Anti-vascular endothelial growth factor drugs combined with laser photocoagulation maintain retinal ganglion cell integrity in patients with diabetic macular edema: study protocol for a prospective,non-randomized,controlled clinical trial
2024-02-11XiangjunLiChunyanLiHaiHuangDanBaiJingyiWangAnqiChenYuGongYingLeng
Xiangjun Li ,Chunyan Li ,Hai Huang ,Dan Bai ,Jingyi Wang ,Anqi Chen ,Yu Gong,Ying Leng,
Abstract The integrity of retinal ganglion cells is tightly associated with diabetic macular degeneration that leads to damage and death of retinal ganglion cells,affecting vision.The major clinical treatments for diabetic macular edema are anti-vascular endothelial growth factor drugs and laser photocoagulation.However,although the macular thickness can be normalized with each of these two therapies used alone,the vision does not improve in many patients.This might result from the incomplete recovery of retinal ganglion cell injury.Therefore,a prospective,non-randomized,controlled clinical trial was designed to investigate the effect of anti-vascular endothelial growth factor drugs combined with laser photocoagulation on the integrity of retinal ganglion cells in patients with diabetic macular edema and its relationship with vision recovery.In this trial,150 patients with diabetic macular edema will be equally divided into three groups according to therapeutic methods,followed by treatment with anti-vascular endothelial growth factor drugs,laser photocoagulation therapy,and their combination.All patients will be followed up for 12 months.The primary outcome measure is retinal ganglion cellinner plexiform layer thickness at 12 months after treatment.The secondary outcome measures include retinal ganglion cell-inner plexiform layer thickness before and 1,3,6,and 9 months after treatment,retinal nerve fiber layer thickness,best-corrected visual acuity,macular area thickness,and choroidal thickness before and 1,3,6,9,and 12 months after treatment.Safety measure is the incidence of adverse events at 1,3,6,9,and 12 months after treatment.The study protocol hopes to validate the better efficacy and safety of the combined treatment in patients with diabetic macula compared with the other two monotherapies alone during the 12-month follow-up period.The trial is designed to focus on clarifying the time-effect relationship between imaging measures related to the integrity of retinal ganglion cells and best-corrected visual acuity.The trial protocol was approved by the Medical Ethics Committee of the Affiliated Hospital of Beihua University with approval No.(2023)(26) on April 25,2023,and was registered with the Chinese Clinical Trial Registry (registration number: ChiCTR2300072478,June 14,2023,protocol version: 2.0).
Key Words: choroidal thickness;diabetic macular edema;laser photocoagulation;retinal ganglion cellinner plexiform layer thickness;retinal ganglion cells;retinal nerve fiber layer thickness;thickness of the macular area;vascular endothelial growth factor;visual acuity
Introduction
Diabetic macular edema is a local pathological manifestation of diabetic retinopathy (Arevalo and Garcia-Amaris,2009;Romero-Aroca et al.,2016;Sun et al.,2019;Li et al.,2021;Gurreri and Pazzaglia,2021;Zhang et al.,2022).Long-term macular edema can lead to severe vision loss (Browning et al.,2018;Baker et al.,2019).Diabetic macular edema has a complex pathogenesis involving various factors,including neuroinflammation,metabolic disorders,slowed blood flow,vascular leakage,and retinal cell apoptosis (Daruich et al.,2018;Antonetti et al.,2021;Zhang et al.,2022).Neuroinflammation is one of the key factors in diabetic macular edema development and progression (Frizziero et al.,2021;Tang et al.,2023).A previous study by this group confirmed that advanced glycation end-products (AGEs) increase the permeability of calcium channels through the upregulation of vascular endothelial growth factor (VEGF) in the diabetic rat’s retina,which is one of the pathogenic mechanisms of diabetic retinopathy (Zhang et al.,2018).
Neuronal cell degeneration disrupts the blood-retinal barrier and increases vascular permeability,leading to diabetic macular edema (Verma et al.,2012).Retinal ganglion cells transmit visual information to the visual cortex;therefore,when the integrity of these cells is impaired,visual dysfunction occurs (Mead and Tomarev,2022;Gan et al.,2023).The ganglion cell layer and inner plexiform layer consist of nuclei and dendrites of retinal ganglion cells,respectively.The retinal nerve fiber layer contains the axons of retinal ganglion cells,and the retinal ganglion cell-inner plexiform layer thickness is diffusely thinned in neuronal cell degeneration.Axons are also affected,resulting in thinning of the retinal nerve fiber layer (Barber,2015).The integrity of the retinal ganglion cells is essential to maintain visual function (Dimitriu et al.,2008;Jehle et al.,2010;Ren et al.,2012;Feng et al.,2016;Gu et al.,2022).Many patients with diabetic macular edema do not have complete vision recovery after treatment,although the macular thickness becomes normalized (Li,2012).Thus,assessing the thickness of the retinal nerve fiber layer and macular ganglion cell-internal plexiform layer (GCIPL) at the time of receding diabetic macular edema is considered meaningful (Ge,2020).
