滇金丝猴夜宿树的选择及夜宿地的利用方式
2024-02-26任宝平吴瑞东黎大勇PaulGarber李明
任宝平 吴瑞东 黎大勇 Paul A.Garber 李明
(1 海南师范大学生命科学学院,热带岛屿生态学教育部重点实验室,海南省热带动植物生态学重点实验室,海口 571158)(2 中国国科学院动物研究所,动物生态与保护生物学院重点实验室,北京 100101) (3 云南大学国际河流与生态学研究院,昆明 650091) (4 西华师范大学,西南野生动植物资源保护教育部重点实验室,南充 637009) (5 Department of Anthropology and Program in Ecology, Evolution, and Conservation Biology, University of Illinois, Urbana 61801, USA) (6 大理大学,国际生物多样性与灵长类保护中心,大理 671003) (7 中国科学院动物进化与遗传前沿交叉卓越创新中心,昆明 650223)
Studies on the spatial and temporal use of sleeping and feeding sites by wild primates offer insights into species-specific patterns of habitat utilization and range use (Hamilton, 1982; Zhouet al., 2007). Nonhuman primates are reported to select sleeping sites based on several factors including protection from predators, the availability of nearby feeding sites, vegetation type (e. g. woodland, forest), physical comfort, proximity to range boundaries, and hygiene (De-Vore and Hall 1965; Hamilton, 1982; Anderson, 1984;Whiten, 1984; Feilen and Marshall, 2014). In taxa that refuge in dens, tree holes, caves, and cliffs, sleeping sites may represent a limiting resource, with a small number of sites used repeatedly across weeks,months, or years (Anderson, 1984). For example, a woodland group of olive baboons (Papio anubis) used the same nighttime sleeping site each evening over an 18-month period. This sleeping site represented the only part of the group’s home range that contained tall emergent trees (Ransom, 1981). Similarly, in white-headed langurs (Trachypithecus leucocephalus), group members used and reused a limited number of caves and rock ledges as sleeping sites (n= 18)over the course of a year (Liet al., 2011). Given the cryptic and vigilant behavior of these langurs when entering a sleeping site, it appears that caves located on steep rock faces provide safety from predators (Liet al., 2011).
In contrast, a 14-year study of 10 groups of golden lion tamarins (Leontopithecus rosalia) found that 73.4% of sleeping trees (1 128 of 1 530) were used on only one occasion (Hankersonet al., 2007). Hankersonet al. (2007) argued that these small-bodied primates avoided reusing the same sleeping site on consecutive nights as part of a strategy of predator avoidance. Finally, in the case of primates such as siamangs (Symphalangus syndactylus, Raemaekers and Chivers, 1980), Kloss’s gibbons (Hylobates klossii,Tenaza and Tilson, 1985), Eastern black-crested gibbons (Nomascus nasutus, Feiet al., 2012), Geoffroyi’s spider monkeys (Ateles geoffroyi, Chapman, 1989),and François langurs(Trachypithecus francoisi, Zhouet al., 2007), the common reuse of the same sleeping sites over the course of consecutive days or weeks appears to coincide with the spatial proximity of these sleeping sites to high quality feeding sites that produced over an extended period of time.
The Yunnan snub-nosed monkey (Rhinopithecus bieti) is an Endangered Asian leaf-eating monkey that inhabits temperate high-altitude (3 000 - 4 400 m above sea level) alpine forests in southeastern Tibet and northwestern Yunnan Province, China (Longet al., 1994). The Yunnan snub-nosed monkey forms a multilevel or modular society (GROUP) composed of several one-male, multifemale, and offspring units(OMUs) and an all-male unit (AMU) composed of juvenile, subadult and adult bachelor males. OMUs feed, forage, travel, and rest together throughout the year (Renet al., 2012). As many as 400 individuals can reside in the same group (Longet al., 1994).
An eight-month study found that preferred sleeping sites for Yunnan snub-nosed monkeys were composed of large stands of emergent trees located in coniferous (Liu and Zhao, 2004; Cuiet al., 2006; Xianget al., 2011) and coniferous and broad-leaved mixed forest (Liet al., 2006). Across their range, a small set of tree species was selected as sleeping sites. For example, trees ofGeorge’s fir (Abies georgei) and Lijiang spruce (Picea likiangensis) were the main sleeping trees used at the site of Nanren, Tibet (3 600 -4 300 m asl., Cuiet al., 2006). Similarly,Abies georgeiwas also the most frequently used sleeping treesat Honglaxueshan in Mangkang, Tibet (3 500 -4 200 m asl., Xianget al., 2011), andPicea likiangensisandTsuga dumosawere the most common sleeping trees used by the Yunnan snub-nosed monkey at the site of Weixi, Yunnan (2 700 - 3 700 m asl.,Liet al., 2006).
Previous studies offer inconsistent conclusions concerning patterns of sleeping site use in Yunnan snub-nosed monkeys. Studies by Liu and Zhao(2004) and Cuiet al. (2006) reported that Yunnan snub-nosed monkeys frequently reused the same sleeping site on consecutive nights. Other studies,however, indicate that sleeping sites were rarely reused (Liet al., 2006, 2013). For example, over the course of three years, Yunnan snub-nosed monkeys reused the same sleeping sites on only 8.9% of nights at the site of Mangkang, Tibet (Xianget al., 2011).These contrasting findings likely reflect site-specific differences in habitat disturbance, the availability of sleeping sites, the distribution of food resources, and/or predation risk. In addition, the extreme difficulties of following Yunnan snub-nosed monkeys across their steep mountainous terrain, may result in data collection biases, as researchers often lose track of the monkeys and cannot conduct all-day following. In this regard, the use of GIS technology and GPS collars represent an important research tool that report accurate group locations, which can be used to identify habitat preferences and patterns of range use.
