High-frequency repetitive transcranial magnetic stimulation promotes neural stem cell proliferation after ischemic stroke
2024-02-11JingLuoYuanFengZhongqiuHongMingyuYinHaiqingZhengLiyingZhangXiquanHu
Jing Luo, Yuan Feng, Zhongqiu Hong, Mingyu Yin, Haiqing Zheng, Liying Zhang,*, Xiquan Hu,*
Abstract Proliferation of neural stem cells is crucial for promoting neuronal regeneration and repairing cerebral infarction damage.Transcranial magnetic stimulation(TMS) has recently emerged as a tool for inducing endogenous neural stem cell regeneration, but its underlying mechanisms remain unclear.In this study,we found that repetitive TMS effectively promotes the proliferation of oxygen-glucose deprived neural stem cells.Additionally, repetitive TMS reduced the volume of cerebral infarction in a rat model of ischemic stroke caused by middle cerebral artery occlusion, improved rat cognitive function, and promoted the proliferation of neural stem cells in the ischemic penumbra.RNA-sequencing found that repetitive TMS activated the Wnt signaling pathway in the ischemic penumbra of rats with cerebral ischemia.Furthermore, PCR analysis revealed that repetitive TMS promoted AKT phosphorylation, leading to an increase in mRNA levels of cell cycle-related proteins such as Cdk2 and Cdk4.This effect was also associated with activation of the glycogen synthase kinase 3β/β-catenin signaling pathway, which ultimately promotes the proliferation of neural stem cells.Subsequently, we validated the effect of repetitive TMS on AKT phosphorylation.We found that repetitive TMS promoted Ca2+ influx into neural stem cells by activating the P2 calcium channel/calmodulin pathway, thereby promoting AKT phosphorylation and activating the glycogen synthase kinase 3β/β-catenin pathway.These findings indicate that repetitive TMS can promote the proliferation of endogenous neural stem cells through a Ca2+ influx-dependent phosphorylated AKT/glycogen synthase kinase 3β/β-catenin signaling pathway.This study has produced pioneering results on the intrinsic mechanism of repetitive TMS to promote neural function recovery after ischemic stroke.These results provide a strong scientific foundation for the clinical application of repetitive TMS.Moreover, repetitive TMS treatment may not only be an efficient and potential approach to support neurogenesis for further therapeutic applications, but also provide an effective platform for the expansion of neural stem cells.
Key Words: AKT/β-catenin signaling; brain stimulation; Ca2+ influx; cell proliferation; ischemic stroke; middle cerebral artery occlusion; neural stem cells;neurological rehabilitation; repetitive transcranial magnetic stimulation
Introduction
Ischemic stroke is a leading cause of both mortality and disability (Benjamin et al., 2019; GBD 2016 Stroke Collaborators, 2019).Despite significant advancements, there are currently only two proven treatments for stroke:thrombolysis and mechanical thrombectomy (Derex and Cho, 2017).However, many patients do not receive recanalization treatment due to either unavailability or ineligibility, resulting in poor outcomes.Therefore, the development of new treatments is essential to broaden the options available to clinicians.Ischemic stroke results in brain damage caused by neuronal necrosis and apoptosis (Rami et al., 2008; Zhou et al., 2010; Feng et al., 2017;Wang et al., 2018a).It was previously believed that damaged neurons in adult mammals are non-renewable and irrecoverable (and therefore could only be replaced by glial cells), and degenerate and diminish with age (Nakayama et al., 2010; GBD 2016 Stroke Collaborators, 2019).However, recent studies have shown that neural stem cell (NSC) proliferation, migration, and differentiation are linked to functional recovery after ischemic stroke (Liu et al., 2009; Guo et al., 2014; Merson and Bourne, 2014; Luo et al., 2017).NSCs form the basis of all neurons and glial cells in the brain and spinal cord (Urbán et al.,2019).After brain injury, two main strategies for NSC-based therapies include activation of endogenous NSCs or transplantation of exogenous NSCs (Huang and Zhang, 2019).However, issues such as poor NSC survival (Othman and Tan, 2020), tumorigenesis (Choi et al., 2018), immune rejections (Bagó et al.,2017), social ethics (Yang et al., 2016), and insufficient sources of NSC donors(Sugaya and Vaidya, 2018) have significantly constrained the application of exogenous NSC transplantation.Therefore, promoting the activation of endogenous NSCs appears to be a safer and more feasible approach.The first step in repairing nerve injuries would be to stimulate the proliferation of NSCs.Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation therapeutic technique that has the potential to treat stroke patients with motor dysfunction, cognitive impairment, and speech disorders(Lorca-Puls et al., 2017; Tang et al., 2017; Rajji, 2019; Shao et al., 2022; Sun et al., 2022; Song et al., 2023).rTMS can promote the repair of brain injury by improving cerebral blood flow, regulating ion balance, promoting synaptic remodeling, and inhibiting cell apoptosis (Liston et al., 2014; Yang et al.,2015).In addition, rTMS has the potential to activate endogenous NSCs in the hippocampus (Ueyama et al., 2011), and promote neuronal regeneration in rats with substantia nigra/striatum injury (Arias-Carrión et al., 2004).Luo et al.(2017) found that rTMS promoted the proliferation of NSCs in the peri-infarct region of rats with cerebral infarction.Therefore, NSC activation may be an important approach for rTMS to improve neurological function after cerebral infarction, but the underlying mechanisms remain unclear.Although some studies have shown that rTMS can promote NSC proliferation (Arias-Carrión et al., 2004; Ueyama et al., 2011), these studies only verified one of the related signaling pathways and did not specify whether the effect was direct or indirect.
