2022, 10(1): 35.
[10]Zeng Z G, Wang X Y, Chen C T A,et al. Boron Isotope Compositions of Fluids and Plumes from the Kueishantao Hydrothermal Field off Northeastern Taiwan: Implications for Fluid Origin and Hydrothermal Processes[J]. Marine Chemistry, 2013, 157: 59-66.
[11]Zeng Z G, Wang X Y, Qi H Y, et al. Arsenic and Antimony in Hydrothermal Plumes from the Eastern Manus Basin, Papua New Guinea[J/OL]. Geofluids, 2018: 6079586. https://doi.org/10.1155/2018/6079586.
[12]Zeng Z G, Wang X Y, Murton B J,et al. Dispersion and Intersection of Hydrothermal Plumes in the Manus Back-Arc Basin, Western Pacific[J/OL]. Geofluids, 2020: 4260806. https://doi.org/10.1155/2020/4260806.
[13]Rong K B, Zeng Z G, Yin X B,et al. Smectite Formation in Metalliferous Sediments near the East Pacific Rise at 13°N[J]. Acta Oceanologica Sinica, 2018, 37(9): 67-81.
[14]Zeng Z G, Wang X Y, Zhang G L, et al. Formation of Fe-Oxyhydroxides from the East Pacific Rise near Latitude 13°N: Evidence from Mineralogical and Geochemical Data[J]. Science in China: Series D: Earth Sciences, 2008, 51(2): 206-215.
[15]Zeng Z G, Ouyang H G, Yin X B,et al. Formation of Fe-Si-Mn Oxyhydroxides at the PACMANUS Hydrothermal Field, Eastern Manus Basin: Mineralogical and Geochemical Evidence[J]. Journal of Asian Earth Sciences, 2012, 60: 130-146.
[16]Zeng Z G, Chen S, Wang X Y,et al. Mineralogical and Micromorphological Characteristics of Si-Fe-Mn Oxyhydroxides from the PACMANUS Hydrothermal Field, Eastern Manus Basin[J]. Science China: Earth Sciences, 2012, 55(12): 2039-2048.
[17]Zeng Z G, Qi H Y, Chen S,et al. Hydrothermal Alteration of Plagioclase Microphenocrysts and Glass in Basalts from the East Pacific Rise near 13°N: An SEM-EDS Study[J]. Science China:Earth Sciences, 2014, 57(7): 1427-1437.
[18]Wang X Y, Zeng Z G, Qi H Y,et al. Fe-Si-Mn-Oxyhydroxide Encrustations on Basalts at East Pacific Rise near 13°N: An SEM-EDS Study[J]. Journal of Ocean University of China, 2014, 13(6): 917-925.
[19]Huang X, Zeng Z G, Chen S,et al. Component Characteristics of Organic Matter in Hydrothermal Barnacle Shells from Southwest Indian Ridge[J]. Acta Oceanologica Sinica, 2013, 32(12): 60-67.
[20]Chen J B, Zeng Z G. Metasomatism of the Peridotites from Southern Mariana Fore-Arc: Trace Element Characteristics of Clinopyroxene and Amphibole[J]. Science in China: Series D: Earth Sciences, 2007, 50(7): 1005-1012.
[21]Wang X M, Zeng Z G, Chen J B. Serpentinization of Peridotites from the Southern Mariana forearc[J]. Progress in Natural Science, 2009, 19(10): 1287-1295.
[22]Zeng Z G, Wang Q Y, Wang X M,et al. Geochemistry of Abyssal Peridotites from the Super Slow-Spreading Southwest Indian Ridge near 65°E: Implications for Magma Source and Seawater Alteration[J]. Journal of Earth System Science, 2012, 121(5): 1317-1336.
[23]Zeng Z G, Li X H, Zhang Y X,et al. Lithium, Oxygen and Magnesium Isotope Systematics of Volcanic Rocks in the Okinawa Trough: Implications for Plate Subduction Studies[J]. Journal of Marine Science and Engineering, 2022, 10(1): 40.
[24]Zeng Z G, Li X H, Chen S,et al. Iron-Copper-Zinc Isotopic Compositions of Andesites from the Kueishantao Hydrothermal Field off Northeastern Taiwan[J]. Sustainability, 2022, 14(1): 359.
[25]Zeng Z G, Chen Z X, Zhang Y X,et al. Geological, Physical, and Chemical Characteristics of Seafloor Hydrothermal Vent Fields[J]. Journal of Oceanology and Limnology, 2020, 38(4): 985-1007.
[26]Fouquet Y, Marcoux E. Lead Isotope Systematics in Pacific Hydrothermal Sulfide Deposits[J]. Journal of Geophysical Research: Solid Earth, 1995, 100 (B4): 6025-6040.