In the present study we used GIS technology(GPS collar and radiotelemetry) and ground truthing over an 11-month period to examine sleeping site use by Yunnan snub-nosed monkeys. We addressed the following research questions. (1) What is the pattern of sleeping site use by Yunnan snub-nosed monkeys?(2) How frequently do these primates re-use the same sleeping sites on consecutive nights? (3) What is the spatial relationship between sleeping sites and feeding sites visited in the late afternoon and the following morning? And (4) are there particular tree species and vegetation types associated with sleeping sites.
1 Methods
1.1 Study site and study group
Field work was conducted at Jinsichang (26º53′N, 99º37′E) in northwest Lijiang City, Yunnan Province, China from December 2003 through October 2004 (Fig. 1). A single group of Yunnan snubnosed monkeys inhabits this area (Yang, 2000; Renet al., 2008, 2009a). The focal group consists of approximately 180 individuals distributed into 21 OMUs and one AMU. The group confined its ranging activities to an elevation of between 3 000 m to 3 900 m and four forest types: deciduous broad leaf forest(3 000 - 3 300 m), mixed coniferous and broadleaf forest (3 300 - 3 800 m), coniferous forest (3 800 -3 900 m) and bamboo forest (2 900 - 3 900 m) (Yang,2000; Renet al., 2009b). Two tree species, George’s fir and Lijiang spruce dominate the landscape and account for 78% of the treespresent (Yang, 2000). The shrub layer is dominated by two species of bamboo,which comprise 90.7% of the vegetation: the evergreenFargesia yunnanensis, at an elevation of 3 300 -3 600 mand the deciduousF.yulongshanersis, at an elevation of 3 650 - 3 800 m (Yang, 2000). The diet of Yunnan snub-nosed monkeys at Jinsichang includes 58.8% bamboo leaves and shoots, 5.0% arboreal lichens (Bryoriaspp.), 28.3% tree leaves, and 4.3% seeds (Yang, 2000). Bamboo shoots, which are available during the spring, represent a critical seasonal component of the Yunnan snub-nosed monkey diet(Renet al., 2009a).
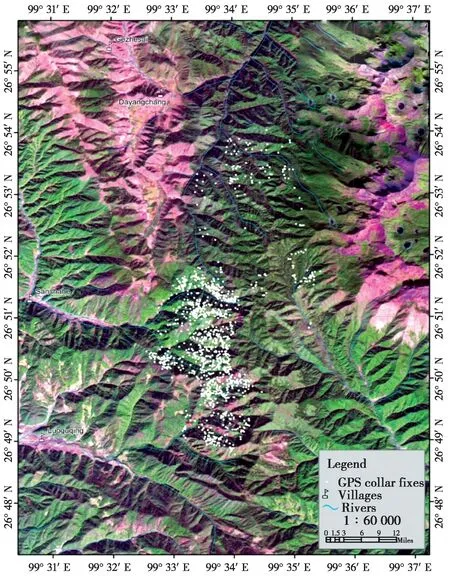
Fig. 1 Map of the study area showing all of the GPS collar fixes (n =1274) at Jinsichang, Lijiang, Yunnan. Green areas are forested and purple indicates bare surfaces without forest
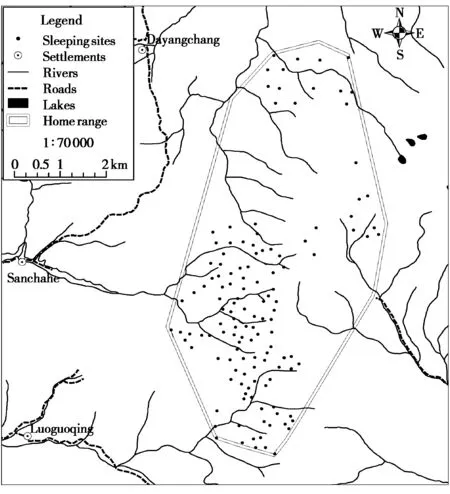
Fig.2 The distribution of 131 sleeping sites based on the location of 272 GPS fixes within the study group’s home range
Anthropogenic changes to the landscape and the extirpation of large carnivores (i. e. black bear,Ursus thibetanus, Yang, 2000) have reduced predation risk to a minimum at all Yunnan snub-nosed monkey field sites (Cuiet al., 2003; Liu and Zhao, 2004; Liet al.,2006; Renet al., 2008, 2009a). Monthly mean temperature at our field station (located at an altitude of 3 280 m asl.) in 2004 was -3.8 ℃ in January (lowest) and 12.7 ℃ in August (highest). Minimum and maximum temperatures were -13 ℃ and -1 ℃ in January and 8 ℃ and 20 ℃ in August. Snow covered the ground from December to April. Only three seasons occur in this region; spring (May to July), autumnn(August to October), and winter (November to April)(Zhang, 1934; Renet al., 2009a).
1.2 Data collection
Given the steep and complex mountainous terrain, it is not possible for humans to continuously track Yunnan snub-nosed monkeys. Therefore, we placed a GPS (Global Positioning System) collar(Type: TGW-3800, Telonics Int., USA) equipped with a VHF beacon on one adult male residing in the AMU. Because males weigh approximately 16 kg,and adult females weigh 9 kg, it was not safe to affix the collar to females or immatures. The GPS collar weighed 890 g.