The Wnt signaling pathway is a classical pathway promoting stem cell proliferation.In the canonical Wnt pathway, β-catenin is actively degraded by a protein complex containing glycogen synthase kinase 3 (GSK3), and binding of Wnt protein to its receptor disrupts functional degradation of the GSK3 complex, leading to stabilization of β-catenin.β-Catenin enters the nucleus and activates genes (Tiwari et al., 2016).A previous study found that phosphorylated AKT (also known as protein kinase B) can activate the Wnt/β-catenin pathway (Dong et al., 2020), while our previous study found that rTMS can activate AKT (Luo et al., 2017).However, it remains unknown whether rTMS can activate the Wnt/β-catenin pathway.In this study, we conducted bothin vitroandin vivoexperiments to explore the effect and mechanism of rTMS on NSC proliferation.
Methods
Isolation and culture of rat NSCs
All animals were fed and animal experiments performed by Laian Technology(Guangzhou) Co., Ltd.(Guangzhou, China).The animal experiments were approved by the Ethical Committee of Laian Technology (Guangzhou) Co.,Ltd.(approval No.G2022003) on November 30, 2021.Pregnant rats were anesthetized using 2% pentobarbital sodium (30 mg/kg, Shanghai Sanshi Biotechnology Co., Ltd., Shanghai, China) by intraperitoneal injection on day 14.5 of gestation (E14.5).NSCs were isolated from fetal rats according to wellestablished protocols (Azari et al., 2011).Briefly, the brains of embryos were removed and subsequently flushed with L-Dulbecco’s modified Eagle medium(L-DMEM) containing 0.3% (v/v) bovine serum albumin.Following dissociation with accutase (Thermo Fisher Scientific, Waltham, MA, USA), brain tissue was filtered through a 70-µm cell strainer to obtain NSCs.Red blood cells were eliminated by ammonium chloride lysis (C3702, Beyotime Biotechnology,Shanghai, China).NSCs were then washed with Hanks’ balanced salt solution and added to culture flasks in NSC culture medium.This medium consisted of L-DMEM with 2% (v/v) B27 (Thermo Fisher Scientific), as well as 20 ng/mL of epidermal growth factor (20 ng/mL, PeproTech, Cranbury, NJ, USA) and basic fibroblast growth factor (20 ng/mL, PeproTech).For adherent cell cultures,cells were harvested and seeded onto culture flasks coated with Matrigel.To inhibit phosphoinositide 3-kinase or calcium signaling, the cells were treated with specified concentrations of specific small molecule compounds(Sigma-Aldrich, Burlington, MA, USA): suramin (50 µM, S2671), LY294002(25 µM, L9908), chlorpromazine (CPZ; 100 µM, BP856), 2-aminoethyl diphenylborinate (2-APB; 10 µM, 100065), and U73122 (5 µM, U6756) prior to the experimental assay.
Differentiation assays
Forin vitrodifferentiation of neural cells from NSCs, specific differentiation media was used, as previously described (Wang et al., 2018b).Briefly, neural differentiation medium contained neurobasal medium, B-27 supplement,and GlutaMAX-I supplement.Astrocyte differentiation medium consisted of DMEM, fetal bovine serum, GlutaMAX-I supplement, and N-2 supplement(all from Thermo Fisher Scientific).Oligodendrocyte differentiation medium consisted of neurobasal medium, B-27 supplement, GlutaMAX-I supplement, NT3 (PeproTech), T3 (Sigma), and ascorbic acid (Sigma).After 7-days of incubation in differentiation medium, the cells were fixed with 4%paraformaldehyde and subjected to immunofluorescence staining.
In vitrooxygen-glucose deprivation model of NSCs
Cobalt chloride (CoCl2) was used to simulate hypoxic conditionsin vitro, as previously described (Page et al., 2016).Briefly, NSC culture medium was replaced with oxygen-glucose deprivation (OGD) medium, which comprised glucose-free DMEM supplemented with 250 µM CoCl2and 250 µg/mL bovine serum albumin.Following a 24-hour incubation in OGD medium, the medium was replaced with NSC culture medium.All groups except the control group were exposed toin vitroOGD.
Animals and experimental grouping
A total of 100 male spontaneously hypertensive rats (SHR) from the Beijing Vital River Laboratory Animal Technology Co., Ltd., (Beijing, China; license No.SCXK [Jing] 2016-0006) were used in this study forin vivoexperiments.All SHR were 200–220 g, aged 8–10 weeks.SHR were randomly divided into a Sham group (n= 20) and middle cerebral artery occlusion (MCAO) group (n= 80).In the Sham group, SHR underwent sham surgery.In the MCAO group, SHR underwent a 90-minute transient MCAO surgery, and were then randomly divided into the TMS (+) group (n= 40) and TMS (–) group (n= 40), based on the following inclusion and exclusion criteria.Inclusion criteria: rats with moderate neurological dysfunction, as assessed by a modified neurological severity score (mNSS; Luo et al., 2017), with scores of 7–12 included.Exclusion criteria: rats were found to have subarachnoid hemorrhage, no paralysis, dying before the sampling time, or with mild or severe damage.Both groups were divided into four subgroups on the 3rd, 7th, 14th, and 28thdays after rTMS (n= 10/group).Different groups maintained the same feeding environment.