Anti-VEGF drugs are commonly used in the treatment of diabetic macular edema,improving visual acuity and retinopathy by inhibiting VEGF activity and reducing vascular leakage and edema in the macula (Banaee et al.,2017;Liu and Wu,2021).The latest study showed that VEGF is not only involved in retinal neovascularization but also in neural damage and inflammatory responses in the macula (Fursova et al.,2022).The expression of VEGF is significantly increased in the retina and macula of diabetic patients,which aggravates neurotoxicity and inflammation,resulting in the loss of retinal function in the macula.Currently,anti-VEGF therapy is applied in most patients with central diabetic macular edema,but the therapeutic effect is still not satisfactory in 40-50% of patients (Zhu et al.,2023).Retinal laser photocoagulation remains the first-line treatment for diabetic macular edema without fovea involvement (Falavarjani et al.,2010;Zhang et al.,2021),which provides better vision improvement than Western therapies,such as intravitreal tretinoin.A previous study confirmed that the combination of anti-VEGF drugs and laser photocoagulation therapy has better outcomes in patients with diabetic macular edema,which is superior to laser photocoagulation alone in reducing macular edema and improving visual acuity (Xi and Fan,2017).However,there are fewer studies addressing the association between the integrity of retinal ganglion cells and vision recovery after the combined treatment.
Study objective
The trial will be conducted in patients with diabetic macular edema undergoing a combination of anti-VEGF drugs and laser photocoagulation.Retinal topography and enhanced depth imaging-optical coherence tomography (OCT) will be used to accurately monitor retinal nerve fiber layer thickness,retinal ganglion cells-inner plexiform layer thickness,retinal macular thickness,and choroidal thickness and analyze visual acuity levels in patients with diabetic macular edema,thereby confirming the efficacy and safety of the combined therapy and clarifying the association between retinal ganglion cell integrity and vision recovery during the follow-up.
Methods/Design
Study design
This is a three-arm prospective,non-randomized,controlled clinical trial.It is planned to include 150 patients with diabetic macular edema who will be divided into three groups according to treatment methods: 50 cases in the drug injection group,50 cases in the laser group,and 50 cases in the combined treatment group.This protocol attempts to confirm the superior efficacy and safety of the combined treatment in patients with diabetic macular edema compared with the other two monotherapies alone and clarify the time-effect relationship between the imaging measures related to retinal ganglion cell integrity and best-corrected visual acuity to allow accurate monitoring of the treatment effect in patients with diabetic macular edema using the above-mentioned non-invasive imaging indicators.
The study protocol was approved by the Medical Ethics Committee of the Affiliated Hospital of Beihua University with approval No.of (2023) 26 on April 25,2023.All patients signed the informed consent form.The study procedures will follow the provisions of theDeclaration of Helsinki(World Medical Association,as amended 2013) and relevant provisions of Age-Related Macular Degeneration Preferred Practice Pattern@formulated by the American Academy of Ophthalmology (Flaxel et al.,2020).The Independent Data and Safety Monitoring Board will audit non-blinded safety and efficacy data from this trial every 3 months.The timing of outcome measurements is shown inTable 1,and the trial flow chart is shown inFigure 1.The trial protocol was written using the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Checklist (Additional file 1;Chan et al.,2013).

Figure 1|Trial flow chart.