We programmed the collar to record spatial coordinates at (1) 08: 00, (2) 10: 00, (3) 15: 00, (4) 17: 00 and (5) 19: 00 (Renet al., 2008). Those hours roughly coincide with the expected position of the monkeys at (1) their previous night’s sleeping site, (2) morning feeding site, (3) diurnal resting site, (4) afternoon feeding site, and (5) night-time sleeping site (the monkeys enter a sleeping site between 16: 00 - 18: 00 in winter-spring and 18: 00 - 19: 00 in summer-autumnn; Liu and Zhao, 2004). Given the expected working life of the battery, the GPS collar was pre-set to release automatically on 22 October 2004. The total number of consecutive days in which data were collected was 310 (from 17 December 2003 to 22 October 2004).
After recovering the released collar, we successfully downloaded and analyzed 82.2% (n= 1274,Fig. 1) of the 1 550 planned GPS locations (Renet al., 2008). We discarded 276 GPS points because their DOP (dilution of precision) value was > 6. Values > 6 indicate the GPS collar was positioned at a poor satellite geometry and this could significantly reduce accuracy (Renet al., 2008). To make certain that the GPS-collared monkey remained part of the study group, we used a radio-telemetry unit (Tr-5 receiver and RA-14K antenna, Telonics, USA) to visually locate the collared individual on 120 sample days in the early morning (before 07: 00), later that day in the afternoon (15: 00), and on 3 occasions after 19: 00(June - August). After locating the collared male, we followed the monkeys until they entered their nighttime sleeping site or the group could not be followed.This allowed us to ground truth the location of resting sites and verify the spatial cohesion between the AMU and resident OMUs of the group. The GPS unit was accurate to 16.1 m.
The data were integrated into the geographical information system in ArcView® 3.2a (ESRI Inc.,Redlands, CA). We assumed that the position of the bachelor male at 19: 00 reflected the general location of the group’s sleeping site. Given the latitude of our field site (26º53′N), 19: 00 was 30.6 minutes after sunset on December 21stand 76.9 minutes before sunset on June 21st. We obtained a total of 272 GPS positions at 19: 00. A digitized map (1: 50 000) of the study area and a SPOT5 satellite image taken on November 2004 (60 km × 60 km, resolution: 2.5 m) provided geo-reference points using the projection of WGS_1984_UTM_ZONE_47N (Renet al., 2009a,Fig.1).
1.3 Sleeping sites identification in Yunnan snubnosed monkeys
Given that only one individual in the 180-member group had a GPS collar, and a sleeping site contained tens of trees used by members of the study group, we defined a sleeping site as the 250 m × 250 m area (62 500 m2, see below) where the study group stopped moving at sunset and remained overnight(Liu and Zhao, 2004; Cuiet al. 2006; Liet al., 2006;Xianget al., 2010, 2011). On any given night, an adult snub-nosed monkey might sleep alone in a single tree; all members of an OMU might occupy the same sleeping tree, or members of the same OMU might be spread across several trees (Cuiet al., 2006;Liet al., 2010). Groups of Yunnan snub-nosed monkeys generally spread out over a distance of 200 -300 m in the daytime (Wu, 2006; Grueteret al.,2008; Renet al., 2009a). Therefore, we assumed that the sleeping site used by the group occupied an area of 40 000 - 90 000 m2. We recorded a sleeping site by both the local name of the area and its GPS position, and we assumed that a given 250 m × 250 m area contained a single sleeping site for the 180 members of the group (Grueteret al., 2009). Sleeping site re-used was scored when the collared male was located in a previously used sleeping area.
We also used a 250 m × 250 m grid-cell method to estimate the home range area occupied by our study group (Cuiet al., 2006; Grueteret al., 2009;Renet al., 2009b). The size of each grid cell was equivalent to the criterion used to distinguish between sleeping sites. From November 15 to December 20 of 2005, we conducted a vegetation analysis of forty 250 m × 250 m grid cells in which at least four GPS fixes were recorded at 19: 00. In each grid cell, we identified the location of each GPS fix and the dominant trees species or vegetation types. Then we classified the vegetation into the following eight plant associations (Tsuga dumosagroup,Abies georgegroup,Abies george-Picea likiangensisgroup,Abies ernestiivar.salouenensisgroup,Larix potaninii-Abies george-Picea likiangensisgroup,Larix potaniniigroup, bamboo-shrub group, and sparse vegetation)and two forest types (coniferous/evergreen broadleaved mixed forest and deciduous forest). Sparse vegetation was defined as scattered oak (Quercus)trees, poplar trees (Populus) and bush mixed forest on large grasslands.
These data were superimposed on a 1: 10 000 map using ArcView 3.2a software (Wu, 2006). We assumed that the monkeys selected the dominant plant association and forest type in a grid-cell as their sleeping site.
Inter-GPS fix distances were calculated in ArcView 3.2a using the Animal Movement Extension V. 1.1 (Hooge and Eichenlaub, 1997). The data were then transferred to Microsoft Excel® for additional analyses. Weather was recorded every day as sunny,cloudy (no sunshine), light snowfall (a small amount of snow that falls for a short period of time and then stops), light rain, heavy rain (persistent rain accompanied by strong winds), or heavy snow (snow lasting several hours accompanied by strong winds) to determine the degree to which weather impacted the daily travel distance of the monkeys.