MCAO surgery
MCAO surgery was performed on SHR, as described in our previous studies(Luo et al., 2014, 2017; Jiang et al., 2017; Pan et al., 2017).To block the left middle cerebral artery, rats were anesthetized, and then a filament(nylon suture with a rounded tip) was inserted into the leftinternal carotid artery.The filament was pulled back to restore blood flow (reperfusion)after 90 minutes of MCAO.Rats in the sham surgery group underwent the same surgery without inserting the filaments.To label dividing cells, daily injections of bromodeoxyuridine (BrdU; 50 mg/kg, Sigma-Aldrich) were given intraperitoneally at 1 day after MCAO until 14 days after MCAO.
Repetitive transcranial magnetic stimulation
Transcranial magnetic stimulation treatment was conducted using a MagPro X100 magnetic stimulator (The MagVenture Company, Copenhagen,Denmark) with a figure-of-eight coil (MCF-B65).Motor evoked potentials were measured as previously described (Luo et al., 2017).In vitro, NSCs were randomly divided into four groups: high-frequency (TMS [H]), low-frequency(TMS [L]), OGD, and control groups (Figure 1A).NSCs in the TMS (H) group were subjected to high-frequency rTMS at a frequency of 10 Hz, an intensity of 1.8T (30% maximum output intensity) of the machine, with 20 pulses per train, a train interval of 10 seconds, and a total of 60 trains (1200 pulses) for 11 minutes and 44 seconds.NSCs in the TMS (L) group were subjected to low-frequency rTMS at a frequency of 1 Hz, an intensity of 30% maximum output intensity of the machine, with 20 pulses per train, a train interval of 5 seconds, and a total of 30 trains (600 pulses) for 11 minutes and 55 seconds.NSCs in the OGD group were not subjected to rTMS.In vivo, rats in the TMS (+)group received 10 Hz rTMS with 20 pulses per train (1200 pulses), 10 seconds inter-train interval, a total of 60 trains for 11 minutes and 44 seconds.Rats in the TMS (–) group experienced the same experimental manipulations and auditory stimuli by placing an inactive coil on the rats’ heads.Animals showed no signs of discomfort.Rats receiving rTMS were stimulated for 3, 7, 14,and 28 days during a 2-day period a week, beginning at 2 days after MCAO(Additional Figure 1).The weight and survival of the rats were observed and recorded for 28 consecutive days.
Morris water maze and novel object recognition tests
The Morris water maze (MWM) and novel object recognition (NOR) tests were used to evaluate rat neurobehavioral cognitive function in each group at 28 days after TMS, as previously described (Jiang et al., 2017; Pan et al.,2017).Briefly, for the MWM, each rat was trained to find the submerged escape platform each day for 4 days.On day 5, the rats swam in the water tank without the platform for 1 minute.In the MWM test, the number oftimes the rats crossed the platform area, time spent in the target quadrant,and escape latency were recorded during the training.
The NOR test was conducted in three stages (habituation, familiarization, and discrimination) for 2 days.In brief, each rat was allowed to explore the open field (40 × 40 × 40 cm3) for 10 minutes during the habituation stage.Then 20 hours later, the rats were placed in the same field containing two identical objects and were allowed to explore for 10 minutes during the familiarization stage.Finally, 6 hours later, one of the two objects was replaced with a novel object of a different shape and color, and the rats were returned to the open field for 10 minutes during the discrimination stage.The NOR test values were recorded during the test, and were expressed as a percentage of the discrimination ratio calculated according to the following formula:Discrimination ratio (%) = (N – F)/(N + F) × 100%, where N represents the time spent exploring the new object, and F represents the time spent exploring the same object.
Tissue preparation
Rats were anesthetized by 2% pentobarbital sodium and perfused with 0.9%normal saline at 4°C, and then sacrificed at 3, 7, 14, and 28 days after rTMS.The brains were carefully removed and fixed for 12 hours, before being immersed sequentially in 20% and 30% sucrose until they sunk, as previously reported (Luo et al., 2014, 2017).
Immunofluorescencence staining
For BrdU immunostaining, frozen brain sections were treated with 2 N HCl at 37°C for 30 minutes.Subsequently, the sections were rinsed twice in 0.1 M borate buffer (pH 8.5) for 10 minutes each, followed by a 30-minute incubation in 3% hydrogen peroxide.After blocking with 5% normal goat serum at room temperature (25°C) for 1 hour, the sections were ready for BrdU immunostaining.