[27]Bjerkgrd T, Cousens B L, Franklin J M. The Middle Valley Sulfide Deposits, Northern Juan de Fuca Ridge: Radiogenic Isotope Systematics[J]. Economic Geology, 2000, 95: 1473-1488.
[28]Kim J, Lee I, Halbach P, et al. Formation of Hydrothermal Vents in the North Fiji Basin: Sulfur and Lead Isotope Constraints[J]. Chemical Geology, 2006, 233: 257-275.
[29]Seal R R II. Sulfur Isotope Geochemistry of Sulfide Minerals[J]. Reviews in Mineralogy and Geochemistry, 2006, 61: 633-677.
[30]Yao H Q, Zhou H Y, Peng X T, et al. Metal Sources of Black Smoker Chimneys, Endeavour Segment, Juan de Fuca Ridge: Pb Isotope Constraints[J]. Applied Geochemistry, 2009, 24: 1971-1977.
[31]Aoyama S, Nishizawa M, Takai K, et al. Microbial Sulfate Reduction within the Iheya North Subseafloor Hydrothermal System Constrained by Quadruple Sulfur Isotopes[J]. Earth and Planetary Science Letters, 2014, 398: 113-126.
[32]McDermott J M, Ono S, Tivey M K, et al. Identification of Sulfur Sources and Isotopic Equilibria in Submarine Hot-Springs Using Multiple Sulfur Isotopes[J]. Geochimica et Cosmochimica Acta, 2015, 160: 169-187.
[33]Halbach P, Nakamura K, Wahsner M, et al. Probable Modern Analogue of Kuroko-Type Massive Sulphide Deposits in the Okinawa Trough Back-Arc Basin[J]. Nature, 1989, 338: 496-499.
[34]Halbach P, Hansmann W, Koppel V, et al. Whole-Rock and Sulfide Lead-Isotope Data from the Hydrothermal JADE Field in the Okinawa Back-Arc Trough[J]. Mineralium Deposita, 1997, 32: 70-78.
[35]Verati C, Lancelot J, Hékinian R. Pb Isotope Study of Black-Smokers and Basalts from Pito Seamount Site (Easter Microplate)[J]. Chemical Geology, 1999, 155: 45-63.
[36]Seal R R Ⅱ, Rye R O, Alpers C N. Stable Isotope Systematics of Sulfate Minerals[J]. Reviews in Mineralogy and Geochemistry, 2000, 40: 541-602.
[37]曾志剛,秦蕴珊,赵一阳,等.大西洋中脊TAG热液活动区中海底热液沉积物的硫同位素组成及其地质意义[J].海洋与湖沼,2000,31(5):518-529.
Zeng Zhigang, Qin Yunshan, Zhao Yiyang, et al. Sulfur Isotopic Composition of Seafloor Surface Hydrothermal Sediments in the TAG Hydrothermal Field of Mid-Atlantic Ridge and Its Geological Implications[J]. Oceanologia et Limnologia Sinica, 2000, 31 (5): 518-529.
[38]曾志刚,蒋富清,翟世奎,等.冲绳海槽Jade热液活动区块状硫化物的铅同位素组成及其地质意义[J].地球化学,2000,29(3):239-245.
Zeng Zhigang, Jiang Fuqing, Zhai Shikui, et al. Lead Isotopic Compositions of Massive Sulfides from the Jade Hydrothermal Field in the Okinawa Trough and Its Geological Implications[J]. Geochimica, 2000, 29 (3): 239-245.
[39]Shanks W C. Stable Isotope in Seafloor Hydrothermal Systems: Vent Fluids, Hydrothermal Deposits, Hydrothermal Alteration, and Microbial Processes[J]. Reviews in Mineralogy and Geochemistry, 2001, 43: 469-525.
[40]Cousens B L, Blenkinsop J, Franklin J M. Lead Isotope Systematics of Sulfide Minerals in the Middle Valley Hydrothermal System, Northern Juan de Fuca Ridge[J]. Geochemistry Geophysics Geosystems, 2002, 3(5): 1-16. doi:10.1029/2001GC000257.
[41]Kim J, Lee I, Lee K Y. S, Sr, and Pb Isotopic Systematics of Hydrothermal Chimney Precipitates from the Eastern Manus Basin, Western Pacific: Evaluation of Magmatic Contribution to Hydrothermal System[J]. Journal of Geophysical Research: Solid Earth, 2004, 109: B12210. doi:10.1029/2003JB002912.
[42]Herzig P M, Hannington M D, Arribas A. Sulfur Isotopic Composition of Hydrothermal Precipitates from the Lau Back-Arc: Implications for Magmatic Contributions to Seafloor Hydrothermal Systems[J]. Mineralium Deposita, 1998, 33: 226-237.