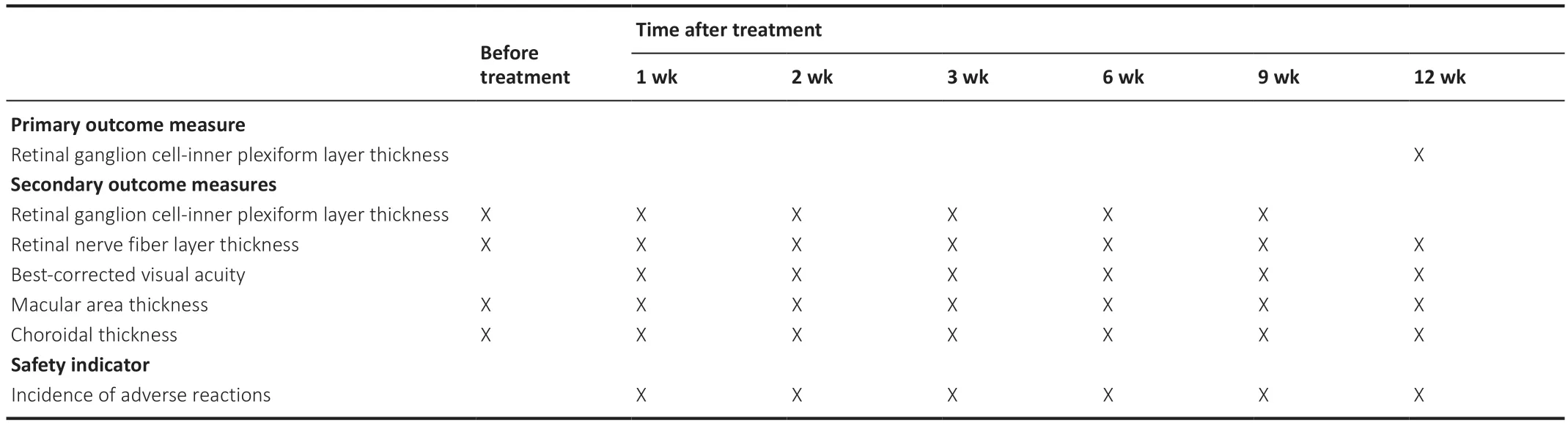
Table 1|Timing of outcome measurements
Recruitment
Patients with diabetic macular degeneration will be mainly recruited from the Affiliated Hospital of Beihua University,China.The recruitment information will be publicized using leaflets advertised to inpatients and posted on hospital bulletin boards.The preferential conditions for participation in the trial are a reduced fee for vision testing during the follow-up period and professional follow-up.Interested patients or relatives will contact the project manager via their attending physicians or by telephone,e-mail,or WeChat.After providing written informed consent,those interested in participating in the trial will be screened by their attending physicians who will be responsible for collecting patients’ medical history,symptoms,signs,imaging,and pathological data and monitoring patients’ data.
Inclusion and exclusion criteria
Inclusion criteria
(1) Type 1 or type 2 diabetic patients,as confirmed by the Standards of Medical Care in Diabetes—2023 issued by the American Diabetes Association,and patients with diabetic macular edema,as confirmed by fundus examination,fundus angiography,and OCT examination;
(2) Patients with monocular lesions;
(3) Patients with a central macular thickness ≥ 250 µm based on OCT;
(4) Best-corrected visual acuity of 20-75 letters in the affected eye as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS).
Exclusion criteria
(1) Patients undergoing any intraocular surgery (such as vitreous surgery and anti-VEGF treatment),retinal or macular laser photocoagulation,within 6 months prior to admission;
(2) Patients with other fundus diseases or active ocular infections (especially those with retinal and macular diseases,abnormalities in or damage to the central structures of the macula) or a past medical history of those;
(3) Presence of iris rubeosis and neovascular glaucoma;
(4) Patients with severe systemic diseases,such as severe cardiovascular,cerebrovascular,and renal insufficiency;
(5) Patients with uveitis,glaucoma,and high myopia above -6.0 D;
(6) Patients with mental disorders;
(7) Patients with language communication disorders.
Withdrawal criteria
(1) The subject develops any clinical adverse event,abnormal test results,or other medical conditions that result in the possibility that the subject might no longer benefit from continued treatment;
(2) The subject is not suitable for further treatment due to changes in his/her disease condition;
(3) The subject voluntarily requests suspension of the treatment;
(4) Significant protocol deviations such as non-compliance or non-adherence after enrollment;
(5) Other reasons due to which the investigators believe that the subject is unable to continue treatment.
Termination criteria
The principal investigator has the right to terminate the study at any time,and the reasons for discontinuing the study include but are not limited to possible harm to the relevant rights and interests of a certain number of subjects caused by continuing the study.
Sample size estimation
Based on pretest results,clinical experience in our department,and previous literature (Ge,2020),at 12 months after treatment,when the retinal ganglion cell-inner plexiform layer thickness reaches 80 µm and 70 µm in combined treatment and laser groups,respectively,the difference between the mean values of the groups is estimated to be approximately 10 µm,and the standard deviation of the two groups is estimated to be 12.0 µm.Based on a significant level ofα=0.05,β≤ 0.1,and power=90%,a sample size of 32 patients (32 eyes) is required for combined treatment and laser groups (Glueck,2008).Assuming a dropout of 25%,the initial sample size of at least 40 patients for combined treatment and laser groups is required.Since the sample size follows the 1:1:1 matching principle and there are three groups in the trial,a minimum of 40 patients per group is required.The final decision was made to include 50 patients (50 eyes) in each group,for a total of 150 patients (150 eyes) in the three groups for this clinical trial,to ensure an adequate sample size for the trial.