For cultured cells, NSCs were first washed with phosphate-buffered saline(PBS) and then fixed with 4% cold paraformaldehyde for 15 minutes.After fixation, cells were washed twice with PBS, and then incubated with primary antibodies overnight at 4°C.Immunofluorescence staining was performed using antibodies against rabbit anti-sex determining region Y-box 2 (SOX-2)(1:200, Abcam, Cambridge, MA, USA, Cat# ab97959, RRID: AB_2195792),mouse anti-Ki67 (1:200, Abcam, Cat# ab279653, RRID: AB_367707), rabbit anti-Nestin (1:200, Abcam, Cat# ab254048, RRID: AB_2235741), rat anti-BrdU (1:200, Abcam, Cat# ab6326, RRID: AB_1659966), rabbit anti-2,3-cyclic nucleotide 3 phosphodiesterase (CNPase) (1:200, Abcam, Cat# ab183500,RRID: AB_471100), rabbit anti-glial fibrillary acidic protein (GFAP; 1:200,Abcam, Cat# ab7260, RRID: AB_2716480), rabbit anti-NeuN (1:200, Abcam,Cat# ab177487, RRID: AB_11083391), and rabbit anti-β-catenin (1:200,Abcam, Cat# ab32572, RRID: AB_2629234) to visualize target proteins.Following primary antibody incubation, the cells were washed twice with PBS and then treated with secondary antibodies: Alexa Fluor 555/488 mouse anti-rabbit IgG (1:1000, Abcam, Cat# 4417S/4416S, RRID: AB_10895269/AB_10894182), Alexa Fluor 488 goat anti-guinea pig IgG-fluorescein isothiocyanate isomer I (FITC) (1:1000, Santa Cruz, Dallas, TX, USA, Cat#374194, RRID: AB_10764638), Alexa Fluor 488/555 rabbit anti-mouse IgG(1:1000, Cell Signaling Technology, Danvers, MA, USA, Cat# 4408S/4409S,RRID: AB_142495/AB_1500655), or Alexa Fluor 555/488 rabbit anti-rat IgG(1:1000, Cell Signaling Technology, Cat# 4416S/4417S, RRID: AB_10894907/AB_10893616) for 1 hour at room temperature.Finally, stained cells were imaged using a Zeiss Observer Fluorescence Microscope (Oberkochen,Germany) and Axiovision imaging software (Beijing Puresisi Instrument Co.,Ltd., Beijing, China).
For tissue analysis, immunofluorescence staining was used to observe the expression of Ki67, Nestin, BrdU, SOX-2, and β-catenin.Sections were initially treated with 5% normal goat serum for 1 hour at room temperature.Following this, sections were incubated overnight at 4°C with a mixture of the primary antibodies mentioned above.The following day, sections were incubated for 1 hour at 37°C with the secondary antibodies mentioned above.Sections were then rinsed and mounted in 4,6-diamidino-2-phenylindole(DAPI).Fluorescence signals were examined under a microscope (Thermo Fisher Scientific).
Magnetic resonance imaging
Rats were anesthetized with isoflurane (Hebei Yipin Pharmaceutical Co.,Ltd., Shijiazhuang, Hebei, China) while monitoring their respiration and body temperature.Anesthesia was induced at a concentration of 1.5% to 2.5%, and maintained at a concentration of 1.0% to 2.0%.Magnetic resonance imaging(MRI) was performed 9 days after MCAO using a 7.0 Tesla system with a Bruker console (Bruker Biospin, PharmaScan70/16, Atlanta, GA, USA).For T2-weighted imaging, an initial anatomical scan was acquired with a 35 × 35 mm2field of view and 128 × 128 image matrix.All six echoes were acquired with equal intervals of echo time (500 ms) and under the same readout gradient polarity in T2* weighted imaging (T2*WI).The total scanning time for each sequence was approximately 5–10 minutes.The infarct volume was calculated using MRIcron software (www.mricro.com).The infarct area was identified layer by layer using a scale to calculate the infarction area.Each infarct volume = product of each infarct area × thickness of a single layer,and the total infarct volume was the summation of all infarct areas across all layers.
RNA sequencing
RNA was extracted from the brains of five rats from sham, TMS (–), and TMS (+) groups, and 30 days after MCAO.The VAHTSTM Stranded mRNAseq Library Prep Kit for Illumina v2 (Cat# NR612, Vazyme Biotech, Nanjing,China) was used for RNA library preparation and sequencing, following the manufacturer’s instructions.The quality of the libraries was evaluated using the Bioptic Qsep100 Analyzer (Bioptic, Taibei, Taiwan, China).RNA sequencing(RNA-seq) was performed using Illumina NovaSeq 6000 (Illumina, San Diego,CA, USA).
Differentially expressed mRNAs were annotated for potential functions in various signaling pathways using the Database for Annotation, Visualization and Integrated Discovery (DAVID).Parental gene functional annotations were predicted using Gene Ontology (GO) functional annotation, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation was used to identify relevant pathways.KEGG analysis results were visualized using scatter plots.GO annotations were downloaded from NCBI (http://www.ncbi.nlm.nih.gov/), UniProt (http://www.uniprot.org/), and Gene Ontology (http://www.geneontology.org/).Pathway analysis was used to identify significant pathways of the differential genes according to the KEGG database (https://www.kegg.jp/kegg/kegg1.html).