[43]Shanks W C Ⅲ, Bischoff J L, Rosenbauer R J. Seawater Sulfate Reduction and Sulfur Isotope Fractionation in Basaltic Systems: Interaction of Seawater with Fayalite and Magnetite at 200-350℃[J]. Geochimica et Cosmochimica Acta, 1981, 45: 1977-1995.
[44]Solomon M, Eastoe C J, Walshe J L, et al. Mineral Deposits and Sulfur Isotope Abundances in the Mount Read Volcanics Between Que River and Mount Darwin, Tasmania[J]. Economic Geology, 1988, 83: 1307-1328.
[45]Peter J M, Shanks W C. Sulfur, Carbon, and Oxygen Isotope Variations in Submarine Hydrothermal Deposits of Guaymas Basin, Gulf of California, USA[J]. Geochimica et Cosmochimica Acta, 1992, 56: 2025-2040.
[46]Arnold M, Sheppard S M F. East Pacific Rise at Latitude 21°N: Isotopic Composition and Origin of the Hydrothermal Sulphur[J]. Earth and Planetary Science Letters, 1981, 56: 148-156.
[47]Goldhaber M B, Kaplan I R. Mechanisms of Sulfur Incorporation and Isotope Fractionation During Early Diagenesis in Sediments of the Gulf of California[J]. Marine Chemistry, 1980, 9: 95-143.
[48]Brunner B, Bernasconi S M. A Revised Isotope Fractionation Model for Dissimilatory Sulfate Reduction in Sulfate Reducing Bacteria[J]. Geochimica et Cosmochimica Acta, 2005, 69: 4759-4771.
[49]Brévart O, Dupré, Allègre C J. Metallogenesis at Spreading Centers: Lead Isotope Systematics for Sulfides, Manganese-Rich Crusts, Basalts, and Sediments from the Cyamex and Alvin Areas (East Pacific Rise)[J]. Economic Geology, 1981, 76: 1205-1210.
[50]Vidal Ph, Clauer N. Pb and Sr Isotopic Systematics of Some Basalts and Sulfides from the East Pacific Rise at 21°N (Project RITA)[J]. Earth and Planetary Science Letters, 1981, 55: 237-246.
[51]Chen J. U, Th and Pb Isotopes in Hot Springs on the Juan de Fuca Ridge[J]. Journal of Geophysical Research: Solid Earth, 1987, 92: 11411-11415.
[52]Hegner E, Tatsumoto M. Pb, Sr, and Nd Isotopes in Basalts and Sulfides from the Juan de Fuca Ridge[J]. Journal of Geophysical Research: Solid Earth, 1987, 92 (B11): 11380-11386.
[53]LeHuray A P, Church S E, Koski R A, et al. Pb Isotopes in Sulfides from Mid-Ocean Ridge Hydrothermal Sites[J]. Geology, 1988, 16: 362-365.
[54]Charlou J L, Donval J P, Fouquet Y, et al. Geochemistry of High H2and CH4Vent Fuids Issuing from Ultramafic Rocks at the Rainbow Hydrothermal Field (36°14′N, MAR)[J]. Chemical Geology, 2002, 191: 345-359.
[55]Shirey S B, Walker R J. The Re-Os Isotope System in Cosmochemistry and High-Temperature Geochemistry[J]. Annual Review of Earth and Planetary Sciences, 1998, 26: 423-500.
[56]Brügmann G E, Birck J L, Herzig P M, et al. Os Isotopic Composition and Os and Re Distribution in the Active Mound of the TAG Hydrothermal System, Mid-Atlantic Ridge [C]//Proceedings of the Ocean Drilling Program: Scientific Results. TX: College Station (Ocean Drilling Program), 1998: 91-100.
[57]Morelli R M, Creaser R A, Selby D, et al. Re-Os Sulfide Geochronology of the Red Dog Sediment-Hosted Zn-Pb-Ag Deposit, Brooks Range, Alaska[J]. Economic Geology, 2004, 99: 1569-1576.
[58]Ravizza G, Martin C E, German C R, et al. Os Isotopes as Tracers in Seafloor Hydrothermal Systems: Metalliferous Deposits from the TAG Hydrothermal Area, 26°N Mid-Atlantic Ridge[J]. Earth and Planetary Science Letters, 1996, 138: 105-119.
[59]Nozaki T, Kato Y, Suzuki K. Late Jurassic Ocean Anoxic Event: Evidence from Voluminous Sulphide Deposition and Preservation in the Panthalassa[J]. Scientific Reports, 2013, 3: 1889. doi:10.1038/srep01889.