Blinding
The trial protocol will be unknown to the outcome indicator collectors,outcome assessors,and statistical analysts.
Interventions
A total of 150 eyes of 150 patients with diabetic macular edema after fundus examination,fundus angiography,and OCT will be enrolled and divided into three groups.
Drug injection group
Intraocular injection of conbercept (0.5 mg,0.05 mL),an anti-VEGF fusion protein injection,will be performed once a month.Regarding the method,antibiotic eye drops are applied to the eyes 3 days before surgery to prevent infection.Pre-operative ocular ultrasound and dilated fundus examination are performed to determine whether there is a proliferative membrane in the retina that can cause retinal detachment,and the injection site should not be chosen at the proliferative membrane.The injection site is selected at 3.5 mm away from the corneal edge for the eyes without a lens and 4.0 mm away from the corneal edge for the eyes with the lens.The needle tip is beveled toward the direction of the optic nerve when injection,and when removing the needle,the pinhole is clamped using microsurgery forceps for 5 seconds.Injections will be given as needed after 3 consecutive months.The indication for re-injection is the central macular thickness >250 µm.
Laser group
Patients will undergo pan-retinal photocoagulation using a 532 nm laser (Quantel Medical,France) in four sessions at an interval of 7 days.Pan-retinal photocoagulation will be performed as follows: green light at a wavelength of 532 nm is applied to disseminate photocoagulation from beyond the vascular arch of the macula to the periphery of the four quadrants,completed in 3-4 sessions,with a spot diameter of 200-300 µm,exposure time of 0.20-0.25 seconds,energy of 200-300 mW,class II-III spot,and 2000-2200 spot counts.All procedures will be performed by the same physician.
Combined treatment group
Intraocular injection of conbercept (0.5 mg,0.05 mL) will be given once a month for 3 consecutive months,during which pan-retinal photocoagulation will be performed 3-4 times in total.
All groups will be followed up for 1 year using the same equipment and methods before and after treatment to perform ophthalmologic examinations.Changes in outcome measures will be recorded and compared between the three groups before and 1,3,6,9,and 12 months after treatment.Fluorescein fundus angiography (FFA) will be reviewed at 3 and 6 months after treatment to assess fundus vascularization.
Outcome measures
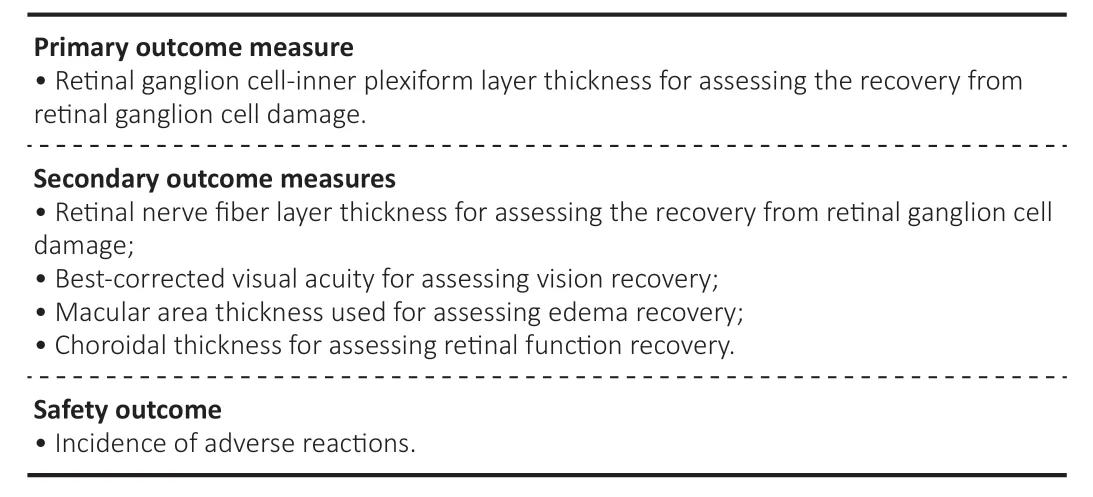
Primary outcome measure
OCT can measure the retinal ganglion cell-inner plexiform layer thickness (Merten et al.,2020;Yu et al.,2022),which is reproducible,non-invasive,and easy to perform.By shining light onto the retina,OCT can produce high-resolution images of the retina to calculate the retinal ganglion cellinner plexiform layer thickness.The testing instrument is a SPECTRALIS HRA (Heidelberg,Germany).The observation time frame is 12 months after treatment.