Western blot analysis
Peri-infarcted cerebral cortical tissue was rapidly removed and homogenized in cell lysis buffer (Fermentas, Burlington, Canada) containing a complete protease inhibitor cocktail (Thermo Fisher Scientific).Next, cytoplasmic and nuclear proteins were extracted using an extraction kit (Cat#P0028, Beyotime Biotechnology).To prepare tissue homogenization solution, an appropriate amount of cytoplasmic protein was mixed with extraction reagents A and B in a 20:1 ratio.Phenyl methane sulfonyl fluoride was added to achieve a final concentration of 1 mM.The homogenization process was conducted at 4°C,followed by centrifugation at 1500 ×gfor 5 minutes at the same temperature.The resulting supernatant was transferred to a pre-cooled plastic tube for extraction of cytoplasmic proteins.To precipitate the remaining supernatant,vortexing was performed at the highest and most intense setting for 15–30 seconds, and repeated for a total of 30 minutes.Subsequently, the solution was centrifuged at 12,000–16,000 ×gfor 10 minutes at 4°C.The supernatant was immediately transferred to a pre-cooled plastic tube, which contained the extracted nuclear protein.
Equal amounts of protein were separated using 10% sodium dodecyl sulfatepolyacrylamide gels and transferred onto polyvinylidene fluoride membranes(Millipore, Billerica, MA, USA).The membranes were blocked with 5%skimmed milk in Tris-buffered saline with Tween 20 (TBST) for 1 hour at 25°C.They were then incubated overnight at 4°C with primary antibodies against: rabbit anti-β-catenin (Abcam, Cat# ab32572, RRID: AB_2629234),rabbit anti-H3 (Abcam, Cat# ab1791, RRID: AB_11184913), rabbit antiphosphorylated-GSK3β (p-GSK3β) (phospho Ser9, Abcam, Cat# ab93926,RRID: AB_2928098), rabbit anti-GSK3β (Abcam, Cat# ab280376, RRID:AB_2928097), rabbit anti-AKT (Abcam, Cat# ab188099, RRID: AB_1662415),rabbit anti-phosphorylated-AKT (p-AKT) (phospho S473, Abcam, Cat#ab81283, RRID: AB_1662951), and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Abcam, Cat# ab181602, RRID: AB_1726478); all at a dilution of 1:500.The membranes were then washed in TBST and incubated with an anti-rabbit horseradish peroxidase-conjugated secondary antibody(1:10,000, Cell Signaling Technology, Cat# ab288151, RRID: AB_420028)for 1 hour at room temperature.Membrane exposure was performed by chemiluminescence using Efficient chemiluminescence kit (P0018AS,Beyotime Biotechnology) under an automatic imager (Shanghai Tianneng Life Science Co., Ltd., Shanghai, China), and digital images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA; Schneider et al., 2012).
Quantitative polymerase chain reaction
RNA was extracted from peri-infarcted rat cortex using an RNA extraction kit(ES-QP002, Shanghai Yishan Biotechnology Co., Ltd., Shanghai, China).cDNA was synthesized using a reverse transcription kit and then amplified using a SYBR Green PCR kit (ES-QP002, Shanghai Yishan Biotechnology Co., Ltd.,) in a LightCycler 96 instrument (06713106001, Roche, Basel, Switzerland).The following procedure was used: initial denaturation at 95°C for 30 seconds;40 cycles at 95°C for 5 seconds, and 60°C for 20 seconds, followed by 65°C for 15 seconds.Fluorescence quantitative PCR (qPCR) detection was then performed.Primer sequences are provided in Additional Table 1.
Cell counting kit-8 assay
NSCs were resuspended and seeded onto 96-well plates at a density of 3 ×103cells per well.Following OGD stimulation, 100 µL PBS containing 10 µL of Cell Counting Kit-8 (C0037, Beyotime Biotechnology) was added to each well.After 3-hour culture, the absorbance of each well at 450 nm was measured using the BioTek Synergy (H1MF, Montpelier, VT, USA).
Calcium imaging
Cells were resuspended and seeded to a 96-well plate at 3 × 103cells per well.Medium was changed 24 hours later, and NSCs were washed twice before being treated with medium containing 1 µM Fluo-4AM (S1060, Beyotime Biotechnology) at 37°C.After 30-minute incubation, cells were stimulated by rTMS before ratiometric fluorescent values were recorded by a fluorescence microplate reader.
Flow cytometry assay
NSCs were washed twice and then treated with 1 µM Fluo-4AM (S1060,Beyotime Biotechnology) in culture medium.After a 30-minute incubation at 37°C, the cells were stimulated with rTMS and imaged using a Zeiss Observer Fluorescence Microscope and Axiovision imaging software.
TdT-mediated dUTP nick end labeling
First, frozen sections from the damaged side of the brain were fully hydrated and incubated with protease K solution for 10 minutes.Subsequently, the sections were incubated at 37°C for 1 hour in the dark, using TdT-mediated dUTP nick end labeling (TUNEL) reaction solution (11684795910, Roche).Following that, the sections were treated with diaminobenzine (DAB) for approximately 10 minutes.The observation method was the same as for immunofluorescence.
Statistical analysis
No statistical methods were used to predetermine sample sizes; however, our sample sizes were similar to those reported in previous publications (Guo et al., 2014; Luo et al., 2017).No animals or data points were excluded in the analysis.Most results are expressed as mean ± standard deviation (SD).The evaluator was blinded to group management.
All results are expressed as mean ± SD.Statistical comparisons were made using a two-tailed Student’st-test (between two groups) or a one-way analysis of variance (for multi-group comparisons).Changes in body weight andMNSS were compared using a repeated measures analysis of variance.P< 0.05 was considered to represent a significant difference.Data analysis and graphing were performed using the GraphPad Prism 6.01 (GraphPad, San Diego, CA, USA, www.graphpad.com).