Only multi-point (≥ 3 GPS fixes per day) travel distances were used to calculate day range (Renet al., 2008). Other measures including distances between sleeping sites on consecutive nights, distance between two adjacent feeding sites, and distance between a sleeping site and a feeding site were computed as a straight line (Appendix A). This method calculates the minimal distance traveled between a set of points. We note that there exist gaps in our data due to the fact that 17.8% of GPS collar fixes had to be discarded. All distances are presented as the mean ±SD.
1.4 Data analysis
When the data exhibited a normal distribution,parametric statistical tests such as ANOVA, and Student’st-test were used. In those cases in which the data were not normally distributed, we used nonparametric tests (Chi-squared test and one sample Kolmogorov-Smirnov test). A one-way ANOVA was used to compare differences in the length of daily travel among seasons, individual months of the year, and across consecutive days. A student’st-test was used to compare variations in daily travel distances across seasons and days. A Spearman’s Rank correlation was used to examine the degree to which the frequency of sleeping site use predicted the frequency that the same location was used as a sleeping site on sequential days (19: 00 GPS fixes on consecutive days). A one sample Kolmogorov-Smirnov test was used to determine whether the distribution of plant associations fit a random uniform-scattered distribution. A Binomial test was run to determine the probability of sleeping site re-use within and across seasons. All tests were two-tailed with a significance level set at 0.05.
2 Results
2.1 Spatial distribution of sleeping sites based on vegetation type
We obtained data on sleeping site locations for 272 nights during the 11-month study period (Appendix C). These sleeping sites were distributed across 10 tree species associations.Tsuga dumosagroup(common name: Himalayan hemlock) was the most common plant association used by the monkeys as sleeping sites, accounting for 47.1% of all sleeping site locations. Himalayan hemlock trees were distributed across a 3 km2area, or 16.9% of the monkey’s home range. The second and third most frequently used plant associations for sleeping were theAbies georgegroup (George’s fir group; 16.9% of nights) andAbies george-Picea likiangensisgroup(mixed George’s fir and Lijiang spruce group, 16.5%of nights). In contrast, bamboo-shrub vegetation was slept in on only two nights. Bamboo-shrub vegetation accounted for 11.2% of the monkey’s home range. Tree species slept in the bamboo vegetation includedAcanthopanax evodiaefoliusandQuercus rehderiana(alpine oak).
Our analysis indicated that the snub-nosed monkeys did not sleep in a given plant association in proportion to the availability of that plant association in their home range (Spearman’s correlation test: ρ =0.473,n= 10,P= 0.168). Tree associations dominated byTsuga dumosawere used as sleeping sites more than expected (χ2= 377.82,df= 127,P<0.001) (Table 1).
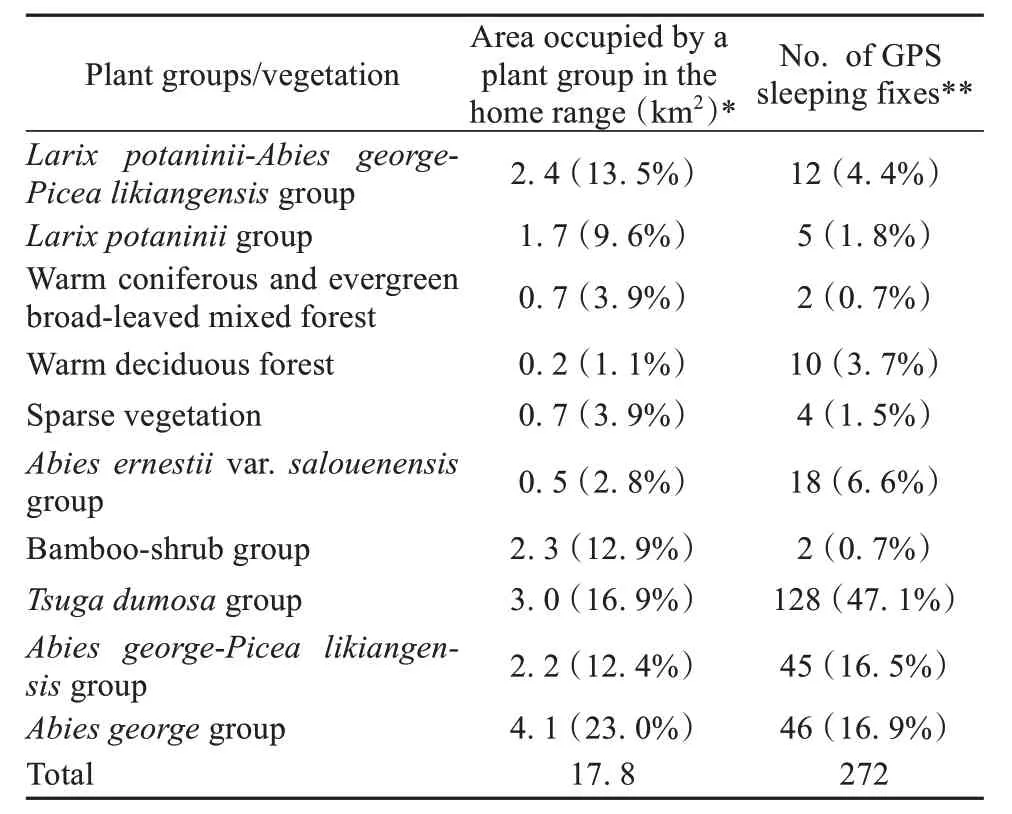
Table 1 Number of sleeping sites associated with different vegeta‐tion types
2.2 Reuse of sleeping sites: number and interval
The home range of the study group consisted of 285 grid-cells (250 m × 250 m = 6.25 hm2) and a total area of 17.8 km2. Based on the location of GPS fixes at 19: 00, field observations using a handheld GPS receiver, and assuming that different GPS locations within the same grid-cell represented the same sleeping site (63 of 271 GPS fixes at 19: 00 were located in the same grid-cell) and GPS fixes at 19: 00 located 250 m of each other represented the same sleeping site, we identified 131 sleeping sites used by the study group (Fig. 2). Seventy (53.4%) of these sleeping sites were used by the monkeys on only one occasion, 25 (19.1%) were used on two occasions, 13(9.9%) were used on 3 occasions, and one sleeping site was used on 9 different nights (Table 2). Of those sleeping sites that were reused by the snub-nosed monkeys, 16 (12.2%) were slept in on two consecutive nights, and three locations were slept in on three consecutive nights (2.3%) (Fig. 2 and Table 2). Reuse of the same sleeping site on 2 and 3 consecutive nights occurred on only 7.0% of nights (19 of 272 nights).