Secondary outcome measures
(1) Regarding the retinal ganglion cell-inner plexiform layer thickness,the details are as provided above,with observations before and 1,3,6,and 9 months after treatment.
(2) Retinal nerve fiber layer thickness is measured using OCT,a non-invasive examination method.The principle of OCT is to shine light onto the fundus,which is partially reflected to form a moving lens,finally generating retinal images by imaging software.The test instrument is the SPECTRALIS HRA.Based on the retinal images,the retinal nerve fiber layer thickness can be calculated by analyzing the intensity and location of the reflections in a specific area (in a Glaucoma mode,CircleScan scans the optic nerve).OCT has a high accuracy for the measurement of retinal nerve fiber layer thickness and can detect minor injuries of the retinal nerve fiber layer (Shin and Cho,2011;Yeo and Kim,2020).
Observation will be held before and 1,3,6,9,and 12 months after treatment.(3) Changes in the best-corrected visual acuity will be detected before and at different observation time points after treatment.Regarding optometry,the optometrist uses different lights and lenses to test the diopter of the patient’s eyes and helps prescribe a pair of corrective lenses that enable the patient to obtain the best-corrected visual acuity.International Vision Chart (Yuejin Medical Optical Instrument Factory,Shanghai,China) will be used in the trial.Observation will be held before and 1,3,6,9,and 12 months after treatment.(4) Macular thickness procedure will be performed as follows.During preparation,patients need to receive anesthetic medication for the eye to ensure that the eye stops moving and remains stable.Patients need to fix their heads on the testing instrument to ensure the accuracy and stability of the examination.The macular area is scanned in EDI mode by OCT using the SPECTRALIS HRA.Regarding positioning,the testing instrument is positioned on the macular fovea.Gazing for a certain time,as in fundus photography,helps measure a strictly defined area of the retina.Regarding lens scanning,the testing instrument emits a harmless laser beam to scan the macular area.The laser beam enters the eye and bounces back,producing a reversedraped image.Regarding data capture,once the scan is complete,the testing instrument will collect and save all the data and generate a digital image.This image will show the various features and layers of the macular area (such as retinal layers and blood vessels) and calculate data such as thickness and pole position.
Observation will be held before and 1,3,6,9,and 12 months after treatment.(5) Choroidal thickness will be assessed as follows.The macular retinal topography technique will be applied to observe the changes in macular thickness before and at various time points after treatment.Scans are taken in EDI mode using the SPECTRALIS HRA.The physician or technical staffwill adjust the lens position and focal length of the testing instrument to obtain the best image quality,depending on the patient’s eye size,deformation,and pigmentation.Patients are required to gaze at the target.The instrument emits a weak laser beam to scan the choroid on the order of microns.The scan usually takes a few seconds to a few minutes.Once the scan is complete,a digital image of the retina and choroid will be produced to record the choroidal thickness.
Observation will be held before and 1,3,6,9,and 12 months after treatment.
Safety indicators
The incidence of adverse reactions will be calculated as follows:
incidence of adverse reactions=(number of cases with adverse reactions/total cases) x 100%.Adverse reactions mainly include ocular safety outcomes: bleeding at the injection site,conjunctival congestion,increased intraocular pressure,conjunctivitis,vitreous opacity,reduced visual acuity,anterior chamber flashes,ocular inflammation,cataracts,corneal epithelial defects,ocular tingling,and visual distortion.Observation will be held at 1,3,6,9,and 12 months after treatment.
Assessment of adverse events
Definition of an adverse eventAn adverse event refers to any adverse medical event that occurs after a clinical trial subject undergoes a drug or laser treatment,but this event is not necessary to have a causal relationship with the treatment.The adverse event can be any unexpected,unfavorable symptom,sign,disease,or abnormal test result,whether or not it is related to the material.
Criteria for determining adverse events
Adverse events include all unanticipated clinical manifestations.As long as these events occur after surgery,they should be reported as adverse events,regardless of whether they are related to medication or laser treatment.
(1) Any uncomfortable reactions reported by the subject or abnormal changes in the laboratory test indicators during treatment should be recorded faithfully.The severity,duration,management,and turnover of any adverse event should also be recorded.
(2) The clinician should also determine whether the adverse event is related to the drug or laser treatment.The causality between the adverse event and target drugs is judged as follows: definitely relevant,probably relevant,probably unrelated,definitely unrelated,and undetermined.“Definitely relevant,” “probably relevant,” and “undetermined” are all classified as adverse events.