[60]Zhu X K, ONions R K, Guo Y, et al. Determination of Natural Cu-Isotope Variation by Plasma-Source Mass Spectrometry: Implications for Use as Geochemical Tracers[J]. Chemical Geology, 2000, 163(1/2/3/4): 139-149.
[61]Sharma M, Polizzotto M, Anbar A D. Iron Isotopes in Hot Springs Along the Juan de Fuca Ridge[J]. Earth and Planetary Science Letters, 2001, 194(1/2): 39-51. https://dx.doi.org/10.1016/s0012-821x(01)00538-6.
[62]Rouxel O, Fouquet Y, Ludden J N. Copper Isotope Systematics of the Lucky Strike, Rainbow, and Logatchev Sea-Floor Hydrothermal Fields on the Mid-Atlantic Ridge[J]. Economic Geology, 2004, 99(3): 585-600. https://dx.doi.org/10.2113/gsecongeo.99.3.585.
[63]Rouxel O, Fouquet Y, Ludden J N. Subsurface Processes at the Lucky Strike Hydrothermal Field, Mid-Atlantic Ridge: Evidence from Sulfur, Selenium, and Iron Isotopes[J]. Geochimica et Cosmochimica Acta, 2004, 68(10): 2295-2311. https://dx.doi.org/10.1016/j.gca.2003.11.029.
[64]John S G, Rouxel O J, Craddock P R, et al. Zinc Stable Isotopes in Seafloor Hydrothermal Vent Fluids and Chimneys[J]. Earth and Planetary Science Letters, 2008, 269(1/2): 17-28. https://dx.doi.org/10.1016/j.epsl.2007.12.011.
[65]Liu S A, Huang J, Liu J, et al. Copper Isotopic Composition of the Silicate Earth[J]. Earth and Planetary Science Letters, 2015, 427: 95-103. https://dx.doi.org/10.1016/j.epsl.2015.06.061.
[66]Severmann S, Johnson C M, Beard B L, et al. The Effect of Plume Processes on the Fe Isotope Composition of Hydrothermally Derived Fe in the Deep Ocean as Inferred from the Rainbow Vent Site, Mid-Atlantic Ridge, 36°14′N[J]. Earth and Planetary Science Letters, 2004, 225(1/2): 63-76. https://dx.doi.org/10.1016/j.epsl.2004.06.001.
[67]Rouxel O, Shanksiii W, Bach W, et al. Integrated Fe- and S-Isotope Study of Seafloor Hydrothermal Vents at East Pacific Rise 9–10°N[J]. Chemical Geology, 2008, 252(3/4): 214-227. https://dx.doi.org/10.1016/j.chemgeo.2008.03.009.
[68]Bennett S A, Rouxel O, Schmidt K, et al. Iron Isotope Fractionation in a Buoyant Hydrothermal Plume, 5°S Mid-Atlantic Ridge[J]. Geochimica et Cosmochimica Acta, 2009, 73(19): 5619-5634. https://dx.doi.org/10.1016/j.gca.2009.06.027.
[69]Mason T F D, Weiss D J, Chapman J B, et al. Zn and Cu Isotopic Variability in the Alexandrinka Volcanic-Hosted Massive Sulphide (VHMS) Ore Deposit, Urals, Russia[J]. Chemical Geology, 2005, 221(3/4): 170-187. https://dx.doi.org/10.1016/j.chemgeo.2005.04.011.
[70]Markl G, Lahaye Y, Schwinn G. Copper Isotopes as Monitors of Redox Processes in Hydrothermal Mineralization[J]. Geochimica et Cosmochimica Acta, 2006, 70(16): 4215-4228. https://dx.doi.org/10.1016/j.gca.2006.06.1369.
[71]Vance D, Archer C, Bermin J, et al. The Copper Isotope Geochemistry of Rivers and the Oceans[J]. Earth and Planetary Science Letters, 2008, 274(1/2): 204-213. https://dx.doi.org/10.1016/j.epsl.2008.07.026.
[72]Little S H, Vance D, Walker-Brown C, et al. The Oceanic Mass Balance of Copper and Zinc Isotopes, Investigated by Analysis of Their Inputs, and Outputs to Ferromanganese Oxide Sediments[J]. Geochimica et Cosmochimica Acta, 2014, 125: 673-693. https://dx.doi.org/10.1016/j.gca.2013.07.046.
[73]Huang J, Liu S A, Gao Y, et al. Copper and Zinc Isotope Systematics of Altered Oceanic Crust at IODP Site 1256 in the Eastern Equatorial Pacific[J]. Journal of Geophysical Research: Solid Earth, 2016, 121(10): 7086-7100. https://dx.doi.org/10.1002/2016jb013095.
