In vivo imaging of the neuronal response to spinal cord injury: a narrative review
2024-02-11JunhaoDengChangSunYingZhengJianpengGaoXiangCuiYuWangLichengZhangPeifuTang
Junhao Deng ,Chang Sun ,Ying Zheng,Jianpeng GaoXiang CuiYu Wang,Licheng Zhang,Peifu Tang
Abstract Deciphering the neuronal response to injury in the spinal cord is essential for exploring treatment strategies for spinal cord injury (SCI).However,this subject has been neglected in part because appropriate tools are lacking.Emerging in vivo imaging and labeling methods offer great potential for observing dynamic neural processes in the central nervous system in conditions of health and disease.This review first discusses in vivo imaging of the mouse spinal cord with a focus on the latest imaging techniques,and then analyzes the dynamic biological response of spinal cord sensory and motor neurons to SCI.We then summarize and compare the techniques behind these studies and clarify the advantages of in vivo imaging compared with traditional neuroscience examinations.Finally,we identify the challenges and possible solutions for spinal cord neuron imaging.
Key Words: anterior horn neurons;calcium imaging;central nervous system;dorsal horn neurons;dorsal root ganglion;in vivo imaging;neuronal response;spinal cord injury;spinal cord;two-photon microscopy
Introduction
Spinal cord injury (SCI) is a devastating neurological trauma,often causing permanent disability with motor,sensory,and autonomic dysfunction.Currently,there are at least 3 million patients with SCI worldwide (~180,000 new cases each year),and the societal burden is heavy (Assinck et al.,2017;Deng et al.,2021).Due to the complicated pathophysiological processes following SCI,no treatment has yet shown any striking therapeutic effects.
Generally,an SCI involves both the primary injury and secondary injuries of complex pathophysiological cellular activities including neuron death,axon degeneration,inflammatory reactions,and glial scar formation.Among these pathophysiological events,the neuronal response is key to neural regeneration failure and locomotor dysfunction following SCI (Hutson and Di Giovanni,2019).Neurons in the adult mammalian central nervous system (CNS) are often impaired and over-reactive,with axonal collapse and demyelination that results in neural regeneration failure and functional deficits (Zhou et al.,2019;Kang et al.,2023;Li et al.,2023;Li and Jia,2023).An understanding of the role of post-SCI neuronal responses and cellular activity would aid in the search for neuro-regenerative strategies.
Classic neuroscience research has predominantly used electrophysiology to record,measure,and analyze the bioelectricity of electrically excitable cells with millisecond scale temporal resolution.Such electrophysiological examination has contributed to the discovery of relationships between specific behaviors and the response of central pattern generator neurons in the spinal cord (Zhao et al.,2022;Liu et al.,2023).However,these approaches require the invasive intracellular insertion of microelectrodes to record electrical activity at the cellular level,and cannot identify cell types or precise spatial positions.Furthermore,long-term observation is precluded by tissue drift,glial scarring,and probe disintegration.
Recent progress in molecular labeling andin vivoimaging approaches has enabled the observation of neuronal dynamics in the healthy and injured CNS (Chen et al.,2019;Wu et al.,2022;Zeng et al.,2022).These innovations provide direct optical access to neuronal cellular structures (neuron bodies,dendritic spines,and axonal boutons),astrocytes,and microglia with high temporal and spatial resolutions.Concurrent advances in high-performance calcium indicators have made available the functional evaluation of neuronal responses by measuring intracellular changes in neuronal calcium ion concentrations in the CNS (Bharioke et al.,2022;Skwarzynska et al.,2023).Unlike the single time-point snapshots of traditional histology,these innovative technologies allow minimally invasive long-term imaging of neuron structure and firing.Thus,they are particularly conducive to the imaging of neural responses and cell-cell relationships following SCI,in which cellular pathological events like structural or functional changes occur across a wide range of timescales.
There are severalin vivoimaging techniques currently used to evaluate the spinal cord,including magnetic resonance imaging (MRI),positron emission tomography (PET),and two-photon microscopy.MRI is a widely used imaging modality for evaluating the structure and function of both normal and abnormal spinal cords,as well as for investigating post-SCI neuroplasticity (Ahuja et al.,2017).However,while MRI provides macroscopic information about the spatial location of structures,it cannot provide structural information at the cellular or subcellular level to directly observe cell bodies or axons,nor can it evaluate calcium signaling.Moreover,MRI usually involves lengthy imaging acquisitions.In contrast,PET is a functional imaging modality that uses a radioactive tracer to visualize metabolic and physiological processes in living organisms.PET imaging involves the injection of a radiotracer into the body,which emits positrons that interact with surrounding tissue and produce gamma rays that can be detected by PET scanners.PET can provide information on metabolic activity and blood flow in the spinal cord,which can be useful for detecting and monitoring SCIs and diseases.However,PET has limited spatial resolution and cannot provide detailed information on anatomical structures,making it less suitable for assessing structural changes in the spinal cord.Additionally,PET imaging involves exposure to ionizing radiation,which can be a concern for some patients (Lu and Yuan,2015).In contrast,two-photon microscopy is a high-resolution imaging technique that uses a focused laser beam to stimulate fluorescent molecules in biological tissues.Unlike traditional microscopy methods,two-photon microscopy can penetrate deeper into biological tissues with less photodamage,allowing for the visualization of subcellular structures and dynamic processesin vivo.Twophoton microscopy provides higher spatial and temporal resolution,which allows visualization of cellular dynamics in synaptic plasticity and neural activity.Additionally,two-photon microscopy involves no ionizing radiation,making it a safer alternative for longitudinal studies and repeated imaging of the same subject (Carrier et al.,2020).