(3) The incidence of adverse events is calculated using the total of these three as the numerator and the total number of subjects as the denominator.
Recording and reporting adverse events
Any adverse event that occurs in the subjects should be recorded in detail,including a description of adverse events and all associated symptoms or signs,time of occurrence,severity,duration,measures taken,and final outcomes (disappearance,remission,and persistence).
Statistical analysis
Principal analysis and sensitivity analysis
The principal analysis will follow the intention-to-treat principle and be conducted according to the treatment method,including all eyes enrolled in the trial.The value of vision loss at follow-up will be evaluated using the Markov Chain Monte Carlo method (Jhaveri et al.,2022).
Data description
The trial results will be analyzed based on the basic principles of intentional analysis,and no interim analysis will be performed.Statistical analyses will be completed by statistical experts applying SPSS 24.0 software (SPSS,IBM,Armonk,NY,USA).The measurement data will be statistically described as means,standard deviations,medians,minimum and maximum values,and interquartile range,while the count data will be statistically described as the number of cases and percentages.
Analytical methods
Differences in the incidence of adverse events between groups will be compared using the Pearson’s chi-squared test or Fisher’s exact probability method.Differences in retinal nerve fiber layer thickness,retinal ganglion cell-inner plexiform layer thickness,best-corrected visual acuity,macular thickness,and choroidal thickness between groups at the same follow-up time will be compared using one-way analysis of variance combined with the Bonferronipost hoctest (data normally distributed) or the Kruskal-WallisHtest (data non-normally distributed).Correlations between outcome indicators will be analyzed using Pearson correlation analysis (data normally distributed) or Spearman correlation analysis (data non-normally distributed).Measurement data at different follow-up time points will be analyzed using the mixed model for repeated measures.Repeated measures one-way analysis of variance and one-way analysis of variance of covariance will be performed for intergroup comparison at different follow-up time points to observe group and time effects on outcomes and analyze differences in main and interaction effects.Pvalues and two-sided 95% confidence intervals (CIs) are required for intergroup comparison.Pre-determined subgroup analyses will be performed only if there are significant differences in the primary outcome between groups.
Data set
All demographic data at baseline will be analyzed based on the full analysis set (FAS),and a safety assessment will be conducted on the safety set (SS).The therapeutic efficacy indexes of the trial will be performed for a full analysis data set and per-protocol set analyses.For the per-protocol population,the point estimates and 95% CIs for the effective measures of each treatment group will be calculated,and differences in the therapeutic efficacy measures among the groups will be calculated.
Data collection and management
Data collection
Patient records as original files will be completely preserved.The data in any case report form will be from and consistent with the original files,and the investigator must ensure that all data are true,complete,and accurate.All items on the clinical trial record form will be filled in,and no blank items or missing items will be allowed (no records should be marked with a slash).Corrections will be marked by underlining,with the revised data marked next to it and explaining the reason,and signed and dated by the recorder.The original record will not be erased or overwritten.
Data management
All raw data,files,laboratory reports,summary reports,and the results of clinical trials will be input into the Electronic Data Capture (EDC) system and preserved in archives in an orderly manner;therefore,all raw data,laboratory reports,trial protocols,and summary reports can be quickly retrieved and easily obtained.A special person will be appointed to manage the archives room.No entry to the archives room without permission will be allowed.Data kept or referred to in the archives will be indexed for easy retrieval.To protect the subject’s privacy,the subject’s name will not appear on the case report form.Furthermore,the investigator will identify and record the subject’s code.
Data monitoring
The inspector will review whether the trial follows relevant regulations,Good Clinical Practice and trial protocol,whether all case report forms are completed correctly,completely,and consistent with the original files,and whether there are not any data errors or omissions.The inspector will crosscheck the contents of the electronic case report form and original files to ensure that data in the electronic case report form are consistent with the original data,which is also called source data verification.
Protocol violation
All requirements specified in the study protocol will be strictly implemented.Any intentional or unintentional deviation from or violation of the study protocol and Good Clinical Practice principles can be classified as a deviation from or violation of the protocol.During the inspection,if the inspector finds deviation from the plan,the investigator or the inspector will fill in the record of violation to record the time of discovery,the time and process of the event,the reasons,and corresponding measures in detail.Then,the record will be signed by the investigator and submitted to the Ethics Committee.
Data release
All articles and reports related to the study will be approved by the principal investigator before publication.
Ethical considerations
Ethical approval
The trial will comply with the relevant requirements of theDeclaration of Helsinki(2008 version).The study protocol was approved by the Medical Ethics Committee of the Affiliated Hospital of Beihua University.During the trial,any modifications to the study protocol will be reported to the Ethics Committee and recorded.It is the responsibility of the investigator to submit interim reports regularly according to the relevant requirements of the Ethics Committee,and the Ethics Committee will be informed of the completion of the trial.