Results
rTMS promotes the proliferation of NSCs after OGD treatment
Most of the cells isolated from fetal rat brain developed into NSC aggregates within 48 hours.Nestin and SOX-2, common markers of NSCs (Urbán et al.,2019), were found to be expressed in NSC aggregates and adherent NSCs(Figure 1B).After the addition of a specific differentiation medium, NSCs were able to develop into GFAP+astrocytes, CNPase+oligodendrocytes, or NeuN+neurons (Figure 1C).These findings indicate that we were successful in differentiating NSCs and characterizing their phenotype.
To verify whether rTMS contributes to the proliferation of NSCs, we investigated the effects of different frequencies (1 and 10 Hz) of rTMS on NSCs.We evaluated the proliferation of NSCs by Ki67 staining and identified NSCs using Nestin.Ourin vitrorTMS experiments showed that the proliferation percentage of NSCs was higher in the TMS (H) group compared with the TMS (L) group, as indicated by population analysis of Nestin+/Ki67+NSCs (Figure 1D and E).Cell counting kit-8 assay also indicated higher cell viability in the TMS (H) group than in the TMS (L) group (Figure 1F).Moreover,different rTMS frequencies did not visibly affect NSC apoptosis, as shown by TUNEL assay (Additional Figure 2A) and mRNA expression analysis of apoptosis-related markers (Bax and Bcl-2; Additional Figure 2B).
Brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) are two neurotrophic factors that have been shown to stimulate NSC proliferation and neurogenesis (Penner et al., 1993; Luo et al., 2017).Our qPCR results showed increased Bdnf and Ngf mRNA levels in the TMS (H) group (Additional Figure 3A).P21 and P57 are cyclin-dependent kinase inhibitors that control the G1 to S phase of the cell cycle by repressing downstream protein complexes (cyclinD1–cdk4, cyclin A–cdk2, and cyclin E–cdk2), and thereby influence NSC proliferation (Ivanovska et al., 2008; Joaquin et al., 2012; Liu et al., 2015).We found upregulated mRNA expression levels of these cell cyclerelated proteins (Cdk2 and Cdk4) in the TMS (H) group compared with the TMS (L) group, while mRNA expression levels of inhibitors (P21 and P57) were lower (Additional Figure 3B).From these experiments, our data indicate that rTMS can effectively promote NSC proliferation.
rTMS reduces brain infarct volume and improves recovery of neurological function in a rat model of experimental cerebral ischemia
The body weight and survival rate of rats were higher in the TMS (+) group than in the TMS (–) group (Figure 2A and B).To evaluate the impact of rTMS on the neurological function of rats following ischemia, we used the mNSS to measure motor recovery and overall neurological function.Notably, mNSS were consistently zero across all time points in the Sham group.In comparison with the TMS (–) group, the TMS (+) group showed a significantly greater improvement in the mNSS scale at 7, 14, and 28 days post-TMS (Figure 2C).No infarct areas were observed in the sham-operated group at any time point.Notably, at 7 days after rTMS, the relative infarct volume was significantly smaller in the TMS (+) group compared with the TMS (–) group (Figure 2D and E).These findings suggest that rTMS may have a beneficial effect on reducing infarct volume following brain infarction.
The TMS (+) group also showed significant improvements in neurocognitive behavioral function, as assessed by the NOR and MWM tests (Figure 2F–H).Specifically, at 28 days post-TMS stimulation, rats in the TMS (+) group showed a higher discrimination ratio compared with those in the TMS (–)group, indicating that rTMS improved NOR memory in MCAO rats.Moreover,on the 5thday, the TMS (+) group exhibited a shorter escape latency than the TMS (–) group (Figure 2I).In the spatial probe test, the TMS (+) group displayed a greater number of platform crossings and spent more time in the target quadrant than the TMS (–) group.Importantly, rTMS was able to reverse this deficit due to MCAO, as evidenced by the increased number of platform crossings (Figure 2J) and longer time spent in the target quadrant(Figure 2K).
rTMS promotes the proliferation of NSCs in the peri-infarct area of a rat model of experimental cerebral ischemia
We further assayed NSC proliferation in peri-infarct tissue from experimental ischemia rats at 7 days after rTMS.Overall, sham group rats exhibited less NSC proliferation compared with the TMS group (Figure 3A and B).The proliferation of NSCs was significantly higher in the TMS (+) group than in the TMS (–) group, as assessed by Nestin+/Ki67+(Figure 3A and B) and SOX-2+/BrdU+(Figure 3C and D) staining.These findings suggest that cerebral ischemia can stimulate the proliferation of NSCs, and that rTMS can improve the proliferative efficiency of these cells.
Gene expression profiling of the peri-infarct area in a rat model of cerebral ischemia
To further delineate differences between the peri-infarct area of rats subjected to experimental ischemia with and without rTMS treatment, we isolated total RNA from rat brains and performed RNA-seq.Cluster analysis of differentially expressed mRNAs revealed significant differences between rTMS-administered samples (TMS [+] group) and ischemic experimental tissues (TMS [–] group) or non-ischemic tissues (Sham group; Figure 4A and B).