In this study,we review updated approaches forin vivoimaging of the mouse spinal cord,with a focus on spinal cord neuron observation via twophoton microscopy.We then summarize the latest findings on the dynamic nature of spinal cord neuron responses following SCI,and we highlight the advantages ofin vivoimaging in these investigations.Next,we discuss current challenges forin vivoobservation of the neuronal response to SCI,and we discuss possible solutions.Finally,we provide a comprehensive overview of the current state of research in this field and discuss potential future research directions in the hope of stimulating progress in this important area.
Search Strategy and Selection Criteria
For this narrative review,we conducted a systematic literature search using PubMed (https://www.ncbi.nlm.nih.gov/pubmed) to identify relevant papers published from inception to 2023.Our search strategy employed a range of keywords,including but not limited to: “In vivoimaging”,“Two-photon microscopy”,“Neuronal response”,“Central nervous system”,“Spinal cord”,“Spinal cord injury”,“Dorsal horn neurons”,“Anterior horn neurons”,“Dorsal root ganglion”,and “Calcium imaging”.After removing duplicates from the retrieved studies,we performed a preliminary screening of each article’s title and abstract,followed by a full-text assessment to exclude studies that were not relevant toin vivospinal cord imaging and neuronal responses.The literature search process was primarily undertaken by author JD.
Current Strategies for In Vivo Imaging of Mouse Spinal Cord Neurons
In vivotime-lapse imaging of the mouse spinal cord was pioneered by Kerschensteiner’s group (Kerschensteiner et al.,2005),who discovered the dynamic axonal changes that follow SCI (early degeneration followed by axonal regeneration).Since then,several studies have offered unprecedented insights into SCI viain vivomicroscopy,especially multiphoton microscopy.Dray et al.(2009) designed a more comprehensivein vivoimaging paradigm for chronic post-SCI investigation of vascular and axonal networks.They confirmed the intrinsic regenerative potential of dorsal root ganglion (DRG) axons and the synergy of angiogenesis and axonal growth for tissue repair after SCI,and they highlighted the potential of their platform for monitoring CNS diseases and evaluating treatment efficacy.Similarly,Di Maio et al.(2011) introducedin vivoimaging for post-injury evaluation of DRG axon regeneration,which allowed study of the same axon for up to 4 months.Also,Bareyre et al.(2011) used repetitivein vivomicroscopy to identify a phasespecific key regulator of axon regeneration—signal transducer and activator of transcription 3—that selectively promotes axon growth at the initial phase.Evans et al.(2014) applied high-resolution intravital imaging to inflammatory cells and found that blood-derived macrophages (rather than microglia) cause axonal dieback after SCI.
While these previous studies succeeded with long-term observation of the spinal cord,their methods required repeated surgical procedures to obtain optical access to the spinal cord,which inevitably increases the risk of further tissue injury,inflammation,pain,or infection.Therefore,to enable chronic spinal cord imaging without repeated surgeries,implantable chambers were invented to provide long-term optical access.Three studies (Farrar et al.,2012;Fenrich et al.,2012;Figley et al.,2013) independently reported chronic windows and relevant surgery protocols for long-term imaging without repeated surgeries.The surgical procedures have some similarities (skin incision,muscle and tendon removal,and laminectomy),though there are subtle differences in the methods of mechanical stabilization and sealing-offof the spinal cord with the implanted chamber.These implanted windows allowed researchers to observe the spinal cord over months while minimizing surgical complexity and repetition,which also enables spinal cord imaging in awake mice (Cheng et al.,2019).Recently,Wu et al.(2021) further refinedin vivoimaging to achieve long-term mouse spinal cord imaging through an optically clear intervertebral window;compared with vertebrae laminectomy,the method is minimally invasive and creates no overt immunological artifacts.With this method,they determined the neuron-glia-microglia dynamics for months after injury.For the synchronous imaging of the spinal cord and DRG,our team developed a method for stabilizing the spinal cord via an intervertebral fusion mount and a vertebral glass window,by which we revealed that increased neuronal activity correlated with animals’ phasic pain behavior (Chen et al.,2019).