Informed consent
Patients and their relatives recruited for the trial will participate in the trial on a voluntary basis.All patients will be fully informed of the trial procedure and sign the informed consent.Before the clinical trial begins,the study protocol will follow the principle of maximizing the protection of subjects’ rights and interests,safety,and health.The study protocol will not be implemented until it is reviewed and approved by the Ethics Committee.
Protocol amendments
No one other than the principal investigator will be able to make changes to the protocol.Any necessary modifications to the protocol will be made in the form of a protocol amendment,which will be submitted to the Ethics Committee for approval or record after obtaining the signature of the principal investigator.Meanwhile,details of previous modifications will be included in the protocol.
Risks and precautionsDrug therapy,laser photocoagulation therapy,and imaging examinations involved in the trial have been widely used in clinical practice.Movement monitoring equipment and instruments used are mature products with sufficient data on their safety.The related risks will not be increased because of the trial.Patients will be asked to immediately inform the patient’s attending physician if the patient experiences any symptoms of discomfort during the trial,and the physician will judge or manage the discomfort as soon as possible.This trial will not disclose the privacy of patients and will not promote any home-based rehabilitation products to patients.If functional improvement after treatment is not entirely consistent with the patient’s expectations,the trial will respond to different functional recoveries with appropriate follow-up treatments.
Compensation
The test fee will be free for the subjects.Furthermore,the relevant vision evaluation (such as visual acuity test and fundus examination) will also be free,and no additional fees will be required.Accordingly,the subjects will receive no additional compensation for their participation in the trial.In the event of a serious adverse event related to the trial,the sponsor will pay subsequent medical expenses.In the event of hospitalization due to a serious adverse event,the sponsor will also provide reasonable compensation for nutrition,wages,and bonuses lost from work.However,treatments and tests for other conditions will not be compensated.
Confidentiality
All information about subjects will be kept strictly confidential during the study.If necessary,relevant administrative departments,ethics committees,or sponsors may have the right to access the subjects’ data according to regulations.However,the subjects’ data will not be used for any other purposes or shared with other groups without permission.Any information transmitted electronically will be renamed to ensure confidentiality.Information on all computers will be password-protected.The study results may be presented through publications in peer-reviewed academic journals or at medical conferences,but no personally identifiable data will be used.All test results (including personal data and laboratory documents) that appear in the original medical records will be kept completely confidential to the extent permitted by law.
Auditing
The Data Monitoring Committee includes experts engaged in neurology,ophthalmology,epidemiology,clinical imaging,clinical pathology,clinical trial management,statistics,and ethics.Clinical inspectors visit the test unit regularly or as needed to conduct clinical quality monitoring.The principal investigator will have a degree in clinical medicine,pharmacy,imaging,or a related field and necessary professional training.Personnel familiar with the standards of clinical trial management and relevant laws and regulations will act as inspectors to monitor and report the progress of the trial,verify the data,and ensure that the rights and interests of the subjects are protected,the trial records and reported data are accurate and complete,and the trial conforms to the approved protocol and relevant laws and regulations.Any unexpected serious adverse events or outcomes will be discussed by the Management Committee.In addition,the Management Committee will monitor recruitment,treatment,consumption rates,and any concerns related to the trial.All drugs and devices will be preserved according to the Good Clinical Practice of China and the Management Measures for Clinical Application of Medical Technology issued by the National Health Commission of China in 2018.
Dissemination and protocol revision
Major trial results will be submitted for publication in an international peerreviewed journal,whether the results are positive,negative,or inconclusive for the study hypothesis.Study results will be published in peer-reviewed journals or presented at an academic conference.Published data will be publicly available at www.figshare.com.Authorship will be based on the recommendations of the International Committee of Medical Journal Editors (ICMJE).
Discussion
Trial significance
The retinal ganglion cell-inner plexiform layer and retinal nerve fiber layer are composed of nuclei and dendrites of retinal ganglion cells,respectively.The retinal nerve fiber layer contains the axons of retinal ganglion cells.These cells collectively transmit visual information from the retina to several regions of the brain in the form of action potentials.Therefore,the integrity of retinal ganglion cells is essential for maintaining visual function.