[74]Chu N C, Johnson C M, Beard B L, et al. Evidence for Hydrothermal Venting in Fe Isotope Compositions of the Deep Pacific Ocean Through Time[J]. Earth and Planetary Science Letters, 2006, 245(1/2): 202-217. https://dx.doi.org/10.1016/j.epsl.2006.02.043.
[75]Beard B L, Johnson C M, Von Damm K L, et al. Iron Isotope Constraints on Fe Cycling and Mass Balance in Oxygenated Earth Oceans[J]. Geology, 2003, 31(7): 629-632. https://dx.doi.org/10.1130/0091-7613(2003)031<0629:iicofc>2.0.co;2.
[76]Shields W R, Goldich S S, Garner E L, et al. Natural Variations in the Abundance Ratio and the Atomic Weight of Copper[J]. Journal of Geophysical Research: Solid Earth, 1965, 70(2): 479-491. https://dx.doi.org/10.1029/jz070i002p00479.
[77]Fernandez A, Borrok D M. Fractionation of Cu, Fe, and Zn Isotopes During the Oxidative Weathering of Sulfide-Rich Rocks[J]. Chemical Geology, 2009, 264(1/2/3/4): 1-12. https://dx.doi.org/10.1016/j.chemgeo.2009.01.024.
[78]P?kala M, Asael D, Butler I B, et al. Experimental Study of Cu Isotope Fractionation During the Reaction of Aqueous Cu(II) with Fe(II) Sulphides at Temperatures Between 40 and 200 ℃[J]. Chemical Geology, 2011, 289(1/2): 31-38. https://dx.doi.org/10.1016/j.chemgeo.2011.07.004.
[79]Turner G, Stuart F M. Helium/Heat Ratios and Deposition Temperatures of Sulphides from the Ocean Foor[J]. Nature, 1992, 357: 581-583.
[80]Stuart F M, Duckworth R, Turner G, et al. Helium and Sulfur Isotopes in Sulfide Minerals from Middle Valley, Northern Juan de Fuca Ridge [C]//Proceedings of the Ocean Drilling Program: Scientific Results. TX: College Station (Ocean Drilling Program), 1994: 387-392.
[81]Stuart F M, Turner G, Duckworth R C, et al. Helium Isotopes as Tracers of Trapped Hydrothermal Fuids in Ocean-Foor Sulfides[J]. Geology, 1994, 22: 823-826.
[82]Jean-Baptiste P, Fouquet Y. Abundance and Isotopic Composition of Helium in Hydrothermal Sulfides from the East Pacific Rise at 13°N[J]. Geochimica et Cosmochimica Acta, 1996, 60: 87-93.
[83]Zeng Z G, Qin Y S, Zhai S K. He, Ne and Ar Isotope Compositions of Fluid Inclusions in Massive Sulfides from the Jade Hydrothermal Field, Okinawa Trough[J]. Acta Oceanologica Sinica, 2004, 23: 655-661.
[84]Hou Z Q, Zaw K, Li Y H, et al. Contribution of Magmatic Fluid to the Active Hydrothermal System in the JADE Field, Okinawa Trough: Evidence from Fluid Inclusions, Oxygen and Helium Isotopes[J]. International Geology Review, 2005, 47: 420-437.
[85]Lüders V, Niedermann S. Helium Isotope Composition of Fluid Inclusions Hosted in Massive Sulfides from Modern Submarine Hydrothermal Systems[J]. Economic Geology, 2010, 105: 443-449.
[86]Webber A P, Roberts S, Burgess R, et al. Fluid Mixing and Thermal Regimes Beneath the PACMANUS Hydrothermal Field, Papua New Guinea: Helium and Oxygen Isotope Data[J]. Earth and Planetary Science Letters, 2011, 304: 93-102.
[87]Lupton J E, Klinkhammer G P, Normark W R, et al. Helium-3 and Manganese at the 21°N East Pacific Rise Hydrothermal Site[J]. Earth and Planetary Science Letters, 1980, 50: 115-127.
[88]Kennedy B M. Noble Gases in Vent Water from the Juan de Fuca Ridge[J]. Geochimica et Cosmochimica Acta, 1988, 52: 1929-1935.
[89]Kodera M, Igarashi G, Ozima M. Noble Gases in Hydrothermal Plumes of Loihi Seamount[J]. Earth and Planetary Science Letters, 1988, 87: 266-272.
[90]Jean-Baptiste P, Charlou J L, Stievenard M, et al. Helium and Methane Measurements in Hydrothermal Fluids from the Mid-Atlantic Ridge: The Snake Pit Site at 23°N[J]. Earth and Planetary Science Letters, 1991, 106: 17-28.