Despite such progress within vivoimaging of the mouse spinal cord,current methods have been limited to superficial imaging (depth <100 µm) of spinal dorsal columns (Laskowski and Bradke,2013).Though two-photon microscopy has improved imaging depth to several hundred microns,spinal cord neurons have been neglected because of their greater depth and the light scattering of white matter and myelinated fiber bundles.Thus,to facilitate deeper access to underlying grey matter,several studies have attempted to dynamically detect neuronal activity in the normal or injured state.Johannssen and Helmchen (2010) pioneered the use ofin vivoimaging to follow neuronal activities by slightly rotating the animal’s spine position to avoid the myelinated fibers and expose the lateral area of the spinal cord.They could directly observe the Ca2+transient in the dorsal horn populations and the enhanced neuronal activity after mechanical stimulation of the paw.These advances enable exploration of spinal cord neuron physiology and pathology in real time.Using a similar strategy,Nishida et al.(2014) developed a direct optical approach to the understanding of information processing in the spinal cord neurons and determined the three-dimensional activity maps of dorsal horn neurons in response to external stimulation.In addition,for recording the activity of spinal cord neuron populations with single-cell resolution,Ran et al.(2016) combined their custom-designed imaging device with a stimulation container to maintain spinal cord stability and regulate stimulus change.They discovered that skin heating and cooling robustly activates spinal cord neuron responses with encoding styles that are distinct for heating and cooling.Furthermore,to achieve steady spinal cord neuron observation in awake behaving mice,Sekiguchi et al.(2016) further developed anin vivoimaging method.They found that activation patterns of spinal cord neurons in the dorsal horn were overlapping for different cutaneous stimuli,and they determined the encoding patterns of stimulus type and its intensity at the single-cell level.More recently,Chen et al.(2018) used a custom-made spinal cord chamber to image lumbar spinal cord neurons at a depth of 25-70 µm (corresponding to lamina I-II intravitally) and found that peripheral pinch evoked a robust neuronal response.An understanding of neuronal activity at greater spinal cord depths (apart from the dorsal horn) would improve our understanding of the neuronal response to locomotor dysfunction and recovery.Cartarozzi et al.(2018) used a novelin vivoimaging approach to study the morphological and functional changes of motor neurons in the deep ventral spinal cord following specific stimuli.However,this kind of imaging technique requires months of substantial training.
Thesein vivoimaging approaches can also be used to test transplanted neuron activity.For instance,Ceto et al.(2020) usedin vivoimaging of grafts to detect focal synaptic responses to behavioral stimuli to determine whether grafted cells form functional synaptic subnetworks after post-SCI neural stem cell transplantation into the spinal cord.Our group previously used two-photon microscopy combined with a self-designed device for long-term observation of early neuronal dynamics in mice with SCI,which clarified the neuroprotective effects of methylprednisolone treatment on SCI mice (Tang et al.,2015).
Taken together,recent developments within vivoimaging (especially two-photon microscopy) have provided direct optical access to both the superficial and deep layers of the spinal cord.Though most studies still focus on superficial neurons and axonal structures,more studies have started to explore neuronal and non-neuronal activities of deeper structures in response to behaviors or specific diseases such as SCI.These studies may improve our understanding of the real-time neuronal response to injury in the mouse spinal cord and help develop strategies for the treatment of SCI (Figure 1).More efforts are required to exploit ventral neuronal activities after SCI.
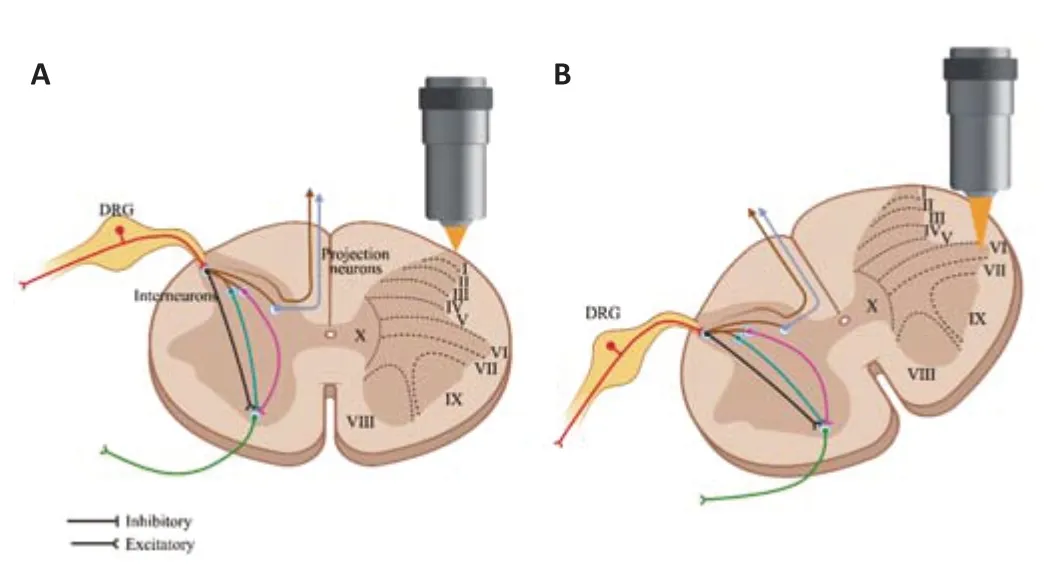
Figure 1|An in vivo two-photon imaging approach for neurons in the spinal cord dorsal horn and ventral horn.