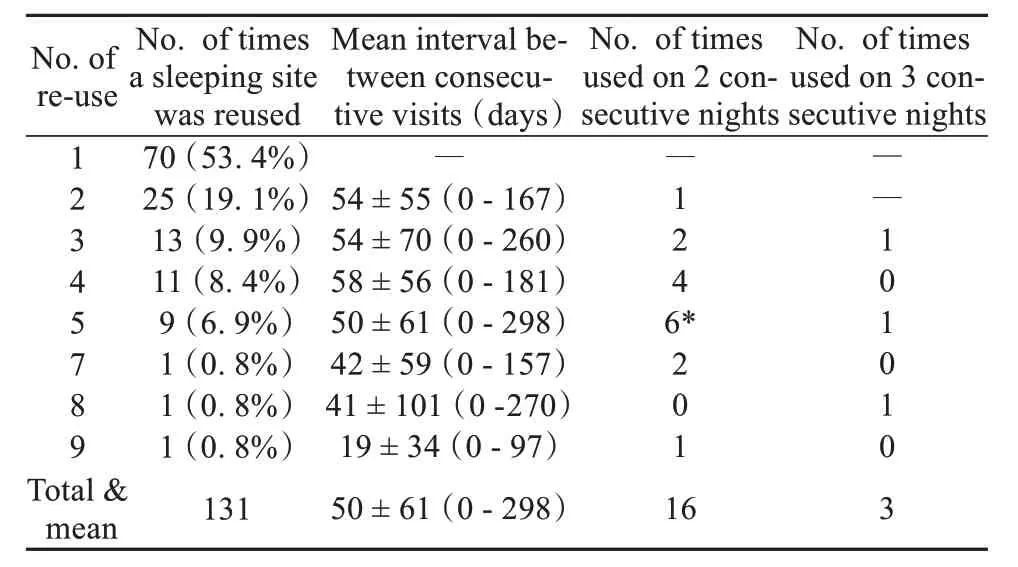
Table 2 Summary of sleeping site re-use across the study period
We found that GPS fixes at 19: 00 significantly autocorrelated with the position of the monkeys at 08: 00 the next morning (Pearson correlation test:r=0.44,df= 209,P< 0.001). The distance between those two fixes (19: 00 & 08: 00) averaged (127.7 ±109.8) m (range: 4 - 592 m,n= 210). The monkeys left their sleeping trees at 08: 00 in the morning. The mean distance the monkeys traveled between 08: 00 and 10: 00 [(182.4 ± 161.4) m, range: 8 - 1 443 m,n= 215], was significantly greater than the distance the monkeys traveled during the early morning hours(19: 00 - 08: 00, Studentt-test:t=4.14,df= 379,P<0.001). The distance traveled between 08: 00 and 10: 00 did not differ from the distance the monkeys traveled between 17: 00 and 19: 00 [(179.5 ±140.0) m; range: 12 - 1 045 m,n= 222; Studentt-test:t= 0.20,df= 422,P= 0.839].
On the 32 days in which the group reused the previous night’s sleeping site, the distance the monkeys traveled in the early morning (from 19: 00 to 08: 00), was (92.8 ± 81.4) m (range:13 - 409 m,n=32). This was significantly shorter than the distance the monkeys traveled from their GPS location at 19: 00 to 08: 00 on days in which they did not reuse the same sleeping site [(134.4 ± 113.4) m,n= 117;one-way ANOVA test:F32,117= 5.14,P= 0.024]. Finally, our results indicate that daily travel distances were significantly shorter when the monkeys reused the same sleeping area on consecutive nights [mean:(527.3 ± 391.4) m,n= 19] compared to using different sleeping sites on consecutive nights [mean:(883.7 ± 439.2) m,n= 42; Studentt-test:t=19.66,P< 0.001].
2.3 Time interval between re-visits of sleeping sites
On those occasions when a previously visited sleeping site was revisited on non-consecutive nights(interval: 2 - 298 days), the mean daily travel distance of the group was (678.4 ± 262.3) m (n= 42),which was significantly shorter than the mean daily travel distance on days in which they entered a new sleeping site [mean: (854.3 ± 426.5) m,n= 278; oneway ANOVA test:F1,276= 8.65,P= 0.004].