[91]Rudnicki M D, Elderfield H. Helium, Radon and Manganese at the TAG and Snake Pit Hydrothermal Fields, 26° and 23°N, Mid-Atlantic Ridge[J]. Earth and Planetary Science Letters, 1992, 113: 307-321.
[92]Charlou J L, Donval J P, Jean-Baptiste P, et al. Gases and Helium Isotopes in High Temperature Solutions Sampled Before and After ODP 158 Drilling at TAG Hydrothermal Field (26°N, MAR)[J]. Geophysical Research Letters, 1996, 23: 3491-3494.
[93]Kurz M D, Jenkins W J, Schilling J-G, et al. Helium Isotopic Variation in the Mantle Beneath the Central North Atlantic Ocean[J]. Earth and Planetary Science Letters, 1982, 58: 1-14.
[94]Stuart F M, Turner G. Mantle-Derived40Ar in Mid-Ocean Ridge Hydrothermal Fluids: Implications for the Source of Volatiles and Mantle Degassing Rates[J]. Chemical Geology, 1998, 14: 77-88.
[95]Kumagai H, Nakamura K, Toki T, et al. Geological Background of the Kairei and Edmond Hydrothermal Fields Along the Central Indian Ridge: Implications of Their Vent Fluids Distinct Chemistry[J]. Geofluids, 2008, 8: 239-251.
[96]Nakamura K, Morishita T, Bach W, et al. Serpentinized Troctolites Exposed near the Kairei Hydrothermal Field, Central Indian Ridge: Insights into the Origin of the Kairei Hydrothermal Fluid Supporting a Unique Microbial Ecosystem[J]. Earth and Planetary Science Letters, 2009, 280: 128-136.
[97]Petersen S, Kuhn K, Kuhn T, et al. The Geological Setting of the Ultramafic-Hosted Logatchev Hydrothermal Field (14°45′N, Mid-Atlantic Ridge) and Its Influence on Massive Sulfide Formation[J]. Lithos, 2009, 112: 40-56.
[98]Eissen J P, Nohara M, Cotten J, et al. North Fiji Basin Basalts and Their Magma Sources: Part I:Incompatible Element Constraints[J]. Marine Geology, 1994, 116: 153-178.
[99]Nohara M, Hirose K, Eissen J P, et al. The North Fiji Basin Basalts and Their Magma Sources: Part Ⅱ:Sr-Nd Isotopic and Trace Element Constraints[J]. Marine Geology, 1994, 116: 179-195.
[100]Koschinsky A, Seifert R, Halbach P, et al. Geochemistry of Diffuse Low-Temperature Hydrothermal Fluids in the North Fiji Basin[J]. Geochimica et Cosmochimica Acta, 2002, 66: 1409-1427.
[101]Gallant R M, Von Damm K L. Geochemical Controls on Hydrothermal Fluids from the Kairei and Edmond Vent Fields, 23°–25°S, Central Indian Ridge[J]. Geochemistry Geophysics Geosystems, 2006, 7: Q06018. doi:10.1029/2005GC001067.
[102]Merlivat L, Pineau F, Javoy M. Hydrothermal Vent Waters at 13°N on the East Pacific Rise: Isotopic Composition and Gas Concentration[J]. Earth and Planetary Science Letters, 1987, 84: 100-108.
[103]Michard G, Albarède F, Michard A, et al. Chemistry of Solutions from the 13°N East Pacific Rise Hydrothermal Site[J]. Earth and Planetary Science Letters, 1984, 67: 297-307.
[104]Bowers T S, Campbell A C, Measures C I, et al. Chemical Controls on the Composition of Vent Fluids at 13°–11°N and 21°N, East Pacific Rise[J]. Journal of Geophysical Research: Solid Earth, 1988, 93(B5): 4522-4536. https://dx.doi.org/10.1029/jb093ib05p04522.
[105]Ishibashi J I, Grimaud D, Nojiri Y, et al. Fluctuation of Chemical Compositions of the Phase-Separated Hydrothermal Fluid from the North Fiji Basin Ridge[J]. Marine Geology, 1994, 116(1/2): 215-226. https://dx.doi.org/10.1016/0025-3227(94)90177-5.
[106]Ishibashi J I, Wakita H, Nojiri Y, et al. Helium and Carbon Geochemistry of Hydrothermal Fluids from the North Fiji Basin Spreading Ridge (Southwest Pacific)[J]. Earth and Planetary Science Letters, 1994, 128(3/4): 183-197. https://dx.doi.org/10.1016/0012-821x(94)90144-9.