The Dynamic Biological Response of Spinal Cord Neurons to Spinal Cord Injury
The combination ofin vivoimaging with histological analyses has revealed that neurons and non-neurons in the spinal cord undergo a series of acute,subacute,and chronic post-SCI responses that range from morphological and functional cellular changes to interactions with other cell types such as glia.Non-neuronal processes,including neuro-inflammation,glial scar formation,and vasculature disruption,are not the focus of this review and have been extensively studied and discussed previously (Dray et al.,2009;Di Maio et al.,2011;Fenrich et al.,2013;Evans et al.,2014),so we focus in this review only on neuronal responses and their interactions with other cell types.Spinal cord neurons are almost entirely within grey matter,which can be further divided into nine layers (laminas) distributed from dorsal to ventral with a tenth lamina around the central canal.Because of the distinct distribution of sensory (dorsal horn lamina 1-6),and motor (ventral horn lamina 8/9) neurons in the spinal cord,the post-SCI responses of both neuron types will be discussed.We excluded from this discussion the intermediolateral horn (lamina 7) containing the autonomic preganglionic neurons,as they are only situated from T2 to L1 and in S1/S2 and comprise a small subset of the spinal cord.The functions and locations of spinal sensory neurons and motor neurons are different,and therefore differentin vivoimaging approaches are used to study them.Sensory neurons detect and transmit sensory information from the periphery to the spinal cord and brain,andin vivoimaging of sensory neurons often uses genetically encoded calcium indicators (GECIs) for real-time monitoring of calcium signals in neurons.Calcium imaging is useful for studying the activity and function of individual neurons or groups of neurons in response to specific stimuli,such as touch or pain.Sensory neurons can also be labeled with fluorescent protein markers,such as green fluorescent protein,to track their location and behaviorin vivo.In contrast,motor neurons are responsible for controlling muscle movement and are located in the spinal cord and brainstem wherein vivoimaging often uses fluorescent dyes or markers injected into the muscles to track the location and behavior of motor neuron terminals.To study the activity and function of motor neurons,electrophysiological techniques such as patchclamp recordings or extracellular recordings can be used to measure the electrical activity of the neurons in response to different stimuli.Overall,in vivoimaging of spinal sensory neurons and motor neurons involves different techniques and approaches tailored to the function,location,and properties of the neurons being studied.
Spinal cord sensory neuron activity in response to SCI
The sensory neuron activities of the spinal cord occur in two places—the neurons in the spinal cord and in the DRG.Clusters of spinal cord sensory neurons are located in the dorsal horn of the spinal cord and synapse with primary afferent fibers to relay their sensory information to corresponding areas within the brain or spinal cord.Sensory neurons of the DRG are the primary sensory neurons along both sides of the spinal cord and carry proprioceptive,mechanoreceptive,nociceptive,and thermoreceptive information to the spinal cord or superior nucleus.In SCI,the superficial spinal cord sensory neurons would be directly damaged and then experience swelling,disintegration,and necrosis,whereas the DRG sensory neurons would develop axonal dieback in the spinal cord but their deeper cell bodies could survive (Chen et al.,2022).Previous SCI studies have mainly observed the DRG sensory neurons,and thus our review focuses on these neurons with limited discussion of spinal cord sensory neurons.
DRG sensory neurons are pseudounipolar neurons with one axon divided into a central branch that enters the spinal cord and a peripheral branch that spreads to the periphery.The central and peripheral branches of DRG sensory neurons have distinct responses to injury despite originating from the same cell body.The peripheral axonal branch of the sensory neuron regenerates robustly after injury,while the central branch shows very limited regenerative capacity (Alsaloum and Waxman,2022).Because SCI causes a range of damage,we summarize only the changes in central branch activity after SCI.Others have suggested that the central axons of DRG neurons would undergo consecutive post-SCI pathological processes.Kerschensteiner et al.(2005) usedin vivoimaging to reveal bidirectional but limited axonal degeneration in the acute phase,followed by long-distance Wallerian degeneration at the distal end and attempted (but failed) regeneration at the proximal end.However,these simple descriptions do not reflect the completein vivopost-SCI response activities of DRG sensory neurons.For example,the structure of the DRG sensory neuron central branch cannot be assumed to be linear and may have intricate and varied axonal patterns.Generally,their central axonal projections present as two bidirectional branches (ascending and descending) that split from one common shaft and form the dorsal column in the spinal cord.Furthermore,these axonal branch patterns may influence the neuronal response to injury (Zheng et al.,2019).Thus,a deep understanding of central branch axonal structure in DRG sensory neurons would support the development of approaches for axonal repair and functional recovery following SCI.
In vivospinal cord imaging has emerged as an excellent method to study the influence of axonal branch structures on post-SCI regeneration by enabling highly precise and localized laser ablation of axonal branches and long-term observation of the axonal injury response.Di Maio et al.(2011) reported the firstin vivoimaging of the axonal response of DRG sensory neurons in the spinal cord and found that dorsal root axons seldom turned around at the dorsal root entry zone,unlike the findings of previous studies (Sorkin et al.,2018;Meltzer et al.,2021).Instead,they observed deep penetration into the spinal cord that stalled and remained immobile following a dorsal root crush.Lorenzana et al.(2015) usedin vivoimaging combined with local laser ablation to systematically investigate the role of injury position relative to the axonal bifurcation points in the DRG sensory neuron response to injury.They discovered that axons undergo rapid bidirectional degeneration following laser ablation of ascending,descending,and main branches,which is consistent with previous studies.However,laser ablation of ascending or descending branches alone resulted in poor regeneration,while regeneration could be detected if the main branch or both ascending and descending branches were injured simultaneously.