We next examined the time interval (i. e. number of days) between re-visits to the same sleeping site. No significant differences were found in the time intervals between revisits to those sleeping sites that were used on multiple occasions (2 - 9 times, oneway ANOVA test:F6,128= 0.48,P= 0.822). The mean time interval between revisits to the same sleeping site was (50.0 ± 6.1) days (range: 0 - 298 days,n= 61). We did not detect any significant correlation between the overall frequency of revisits to the same sleeping location when visited on two consecutive nights (Spearman’s correlation test: ρ = -0.13,n= 7,P= 0.786) compared to when visited on three consecutive nights (Spearman’s correlation test: ρ = -0.14,n= 7,P= 0.758).
2.4 Seasonality and climate impact on re-use patterns of sleeping sites
Seasonality did not influence the pattern of habitat exploitation and feeding site use. Overall, there were no differences in daily travel distance by season(one-way ANOVA test:F2,239= 1.36,P= 0.254) (Appendix B). In addition, the distance the monkeys traveled from 08: 00 to 10: 00 did not vary seasonally [winter distance: (178.5 ± 180.3) m,n= 114;spring distance: (207.6 ± 166.9) m,n= 48; and autumn distance: (167.9 ± 102.4) m,n= 53; one-way ANOVA test:F2,212=0.83,P= 0.437], and the distance the monkeys traveled from 17: 00 to 19: 00, did not vary across seasons [winter distance: (175.1 ±154.4) m,n= 110; spring distance: (189.0 ± 130.0) m,n= 58; and autumn distance: (178.1 ± 120.1) m,n=54; one-way ANOVA test:F2,219= 0.19,P= 0.828].In contrast, the distance the monkeys traveled from their sleeping site at 19: 00 the previous night to their location at 10: 00 was found to differ by season [winter distance: (471.9 ± 304.3) m,n= 105; spring distance: (633.3 ± 361.1) m,n= 38; and autumn distance: (478.1 ± 234.2) m,n= 53; one-way ANOVA test:F2,185= 4.183,P= 0.017]. The monkeys traveled greater distances between 19: 00 and 10: 00 in spring than in either winter or autumn (Turkey HSD test:Pwinter/spring= 0.016,Pautumn/winter= 0.998 andPspring/autumn=0.055).
During May, June, August and November of 2004, the study group did not revisit the same sleeping site on consecutive nights. However, sleeping locations were revisited on consecutive nights during all other months of the year. In Table 3, we provide data on daily travel distance, mean daily temperature, and general weather conditions on days in which the monkeys reused the same sleeping site on two or more consecutive nights. Sixteen of those 19 cases occurred during the winter (December - April). Two of the remaining three cases occurred in autumnn (August to October), and one in the spring (May - June).Winter included the highest frequency of consecutive nightly reuse of the same sleeping sites (Binomial test:n= 19,P= 0.004). However, factors such as weather (Spearman’s correlation test: ρ = -0.06,n=41,P= 0.702), ambient temperature (Spearman’s correlation test: ρ = -0.18,n= 41,P= 0.248), mean ambient temperature (Spearman’s correlation test:ρ = 0.06,n= 41,P= 0.742), season (Spearman’s correlation test: ρ = 0.22,n= 41,P= 0.169) and daily travel distance (Spearman’s correlation test: ρ =-0.16,n= 41,P= 0.320) were not found to effect whether the monkeys reused the same sleeping area on consecutive nights.
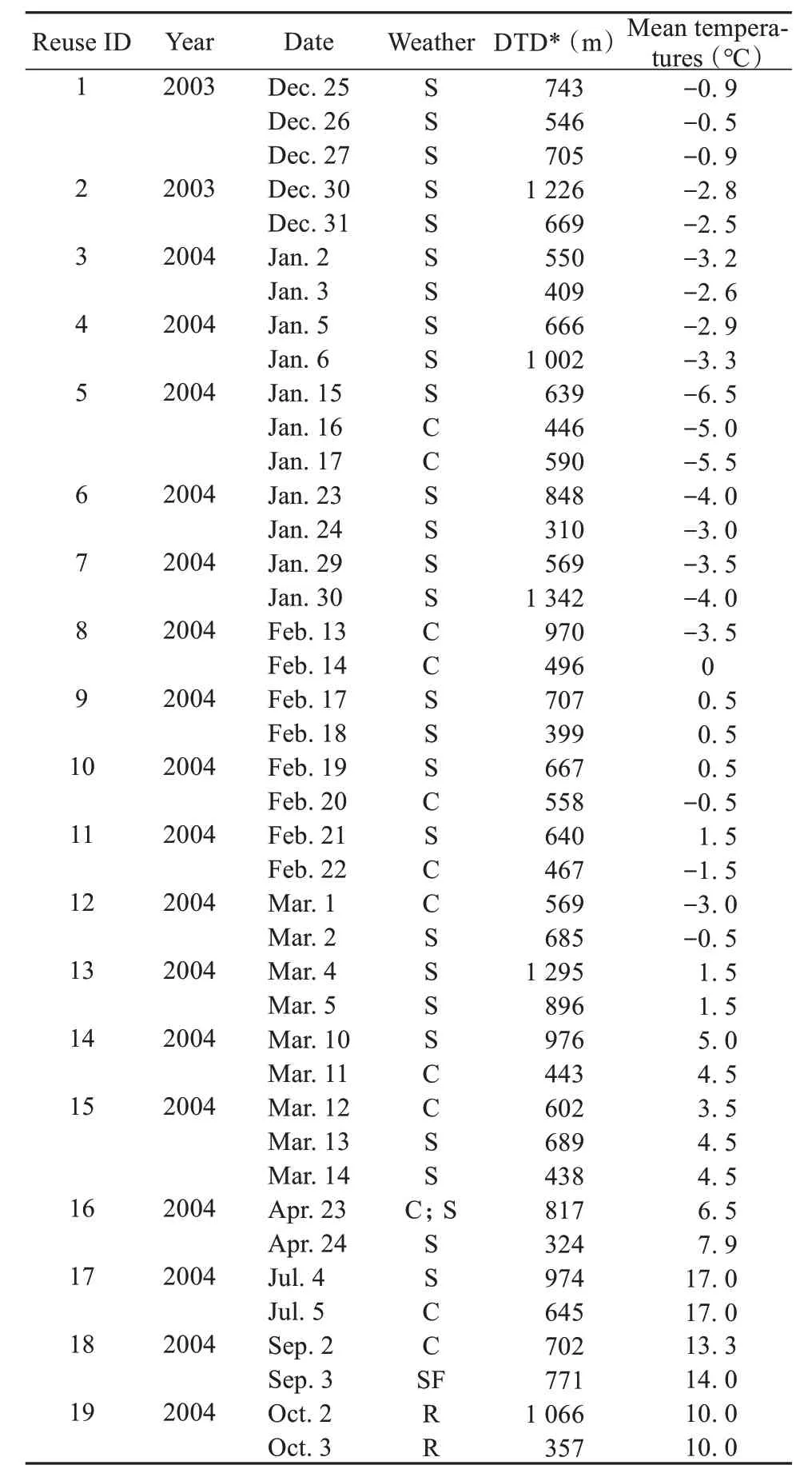
Table 3 Weather and daily travel distance on days in which the monkeys reused the same sleeping site on consecutive nights
3 Discussion