[107]Schmidt K, Koschinsky A, Garbe-Sch?nberg D, et al. Geochemistry of Hydrothermal Fluids from the Ultramafic-Hosted Logatchev Hydrothermal Field, 15°N on the Mid-Atlantic Ridge: Temporal and Spatial Investigation[J]. Chemical Geology, 2007, 242(1/2): 1-21. https://dx.doi.org/10.1016/j.chemgeo.2007.01.023.
[108]Peucker-Ehrenbrink B, Ravizza G. The Marine Osmium Isotope Record[J]. Terra Nova, 2000, 12: 205-219.
[109]Baker E T, Lupton J E. Changes in Submarine Hydrothermal3He/Heat Ratios as an Indicator of Magmatic/Tectonic Activity[J]. Nature, 1990, 346: 556-558.
[110]Butterfield D A, Massoth G J, McDuff R E, et al. Geochemistry of Hydrothermal Fluids from Axial Seamount Hydrothermal Emissions Study Vent Field, Juan de Fuca: Subseafloor Boiling and Subsequent Fluid-Rock Interaction[J]. Journal of Geophysical Research: Solid Earth, 1990, 95: 12895-12921.
[111]Malahoff A, Hammond S R, Naughton J J, et al. Geophysical Evidence for Post-Miocene Rotation of the Island of Viti Levu, Fiji, and Its Relationship to the Tectonic Development of the North Fiji Basin and Lau Basins[J]. Earth and Planetary Science Letters, 1982, 87: 4109-4125.
[112]Auzende J M, Eissen J P, Lafoy Y, et al. Seafloor Spreading in the North Fiji Basin (Southwest Pacific)[J]. Tectonophysics, 1988, 146: 317-351.
[113]Tanahashi M, Kisimoto K, Joshima M, et al. Geological Structure of the Central Spreading System, North Fiji Basin[J]. Marine Geology, 1991, 98: 187-200.
[114]Huchon P, Gracia E, Ruellan E, et al. Kinematics of Active Spreading in the Central North Fiji Basin (Southwest Pacific)[J]. Marine Geology, 1994, 116: 69-87.
[115]Ozima M, Podosek F A. Noble Gas Geochemistry [M]. Cambridge: Cambridge University Press, 2002.
[116]Rubin K H, Macdougall J D, Perfit M R.210Po/210Pb Dating of Recent Volcanic Eruptions on the Sea Floor[J]. Nature, 1994, 368: 841-844.
[117]Von Damm K L, Oosting S E, Kozlowskl R, et al. Evolution of East Pacific Rise Hydrothermal Vent Fuids Following a Volcanic Eruption[J]. Nature, 1995, 375: 47-50.
[118]Fornari D J, Shank T, Von Damm K L, et al. Time-Series Temperature Measurements at High-Temperature Hydrothermal Vents, East Pacific Rise 9°49′–51′N: Evidence for Monitoring a Crustal Cracking Event[J]. Earth and Planetary Science Letters, 1998, 160: 419-431.
[119]Seyfried W E, Seewald J S, Berndt M E, et al. Chemistry of Hydrothermal Vent Fuids from the Main Endeavour Field, Northern Juan de Fuca Ridge: Geochemical Controls in the Aftermath of June 1999 Seismic Events[J]. Journal of Geophysical Research: Solid Earth, 2003, 108 (B9): 2429. doi:10.1029/2002JB001957.
[120]Teng F Z, Dauphas N, Huang S, et al. Iron Isotopic Systematics of Oceanic Basalts[J]. Geochimica et Cosmochimica Acta, 2013, 107: 12-26. https://dx.doi.org/10.1016/j.gca.2012.12.027.
[121]Moeller K, Schoenberg R, Grenne T, et al. Comparison of Iron Isotope Variations in Modern and Ordovician Siliceous Fe Oxyhydroxide Deposits[J]. Geochimica et Cosmochimica Acta, 2014, 126: 422-440. https://dx.doi.org/10.1016/j.gca.2013.11.018.
[122]Polyakov V B, Mineev S D. The Use of M?ssbauer Spectroscopy in Stable Isotope Geochemistry[J]. Geochimica et Cosmochimica Acta, 2000, 64(5): 849-865. https://dx.doi.org/10.1016/s0016-7037(99)00329-4.
[123]Johnson C M, Skulan J L, Beard B L, et al. Isotopic Fractionation Between Fe(Ⅲ) and Fe(Ⅱ) in Aqueous Solutions[J]. Earth and Planetary Science Letters, 2002, 195(1/2): 141-153. https://dx.doi.org/10.1016/s0012-821x(01)00581-7.
[124]Butler I B, Nesbitt R W. Trace Element Distributions in the Chalcopyrite Wall of a Black Smoker Chimney: Insights from Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS)[J]. Earth and Planetary Science Letters, 1999, 167(3/4): 335-345. https://dx.doi.org/10.1016/s0012-821x(99)00038-2.