The injury response of sensory neurons in the spinal dorsal horn remains unclear.These neurons receive sensory information from primary afferents innervating the skin and deeper tissues of the body and respond to specific types of noxious and non-noxious stimuli.The incoming information is processed by complex neural circuits of excitatory and inhibitory interneurons before being relayed via projection neurons to several brain regions (Todd,2010).Previous studies report that 60-80% of SCI patients experience post-SCI pain regardless of injury type or severity (Watson and Sandroni,2016;Hatch et al.,2018),and this pain ranges from deep-seated to surface-level sensations with a possible burning or tingling quality.In fact,SCI patients often experience sensory disturbances and pain below the level of the sensory lesion in areas where no sensation is present in a phenomenon known as allodynia (Huynh et al.,2021).Therefore,sensory neuron activity in the spinal cord dorsal horn would inevitably be affected by SCI.
Several studies have demonstrated that SCI induces structural changes in dendritic spines of lamina II neurons and increases the expression of calcitonin gene-related peptide and growth associated protein 43 in the dorsal horn of the spinal cord,which suggests a possible link with nociceptive neuron hyperexcitability (Lee-Kubli et al.,2016;Cao et al.,2017;Keller et al.,2019).Another study has revealed changes in the electrophysiological properties of dorsal horn nociceptive neurons that may contribute to the development and maintenance of neuropathic pain after SCI (Lütolf et al.,2022).These changes include an elevated proportion of nociceptive-responsive neurons,heightened spontaneous irregular firing,augmented activity in response to both noxious and innocuous stimuli,and increased firing following stimulation.Similarly,Kang et al.(2020) reported that sensory neurons in the lumbar spinal dorsal horn fired more frequently after thoracic ischemic injury,and sensory neurons also experienced redistribution in the spinal cord dorsal horn,medulla,and thalamus.These findings suggest that SCI induces complex changes in sensory processing and transmission pathways,which may contribute to the development and maintenance of chronic pain after injury.
Recently,in vivoimaging has emerged as a powerful tool for studying sensory neurons in the spinal dorsal horn,with some advantages overin vivoelectrophysiology (Johannssen and Helmchen,2013;Ran et al.,2016;Ran and Chen,2019).Althoughin vivoelectrophysiological recordings provide rapid feedback on the responses of spinal neurons to changes in skin temperature,the method requires large sample sizes to gain a functional understanding of the morphology,physiology,transcriptomics,connectomics,and diversity of spinal neurons.For example,in a study by Andrew and Craig (2001),only 10 of 474 recorded neurons were thermoresponsive,and their response properties could not be analyzed in further detail or characterized after loss-of-function manipulations.Calcium imaging is critical forin vivoimaging studies of neurons,but technical limitations have prevented this method from being applied within the dorsal horn of the spinal cord.An early epifluorescence microscopy study revealed overall higher basal calcium indicator fluorescence ipsilateral to transverse spinal cord sections from animals with a chronic SCI model of pathological pain,suggesting increased dorsal horn neuronal activity in the absence of external stimuli (Harding et al.,2020).Moreover,Luo et al.(2008a) have found larger calcium responses to dorsal root stimulation in the ipsilateral superficial dorsal horn after nerve injury,which reflects the hyperalgesic phenomenon of pathological pain.With advances in calcium ion indicators and microscopy,single-neuron and single-event analyses are now available and accessible.Skorput et al.(2018) found that glutamateevoked calcium responses were significantly higher in superficial dorsal horn neurons after application of a peptide derived from VGF nerve growth factor,suggesting that VGF enhances glutamatergic signaling in the spinal cord.Calcium imaging has also enabled effective capture and classification of responses to mild sensory stimuli,such as cold and warm temperatures that activate only a small fraction of neurons (Ran et al.,2016).The combination of calcium imaging with two-photon microscopy holds great potential for the observation of sensory neuron activity and the understanding of post-SCI neuronal responses.
Spinal cord motor neuron activity in response to SCI
The cytoarchitecture of the spinal cord anterior horn comprises motor neurons,interneurons,and non-neuronal cells such as astrocytes and microglia.Since this review focuses on neurons,subsequent discussions will concentrate on anterior horn motor neurons.Anterior horn motor neurons of the spinal cord are subordinate motor neurons of the corticospinal tract pathway and,together with the corticospinal tract,control voluntary muscle movements and play a pivotal role in spinal signaling processes (Picheca et al.,2019).The primary function of motor neurons is to receive impulses transmitted from the cerebral cortex and transmit them to effector organs innervated by axons.Neurons from the cerebral motor cortex and anterior horn whose axons descend to the spinal cord (pyramidal tract) are termed neuronal units (Rickman et al.,2020).