Conbercept is a second-generation recombinant anti-VEGF fusion protein composed of VEGF binding domain 1 of VEGF receptor 1 and domains 2 and 3 of VEGF receptor 2 combined with the Fc portion of human immunoglobulin G-1,which is commonly used to treat macular edema (Cai et al.,2018;Wang et al.,2021;Zhang et al.,2022).Conbercept can effectively inhibit the binding of VEGF receptors in patients,thereby reducing neovascularization and inhibiting leakage and infiltration of blood vessels.Laser photocoagulation is still the first-line treatment for diabetic macular edema that does not involve the macular fovea.In the trial,the combination of anti-VEGF drugs and laser photocoagulation therapy can effectively reduce retinal edema and fully protect the function of the macular area,thus,improving patients’ visual acuity.
In this study,anti-VEGF therapy,retinal laser photocoagulation,and their combined therapy will be applied to observe the changes in retinal thickness,choroidal thickness,retinal ganglion cell-inner plexiform layer thickness,and retinal nerve fiber layer thickness in patients with diabetic macular edema and determine the correlation between the above-mentioned indicators and the best corrected visual acuity.The morphological results will be combined with clinical efficacy to study the effects of the three treatments,explore how to use the above-mentioned non-invasive examination indicators to accurately monitor the diagnostic and therapeutic effects on diabetic macular edema,and solve the practical difficulties in the clinical diagnosis and treatment of diabetic macular edema.
Trial innovation
Most studies focus on the relationship between retinal indicators and vision recovery from the perspective of vascular diseases.Previous studies confirmed that nerve damage occurs earlier than vascular diseases in diabetic retinopathy,indicating the importance of nerve damage.Therefore,this study attempts to investigate the effect of anti-VEGF therapy combined with retinal laser photocoagulation on the integrity of retinal ganglion cells in patients with diabetic macular edema.From the perspective of the relationship between retinal ganglion cell integrity and vision,this study will confirm whether this combination therapy can influence the visual recovery of patients in the follow-up period if the integrity of retinal ganglion cells can be maintained,thereby providing a new predictive monitoring indicator for the visual recovery of patients with diabetic macular edema.
Expected conclusions
(1) The combined therapy will have the best outcomes in diabetic macular edema.Alterations in best-corrected visual acuity will be negatively correlated with choroidal thickness,macular thickness,retinal ganglion cell-inner plexiform layer thickness,and retinal nerve fiber layer thickness.The lower the above-mentioned three indicators,the greater the improvement in bestcorrected visual acuity.(2) Retinal ganglion cell injury in patients with diabetic macular edema may result in poor visual acuity after treatment.The use of OCT to measure retinal ganglion cell-inner plexiform layer thickness or retinal nerve fiber layer thickness during the follow-up period may be considered a prognostic factor for visual acuity.
Limitations and prospects of the trial
This study also has some limitations.First,there might be selection bias since the allocation of the subjects is determined by subjective factors such as treatment methods;therefore,the subjects cannot be randomly assigned to different groups,which affects the reliability of the test results.Second,there is also insufficient control of differences between groups.Specifically,there is no randomization and blindness in the study,which might lead to differences between the result data and the actual data.Therefore,the trial results cannot be guaranteed to be accurate and might be biased.Third,this is a nonrandomized concurrent controlled trial in which patients are allocated into a test group or a control group according to their disease conditions and related factors.In the presence of human factors,the subjects in different groups will be in different baseline states before the initiation of the trial.It is difficult to evaluate the trial results using a blind method,which inevitably leads to selective bias and measurement bias.In the future,a prospective,multicenter,randomized controlled trial with a large sample size will be conducted to verify the results of this study.As a preliminary exploratory clinical trial,this paper has some practical significance,which will provide non-invasive imaging measures for accurate monitoring of diagnosis and treatment in patients with diabetic macular edema.
Recruitment date:June 2023 to August 2023.
Study start date:June 2023.
Estimated data analysis date:September 2024.
Estimated trial completion date:December 2024.
Author contributions:Study design,manuscript writing,and manuscript review and editing: XL.Data collection and analysis,and approval of final version of the manuscript: all authors.
Conflicts of interest:The authors have no conflicts of interest to declare.
Data availability statement:All data generated or analyzed during this study are included in this published article and its Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional file 1:SPIRIT checklist.
杂志排行
中国神经再生研究(英文版)的其它文章
- Could mammalian inorganic polyphosphate be a crucial signaling molecule in neurological disorders?
- Use of an immunocapture device to detect cytokine release in discrete brain regions
- New immune regulators of sciatic nerve regeneration? Lessons from the neighborhood
- Multifunctional glycolipids as multi-targeting therapeutics for neural regeneration
- Astrocytes dynamically regulate the blood-brain barrier in the healthy brain
- Epigenetic memory of drug exposure history controls neural stem cell quiescence in the adult brain