[125]Samanta M, Ellwood M J, Sinoir M, et al. Dissolved Zinc Isotope Cycling in the Tasman Sea, SW Pacific Ocean[J]. Marine Chemistry, 2017, 192: 1-12. https://dx.doi.org/10.1016/j.marchem.2017.03.004.
[126]Urey H C. The Thermodynamic Properties of Isotopic Substances [J/OL]. Journal of the Chemical Society, 1947: 562-581. https://dx.doi.org/10.1039/jr9470000562.
[127]Maréchal C N, Sheppard S M F. Isotopic Fractionation of Cu and Zn Between Chloride and Nitrate Solutions and Malachite or Smithsonite at 30° and 50 ℃[J]. Geochimica et Cosmochimica Acta, 2002, 66(Sup.1): A84. https://dx.doi.org/10.1016/S0016-7037(02)01007-4.
[128]Grimaud D, Ishibashi J I, Lagabrielle Y, et al. Chemistry of Hydrothermal Fluids from the 17°S Active Site on the North Fiji Basin Ridge (SW Pacific)[J]. Chemical Geology, 1991, 93(3/4): 209-218. https://dx.doi.org/10.1016/0009-2541(91)90114-7.
[129]Knott R, Fouquet Y, Honnorez J, et al. Petrology of Hydrothermal Mineralization: A Vertical Section Through the TAG Mound [C]//Proceedings of the Ocean Drilling Program: Scientific Results. TX: College Station, (Ocean Drilling Program), 1998: 5-26.
[130]Rona P A, Scott S D. Seafloor Hydrothermal Mineralization: New Perspective[J]. Economic Geology, 1993, 88: 1935-1976.
[131]Zierenberg R A, Fouquet Y, Miller D J, et al. The Deep Structure of a Sea-Floor Hydrothermal Deposit[J]. Nature, 1998, 392: 485-488.
[132]Rouxel O, Maureen A. Iron Isotope Variations in Coastal Seawater Determined by Multicollector ICP-MS[J]. Geostandards and Geoanalytical Research, 2010, 34(2): 135-144. https://dx.doi.org/10.1111/j.1751-908x.2010.00063.x.
[133]Savage P S, Moynier F, Chen H, et al. Copper Isotope Evidence for Large-Scale Sulphide Fractionation During Earths Differentiation[J]. Geochemical Perspectives Letters, 2015, 1(1): 53-64. https://dx.doi.org/10.7185/geochemlet.1506.
[134]Wilkinson J J, Weiss D J, Mason T F D, et al. Zinc Isotope Variation in Hydrothermal Systems: Preliminary Evidence from the Irish Midlands Ore Field[J]. Economic Geology, 2005, 100(3): 583-590. https://dx.doi.org/10.2113/gsecongeo.100.3.583.
[135]Kelley K D, Wilkinson J J, Chapman J B, et al. Zinc Isotopes in Sphalerite from Base Metal Deposits in the Red Dog District, Northern Alaska[J]. Economic Geology, 2009, 104(6): 767-773. https://dx.doi.org/10.2113/gsecongeo.104.6.767.
[136]Gagnevin D, Boyce A J, Barrie C D, et al. Zn, Fe and S Isotope Fractionation in a Large Hydrothermal System[J]. Geochimica et Cosmochimica Acta, 2012, 88: 183-198. https://dx.doi.org/10.1016/j.gca.2012.04.031.
[137]Lee J Y, Marti K, Severinghaus J P, et al. A Redetermination of the Isotopic Abundances of Atmospheric Ar[J]. Geochimica et Cosmochimica Acta, 2006, 70: 4507-4512.
[138]Fouquet Y, Auclair G, Cambon P, et al. Geological Setting and Mineralogical and Geochemical Investigations on Sulfide Deposits near 13°N on the East Pacific Rise[J]. Marine Geology, 1988, 84: 145-178.
[139]Dekov V M, Kamenov G D, Abrasheva M D, et al. Mineralogical and Geochemical Investigation of Seafloor Massive Sulfides from Panarea Platform (Aeolian Arc, Tyrrhenian Sea)[J]. Chemical Geology, 2013, 335: 136-148.
[140]Trull T W, Kurz M D, Jenkins W J. Diffusion of Cosmogenic3He in Olivine and Quartz: Implications for Surface Exposure Dating[J]. Earth and Planetary Science Letters, 1991, 103: 241-256.
[141]Fourre E, Jean-Baptiste P, Charlou J L, et al. Helium Isotopic Composition of Hydrothermal Fluids from the Manus Back-Arc Basin, Papua New Guinea[J]. Geochemical Journal, 2006, 40: 245-252.