Within a few weeks of SCI,anterior horn motor neurons may be damaged to varying degrees,including the ventral root (Liu and Xu,2010;Karalija et al.,2012;Pastor et al.,2013).Lesions to proximal spinal motor neurons and the distal ventral root can result in denervation and subsequent atrophy of the muscles they innervate (Byers et al.,2012),with the extent of damage and functional deficits dependent on injury severity and location.Motor neuron loss and axonal damage can lead to muscle weakness,paralysis,and spasticity,which significantly impact the life quality of SCI patients.Post-SCI recovery relies not only on the integrity of motor neurons in the spinal cord but also on the health and functionality of their target musculature.Chronic compression of the spinal cord can lead to atrophy and loss of anterior horn cells,which is associated with irreversible injury in the spinal cord (Dias et al.,2018;Badhiwala et al.,2021).Furthermore,permanent axotomy of an upstream axon in adult cats results in a transient increase in spinal motor neuron soma size,followed by normalization and a reduction in dendritic branch area and neuronal length (Duraikannu et al.,2019).The loss of motor neurons is associated with permanent motor impairment after SCI,and research on anterior horn motor neurons is crucial for understanding the underlying mechanisms of SCI and developing effective therapeutic interventions.However,in vivostudies have been limited by the depth of these neurons within the spinal cord,and direct evidence is lacking regarding their functions and mechanisms of damage after SCI.A better understanding of anterior horn motor neurons will ultimately improve outcomes for individuals with SCI,highlighting the importance of this area of research.
Electrophysiological techniques have long been used to study neural activityin vivoand enable the evaluation of motor neurons in the ventral horn of the spinal cord.Early approaches used microelectrodes to record the electrical activity of individual neurons,and these methods have since expanded to include extracellular recordings,intracellular recordings,and whole-cell patch clamp recordings.For instance,Dougherty and Kiehn (2010) were able to observe whole-cell patch recordings from deep spinal cord neurons along the entire lumbar spinal cord by removal of the spinal cord dorsal horn.Li et al.(2022) used patch-clamp recording to investigate the effects of pilocarpine and understand the neurophysiological regulation of the spinal cord ventral horn.Though useful for studying neural activity,these electrophysiology techniques are limited by their invasive tissue-damaging nature and the lack of spatial information they provide.These limitations have inspired the development and use of non-invasive,high-resolution imaging techniques to study neural activityin vivo.Such techniques offer the potential for more detailed and comprehensive insights into neural activity without the risks associated with invasive methods.Muto et al.(2011) demonstrated that the GECI calcium modulated protein (GCaMP) can effectively detect calcium signals in both cell bodies and axons and identify activated neurons within an image containing multiple neurons.Similarly,Tian et al.(2011) utilizedin vivooptical imaging to examine autophagy in motor neurons in the spinal cord of animals.Cartarozzi et al.(2018) successfully applied two-photon laserscanning microscopy to observe the ventral horn of the spinal cord,achieving high spatial resolutionin vivo.This breakthrough will facilitate exploration into motor neuron-glial interactions.However,despite the potential of twophoton microscopy for studying neural activityin vivo,few studies have used this technique to observe motor neurons in the anterior horn of the spinal cord because of the challenge in imaging neurons deep within the spinal cord.Improvements in fluorescent probe designs and adaptive corrective optics may improve anterior horn motor neuron imaging in the future,and refinement of observation window construction for multiphoton microscopy may provide even better access to these neurons.
The Superiority of In Vivo Imaging over Traditional Examination as a Research Tool to Explore the Neuronal Response to Spinal Cord Injury
As previously mentioned,calcium imaging and two-photon microscopy bring many advantages toin vivofunctional imaging.While electrophysiology enables us to directly record the electrical activity of neurons with high signalto-noise ratios using electrode membranes,the direct physical contact of patch clamp methodology also causes significant damage to neuronal tissue.In contrast,calcium imaging allows observations of neuronal function without direct physical contact,as light can pass through biological membranes.This method offers many additional advantages,including better spatial resolution,wider observation fields,and the ability to observe specific cell subtypes and subcellular domains using gene-targeted probes (Hell,2007;Luo et al.,2008b;Klementieva et al.,2020).Calcium imaging uses calcium-sensitive molecules such as calmodulin or troponin fused to fluorescent proteins as indicators.This non-invasive imaging technique is also made possible through the use of optical fiber imaging.For instance,Ran et al.(2016) used calcium imaging to observe neurons in the superficial dorsal horn of the spinal cord via twophoton microscopy and studied the coding of skin temperature in the spinal cord.Similarly,Johannssen and Helmchen (2010) used calcium imaging to observe spontaneous calcium transients in superficial dorsal horn neurons and the evoked response to mechanical stimulation of the paw.