Several researchers have studied sleeping site use by Yunnan snub-nosed monkeys since 2004 (Liu and Zhao, 2004; Cuiet al., 2006; Liet al., 2006,2013; Xianget al., 2011), however, across studies no clear pattern has emerged. Given the conditions of our field site (high elevation, cold winter temperatures complex terrain and dense vegetation), we used a GPS collar to track one adult male, and assumed his pattern of sleeping site use was consistent with other members of the study group. In total, we obtained 1 274 GPS fixes including 272 nighttime locations from which to analyze ranging behavior (Renet al.,2008, 2009a, 2009b). Combined with ground truthing of the vegetation and location of sleeping sites, we examined Yunnan snub-nosed monkey patterns of sleeping site use over an 11-month period.
Our results indicate that Yunnan snub-nosed monkeys rarely re-used on consecutive nights for hygiene, safety, and avoidance of food depletion. However, when the same sleeping area was used on consecutive nights, daily path length was decreased significantly. We found that the monkeys used Himalayan hemlock (Tsuga dumosa) as sleeping trees in greater frequency than their distribution in the home range.
3.1 Sleeping trees and sleeping sites in different forest types
The distribution of tree species across the range of Yunnan snub-nosed monkeys is influenced by several factors including climate, geography, altitude,soil type, longitude, and latitude. The trees used by Yunnan snub-nosed monkeys as sleeping sites are reported to vary from site to site (Cuiet al., 2006; Liet al., 2006, 2013; Xianget al., 2011), and each of those species are present in the home range of our studied group (Jinsichang, Yunnan, China). At our study site, Lijiang spruce (3 100 - 3 700 m asl.)and Himalayan hemlock (2 700 - 3 500 m asl.) dominate the landscape, whereas George’s fir is common at elevations between 3 800 meters to 3 900 meters (Yang,2000). These tree species represent the principal sleeping sites used by our group of Yunnan snubnosed monkeys. Himalayan hemlocks, George’s fir,and Lijiang spruce trees were likely used as sleeping sites because they are large in height and diameter, numerous, serve as a wind break, and are found in close proximity to trees used by the snub-nosed monkeys as major feeding sites (Liu and Zhao, 2004; Liet al.,2006; Cuiet al., 2006; Xianget al., 2011). Himalayan hemlocks have an extensive distribution at our field site, however, they are often illegally logged as their wood has high commercial value. Protecting this tree species is critical to the survival of the Yunnan snub-nosed monkeys.
These primary tree species used by the monkeys as sleeping sites were not used as a food source (Liu and Zhao, 2004; Cuiet al., 2006; Xianget al., 2011;this study). During foraging the monkeys showed a preference to feed in mixed forest and deciduous broad-leaved forest. This could reflect several factors, including that the monkeys usually excrete large amounts of urine and feces under a sleeping site (Cuiet al., 2006; Xianget al., 2010, 2011; Liet al.,2013). Several other primate species, including gibbons (Hylobates lar, Reichard, 1998;Nomascus nasutus, Feiet al., 2012), also avoid sleeping in their common feeding sites.
3.2 Patterns of sleeping site use in snub-nosed monkeys
Yunnan snub-nosed monkeys showed three patterns of sleeping site use: trees only used once during the study period, trees occasionally reused but not on consecutive nights, and trees reused on consecutive nights. This pattern differs across wild groups of Yunnan snub-nosed monkeys at different field sites (Table 2). In our study, the consecutive reuse of sleeping sites was only 7.0%. Similarly, the Honglaxueshan group of Yunnan snub-nosed monkeys exhibited these same three patterns of sleeping site use (Xianget al.,2011) and reused sleeping sites on 8.9% of nights. In other studies, reuse of sleeping sites on consecutive nights was not observed (Cuiet al., 2006; Liet al.,2006).