Heatmap and volcano plot analyses showed that compared with the TMS(–) group or Sham group, the number of differentially expressed mRNAs was significantly upregulated in the TMS (+) group.To investigate the underlying mechanism of rTMS on experimental ischemic rats, we conducted KEGG pathway enrichment analysis.This found that significantly upregulated mRNAs in the TMS (+) group were enriched in 16 pathways compared with the TMS(–) group, and in 46 pathways compared with the Sham group.Using Venn diagram analysis, we identified 13 pathways common to both groups, which were selected for further analysis (Figure 4C).Together, the KEGG pathway and Venn diagram analyses suggested that upregulated expressed mRNAs were significantly associated with several pathways, including ‘Cytokinecytokine receptor interaction’, ‘Neuroactive ligand-receptor interaction’, ‘Wnt signaling pathway’, and ‘Cell adhesion molecules’ (Figure 4D).
rTMS promotes the proliferation of NSCs by activating the p-AKT/GSK3β/β-catenin signal pathway
To explore whether rTMS can promote NSC proliferation through the p-AKT/GSK3β/β-catenin signaling pathway, we analyzed the activity of this pathway in brain tissue following rTMS at various times.Our RNA-seq analysis screened out Wnt family genes in both the TMS (+) and TMS (–) groups.Compared with the TMS (–) group, no members of the Wnt family were significantly upregulated in the TMS (+) group (Additional Figure 4A).We detected the expression of highly expressed Wnt family members (with an average FPKM of > 2) in NSCs using qPCR and confirmed there was no significant difference in the expression levels of Wnt family genes between the TMS (+) group and TMS (-) group (Additional Figure 4B).
We found that p-GSK3β expression significantly increased in the TMS (+)group on days 3 and 28 compared with the TMS (–) group and sham-operated animals.Consistent with this effect, levels of β-catenin in the nucleus, the downstream target of p-GSK3β, were also significantly increased in the TMS(+) group (Figure 5A).Moreover, immunofluorescence staining revealed that β-catenin is more likely to enter the nucleus of Nestin+NSCs in the TMS (+)group than in the TMS (–) group and sham-operated animals (Figure 5B).
Our previous study showed that rTMS can promote the activation of AKT,which is thought to inhibit downstream GSK3β (Luo et al., 2017).To further explore whether rTMS promoted the proliferation of NSCs through the canonical Wnt signaling pathway, we analyzed the activity of this pathway in rat brain tissue following rTMS at various times.To confirm whether rTMS activates the GSK3β/β-catenin signaling pathway by promoting AKT activation, and thereby promoting NSC proliferation, we determined whether NSCs culturedin vitrowere stimulated by rTMS, and whether each pathway activated or inhibited cell proliferation.We found that expression levels of p-AKT increased significantly in the TMS (+) group on days 3, 14, and 28 compared with the TMS (–) group and sham-operated animals (Figure 5A).In addition, rTMS also improved expression levels of p-GSK3β, p-AKT, and β-catenin in the nucleus of NSCs and their fluorescent signals (Figure 5C and D).Moreover, using an inhibitor of AKT activation (LY294002), the function of rTMS in promoting AKT activation and activating GSK3β/β-catenin signaling was inhibited (Figure 5E), as was the effect on promoting NSC proliferation.
rTMS activates AKT through a Ca2+/P2 calcium channel/CaM pathway
To investigate how rTMS affects the p-AKT/GSK3β/β-catenin signaling pathway,a GO term enrichment analysis was conducted.This found that significantly upregulated mRNAs in group 1 (TMS [+]vs.TMS [–]) were enriched in 466 biological processes, while significantly upregulated mRNAs in group 2 (TMS[+]vs.Sham) were enriched in 708 biological processes.Based upon Venn diagram analysis, 229 biological processes in the intersection of both groups were selected for further analysis.
The GO and Venn diagram analyses revealed that the upregulated mRNAs were highly associated with biological processes related to cell proliferation and the Wnt signaling pathway.Among the biological processes, the results revealed that rTMS could significantly affect ‘positive regulation of cytosolic calcium ion concentration’ (Additional Figure 5A and B).As calcium ion (Ca2+)influx rate across the plasma membrane has a rapid influence on cytosolic Ca2+concentration (Willert et al., 2003), this indicates that rTMS may promote the process of Ca2+influx.
Recent studies have demonstrated that the AKT/GSK3β/β-catenin signaling pathway of bone marrow mesenchymal stromal cells can be inactivated by decreased Ca2+flux, leading to the regulation of cell growth and proliferation in various cell types (Pinto et al., 2015).This suggests that Ca2+flux may play a crucial role in the regulation of NSC proliferation by rTMS.Our findings indicate that rTMS can increase Ca2+influx in NSCs (Figure 6A and B) and increase the proportion of Fluo-4 AM+cells (Figure 6C).Moreover,rTMS-induced activation of the AKT/GSK3β/β-catenin signaling pathway was abolished by the calcium channel P2 antagonist, suramin (Figure 6D).Additionally, a phospholipase C inhibitor, U73122, inositol 1,4,5-trisphosphate receptor antagonist, 2-APB, and a recombinant calmodulin (CaM) antagonist,CPZ, all inhibited rTMS-induced AKT and GSK3β/β-catenin signaling pathway activation in NSCs (Figure 6D).Therefore, our results suggest that rTMSinduced activation of the AKT/GSK3β/β-catenin signaling pathway is dependent on Ca2+/P2 calcium channel/CaM signaling, which increases Ca2+influx, potentially through the purinergic receptor Y–phospholipase C pathway and purinergic receptor X calcium activation.