Two-photon imaging offers high spatial localization and high resolution,making it well-suited for imaging scattered tissues (Zipfel et al.,2003;Helmchen and Denk,2005;Wang et al.,2010).Because the signal is collected point by point as the sample is excited,pixel crosstalk in wide-field imaging can be overcome.Furthermore,the longer excitation wavelengths reduce the tissue scattering of single-photon excitation.The most commonly used single-photon fluorophores have excitation wavelengths of 400-500 nm,whereas excitation of the same fluorophore with a two-photon microscope used wavelengths of 800-1000 nm.Thus,two-photon microscopy increases the depth of penetration,reduces photobleaching,and provides higher spatial resolution than single-photon microscopy (Wu et al.,2021).These features have made two-photon microscopy a good alternative for functional imaging in confocal microscopy,and the method is particularly useful in rodents studies of neural activity during behavior,functional connectivity between cortices,neurovascular coupling,and the structure and function of the spinal cord (Johannssen and Helmchen,2013;Meehan et al.,2020).In addition to scan speed,field-of-view size is crucial for systems neuroscience studies that reveal interactions across fields.To maintain single-cell resolution and high temporal resolution,the entire field of view is usually not scanned simultaneously.However,a spinal cord window would enable long-term repetitive imaging of neuronal axons within the same mouse,theoretically allowing non-destructive imaging of the spinal cord for up to 1 year (Fenrich et al.,2012;Table 1).

Table 1| The advantages of two-photon microscopy over electrophysiology and confocal microscopy
Moreover,the combination of optogenetics within vivoimaging allows for targeted and cell-type-specific neuronal excitation or inhibition (Adam et al.,2019;Zhang et al.,2019;Adesnik and Abdeladim,2021).This approach enables real-time detection of mouse behavior and decoding of neural activity,feedback manipulation of specific cell types and brain regions,and relevant behavioral analyses (Chen et al.,2021).This closed-loop system can also be used to detect and suppress the development of epileptiform activity,investigate deep brain stimulation mechanisms,and support braincomputer interfaces (Sorokin et al.,2017).Furthermore,when combined with optogenetics,this closed-loop system has the potential to decode and regulate neural patterning in real time and perform closed-loop optogenetic interventions in freely behaving animals (Kathe et al.,2022).In vivoimaging can be combined with transcriptomics to visualize and capture activated neurons for transcriptomic analysis,which enabled the discovery of cortical inhibitory neurons with multiple fine molecular subtypes (Bugeon et al.,2022).This approach has the potential to reveal novel molecular subtypes of neurons and their corresponding functions,leading to a deeper understanding of the neural mechanisms underlying behavior and disease.Overall,the combination ofin vivoimaging with optogenetics and transcriptomics supports the investigation of neural circuits and molecular mechanisms underlying behavior and disease.As these techniques continue to advance,we can expect to gain even deeper insights into the workings of the nervous system.
Challenges and Solutions for In Vivo Imaging on Spinal Cord Neurons
Despite the advantages of two-photon microscopy (high resolution,subcellular structural imaging,and high spatial localization),shortcomings still exist with regard to neuroimaging,especially after injury.Long-term imaging of the same neuron or axon often requires repeated surgery at the same site,and these surgical manipulations (e.g.,dura mater removal) increase trauma risk and disturb spinal cord tissue (Kerschensteiner et al.,2005;Misgeld et al.,2007).To minimize repeat surgeries and enable long-term imaging in awake or freely-behaving mice,implanting viewing windows have been developed for the spinal cord (Farrar et al.,2012;Figley et al.,2013).However,this approach causes immune activation in the spinal cord,leading to the ascension of relevant inflammatory cells (Farrar et al.,2012;Fenrich et al.,2012),and the required anti-inflammatory drug treatment may impact the study results.More recently,minimally-invasive viewing window construction methods have been developed for the spinal cord in which the dorsal spinal cord is imaged only from the intervertebral space (Wu et al.,2022).Additionally,our group also performed single-cell two-photon imaging of neuron activity in the DRG of awake mice by constructing a minimally invasive DRG viewing window (Chen et al.,2019).
Imaging depth is another challenge in two-photon microscopy;the thick and dense layer of axonal myelin surrounding the spinal gray matter reflects and scatters light across a wide wavelength range (Schain et al.,2014;Kwon et al.,2017).Single-photon microscopy is thus limited to shallow imaging (60-80 µm) in the dorsal spinal cord.Two-photon microscopy with redshifted calcium indicators (Dana et al.,2016;Qian et al.,2019) can reach greater imaging depths (150-200 µm) in lamina I and II (Sekiguchi et al.,2016) and even deeper in lamina IV (Johannssen and Helmchen,2010),but this method is not effective for regions such as the anterior horn of the spinal cord.A single study has acutely imaged motor neurons and peripheral glia in anesthetized mice by ventrolateral laminectomy and dura removal (Cartarozzi et al.,2018).The depth of observation forin vivoimaging is further enhanced by the application of gradient index lens technology.Recently,three-photon microscopy has allowed for deeper observation (500 µm) into lamina V (Ouzounov et al.,2017),but these microscopes use low-frequency lasers with limited output power,thus limiting image frame rates and motion artifact correction (Ouzounov et al.,2017;Guesmi et al.,2018).Thus,further technological advancements are required to improve imaging depth and clarity,particularly for deep structures in the nervous system.