Similar findings have also been reported in other primate species. For example, northern pigtailed macaques reused sleeping sites (n= 107) on 14.0% of nights but never on consecutive nights (Gazagneet al., 2020). In white-handed gibbons (Hylobates lar)sleeping sites were reused on 24.7% (n= 148) of nights (Reichard, 1998). In the case of saddleback tamarins (Leontocebus nigricollis) and mustached tamarins (Saguinus mystax) sleeping sites were reused on only 2.2% - 8.6% nights (Smithet al., 2007).Similarly, bonnet macaques (Macaca radiata, Ramakrishnan and Coss, 2001) and proboscis monkeys (Nasalis larvatus: Feilen and Marshall, 2014) are reported to rarely revisit the same sleeping sites on consecutive nights.
Yunnan snub-nosed monkeys, on average, revisited the same sleeping site once every 50 days. This suggests a pattern in which a large multilevel society of some 200 snub-nosed monkeys is likely to deplete local feeding sites requiring individuals to travel to other areas of their home range to feed and to locate nearby resting sites. The efficient use of such a foraging and ranging pattern necessitates the ability to (1)remember their recent foraging activities and locations of feeding and resting sites, (2) remember the time interval since they last visited a set of feeding sites and sleeping sites, and (3) avoid returning to those areas until they have some expectations that resource availability in that area has sufficiently increased. Several studies of nonhuman primates indicate that individuals actively store, recall, and access information on time, space, and quantity (Garber,2000, in press; Bicca-Marques, 2004).
3.3 Seasonality, climate and other factors impact on patterns of sleeping site use
There are three seasons, spring, autumnn and winter, in the habitat of Yunnan snub-nosed monkeys. Daily travel distances of our study group were not influenced by season but are influenced by daily ambient temperature (Renet al., 2009a). We found that although the monkeys reused the same sleeping site on consecutive nights more during winter, overall the distance traveled from the previous night’s sleeping site to their first feeding site in the morning did not vary seasonally. Thus, despite living at high elevations (> 3 000 m asl.) and experiencing a long and cold winter season (November - April), Yunnan snubnosed monkeys actively select sleeping sites that are in close proximity to the next morning feeding sites throughout the year. Their increased reuse of feeding sites in the winter suggests that certain winter feeding sites may be more productive or exhibit a more clumped distribution than during other times of the year. This is consistent with a recent study on golden snub-nosed monkeys (R.roxellana): during the winter, individuals experienced an 80% increase in their intake of metabolizable energy (from 399 kJ/mbm in spring to 721.5 kJ/mbm in winter (Guoet al., 2018).This difference was the result of consuming a winter diet higher in lipids and carbohydrates, which offset the increased costs of remaining thermoneutral during the long and cold winter (Guoet al., 2018), rather than in response to an increase in daily travel distance. In Yunnan snub-nosed monkeys, re-used the same sleeping site on consecutive nights was found to reduce daily travel distance (from 883.7 m to 527.3 m),which may represent a behavioral strategy to reduce winter energy expenditure.
Liet al. (2013) reported that in Yunnan snubnosed monkeys, 44% of sleeping sites were located in the vicinity of the group’s late afternoon feeding site.Sleeping sites of gray snub-nosed monkeys (R.brelichi) also were located close to their late afternoon feeding sites (Xianget al., 2010). Based on the fact that the distance traveled between 19: 00 and 08: 00 was only 128 m, it appears that monkeys in our study group exhibited a similar pattern. Moreover, by sleeping in relatively close proximity to their late afternoon feeding site, the monkeys can return the next morning to consume any remaining food items.In doing so, and given there were 180 monkeys in their group, they likely deplete local feeding sites and avoid returning to the area for several weeks or months (time interval = 50.0 days). Given the monkey’s large home range (17.8 km2), this sleeping/foraging strategy allowed them to explore much of their habitat over the course of each year. Thus, sleeping site used in Yunnan snub-nosed monkeys is likely determined by their proximity to major feeding sites,similarly as that in pigtailed macaques (Gazagneet al., 2020).
In conclusion, based on GPS technology and ground truthing of the vegetation associated with sleeping site use in a free-ranging group of Yunnan snub-nosed monkeys, we identified 131 sleeping sites used over an 11-month period. Most of sleeping sites were used only once, some were reused but not on consecutive nights and a small number were reused on consecutive nights. The most preferred sleeping tree is Himalayan hemlocks. Availability of productive feeding sites and avoidance of depleting food resources in the vicinity of sleeping sites appear to be the primary factors influencing daily ranging patterns and selection of sleeping sites. Given the results of this study, future investigation of Yunnan snub-nosed monkeys should carefully monitor the effects of daily,monthly and seasonal changes in food distribution and availability, and monthly and seasonal differences in nutrient intake in order to better understand how patterns of range use and foraging strategies enable this Endangered primate species to survive in high elevation human-modified landscapes. This information will contribute to their conservation efforts, especially given that the number of remaining wild individuals is only 3 500 individuals.

Appendix A Distances between GPS fixes of Yunnan snub-nosed monkeys at Jinsichang, Yunnan, China

Appendix C Map of 272 sleeping GPS fixes of the collared male at Jinsichang, Yunnan, China