Movement artifacts (from breathing,heartbeat,and animal behavior) create challenges forin vivoimaging,and adaptive focus control and ratio imaging have been used to reduce axial motion artifacts (Johannssen and Helmchen,2010;Laffray et al.,2011).Nonetheless,in vivospinal cord imaging remains challenging because of the frame distortions caused by tissue deformation during animal behavior.Specifically,animals implanted with dorsal optical windows can exhibit varying degrees of tissue displacement that result in nonlinear image deformations,particularly in regions outside the central venous territory.Advanced imaging algorithms may minimize these artifacts (Sekiguchi et al.,2016),though they do not correct for movement in the Z-axis.
Recently,interest has grown in usingin vivoimaging of freely moving mice to maximize image recovery in a more realistic physiological environment.Miniaturized live-imaging has transformed brain imaging,but these advances cannot be applied directly to the spinal cord because of the irregular movements of spinal cord tissue caused by breathing,heartbeat,and locomotion.Additionally,the limited imaging speed and optical lamellae of two-photon microscopy can result in focal plane drift,image distortion,or loss when imaging the somatic spinal cord (Johannssen and Helmchen,2010;Ran et al.,2016).Thus,most optical imaging studies of the spinal cord are conducted on anesthetized animals,with few studies on awake freelybehaving animals (Sekiguchi et al.,2016;Nelson et al.,2019).Consequently,the mechanisms by which the spinal cord encodes descending central commands and peripheral input signals under real-world conditions remain unclear (Moser et al.,2017),and live spinal cord imaging methods are essential to furthering this understanding.The application of miniaturized high-resolution two-photon fluorescence microscopy has enabled breakthrough progress in related research on somatic imaging in freely moving animals (Zong et al.,2017,2021;Vázquez-Guardado et al.,2020),and this technology provides an opportunity for spinal cord imaging in freely behaving mice.It is critical to overcome the technical barriers for live twophoton spinal cord imaging in freely moving animals to advance research on functional neural networks of the spinal cord.
Improvements in calcium indicators are critical forin vivoimaging,as fluorescent labeling techniques often require specific transgenic murine or viral markers to enable neuron-specific imaging.To achieve more targeted imaging,GECIs can be expressed in specific subtypes of neurons or glia.One such GECI is GCaMP,which comprises calmodulin,green fluorescent protein,calcium,and M13 domains.The GCaMP6 family of GECIs,including GCaMP6f,GCaMP6m,and GCaMP6s,report cytoplasmic free calcium levels as a proxy for neuron firing.GCaMP6s and GCaMP6m produce larger responses to single action potentials,though GCaMP6f responses are faster (Chen et al.,2013;Lin and Schnitzer,2016).Red-shifted GECIs (e.g.,RCaMP) use a red fluorescent protein instead of GFP (Dana et al.,2016),resulting in longer fluorescence excitation wavelengths and reduced tissue absorption,thus improving imaging depth and reducing phototoxicity.More recently,GCamp7 and GCamp8 have been developed to label neuron bodies,axons,and dendrites with high sensitivity (Bharioke et al.,2022;Zhang et al.,2023).
Taken together,there are currently several challenges in two-photon imaging of the spinal cord,and researchers are actively developing innovative solutions to overcome these obstacles.With such ongoing advances,we can expect to gain greater insights into the complex neurological functions of the spinal cord (Table 2).

Table 2|The challenges of in vivo imaging,and potential solutions
Concluding Remarks
In vivoimaging has the potential to reveal novel mechanisms of neuronal,glial,immune,and vascular responses to SCI in the mammalian CNS.As with many other areas of modern biology,we believe that technological advances will drivein vivoimaging studies,particularly when integrated with other emerging technologies and tools.Approaches for repeatedin vivoimaging (Drew et al.,2010;Fenrich et al.,2012),in vivoimaging of freely behaving mice (Sekiguchi et al.,2016),and GECI-basedin vivoimaging of neuronal activity (Lim et al.,2016) may all provide greater insights into post-SCI neuronal responses.By combining structural and functional studies with methods to monitor and manipulate neuronal activity (Kim et al.,2017) and glial-neuronal interactions (Akassoglou et al.,2016),in vivoimaging can continue to uncover more mechanisms of post-SCI neuronal responses.Furthermore,molecular information revealed by multi-omics approaches can be used to discover and generate novel molecular probes to detect neuronal dysfunction,immune cell activation,and vascular abnormalities,which have the potential to reveal new avenues for therapeutic intervention in SCI.
Acknowledgments:The authors are grateful for the valuable and constructive suggestions given by Dr.Jiang Peng from Orthopedics Institute of Chinese PLA.
Author contributions:Conceptualization and literature collection: LZ,PT;manuscript draft: JD,CS;manuscript revision: YZ,JG,XC,YW.All authors have read and agreed to the published version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:The present review article does not include any original data analysis.Any further information is available from the corresponding author upon request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Could mammalian inorganic polyphosphate be a crucial signaling molecule in neurological disorders?
- Use of an immunocapture device to detect cytokine release in discrete brain regions
- New immune regulators of sciatic nerve regeneration? Lessons from the neighborhood
- Multifunctional glycolipids as multi-targeting therapeutics for neural regeneration
- Astrocytes dynamically regulate the blood-brain barrier in the healthy brain
- Epigenetic memory of drug exposure history controls neural stem cell quiescence in the adult brain