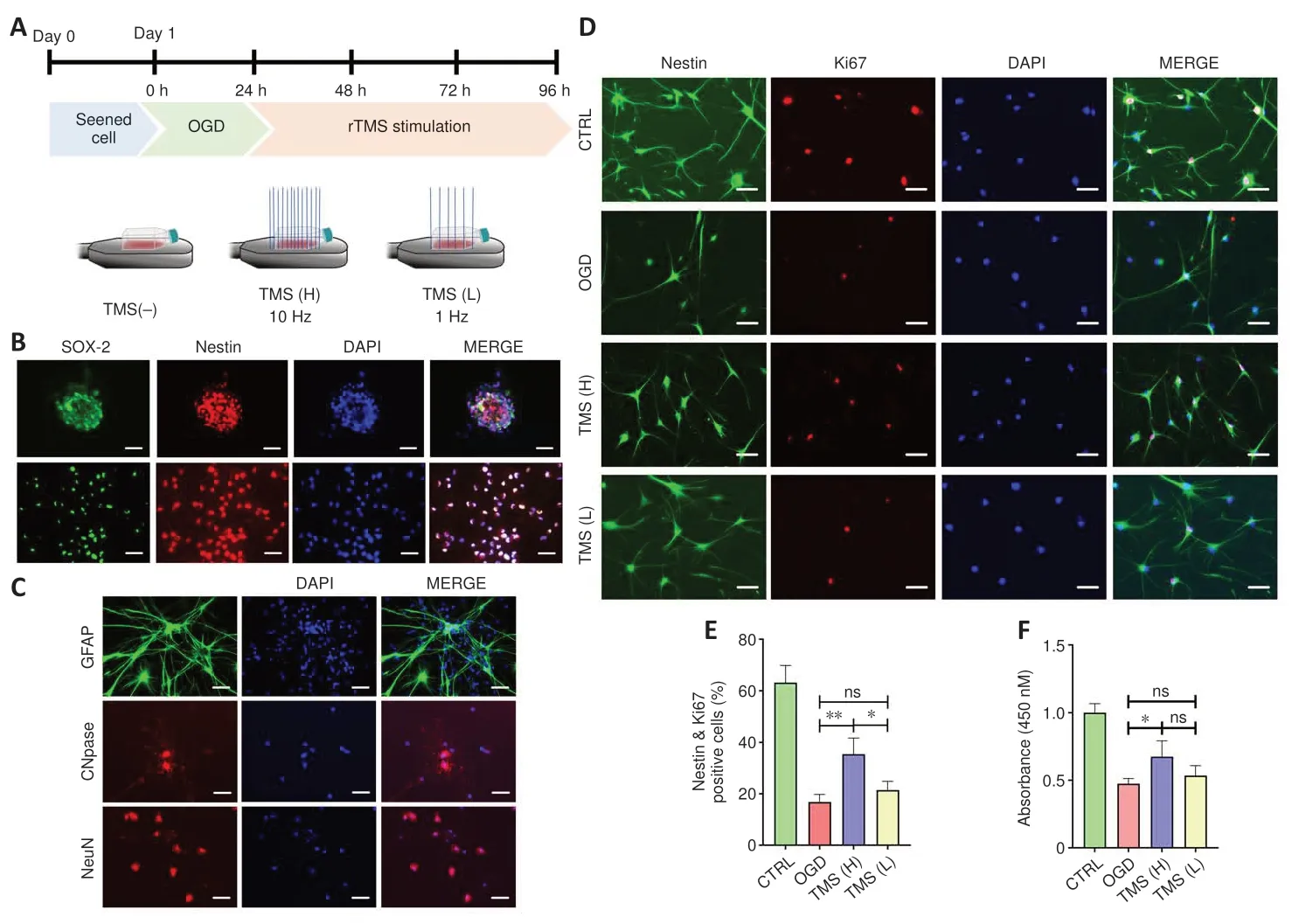
Figure 1 |Effect of different frequencies of rTMS on NSC proliferation.

Figure 2 |rTMS mitigates experimental ischemic brain injury.
Discussion
NSC-based therapy holds promising potential in treating stroke due to the self-renewal capacity and neurodifferentiation potential of NSCs.Studies have shown that rTMS, a noninvasive stimulation method, can help restore neurological function after cerebral infarction and promote NSC proliferation(Luo et al., 2017; Cui et al., 2019).However, the therapeutic effect of rTMS is dependent not only on the brain function of different stroke patients but also on the choice of stimulation scheme.It is crucial to determine the exact conditions and related mechanisms of rTMS to activate NSCs to promote the clinical application of rTMS for brain injury.Different frequencies of rTMS generate different therapeutic effects.Previous studies have shown that highfrequency (5 Hz) rTMS is primarily excitatory, while low-frequency (≤ 1 Hz)is primarily inhibitory (Pinter and Brainin, 2013).Low-frequency (1 Hz) rTMS treatment can improve the motor function of patients with cortical stroke(Kim et al., 2020), while high-frequency rTMS can promote the proliferation of mouse neuroblastoma cells and inhibit the apoptosis of OGD-injured cells(Baek et al., 2018).

Figure 3 |Effect of rTMS on NSC proliferation in the peri-infarct area.
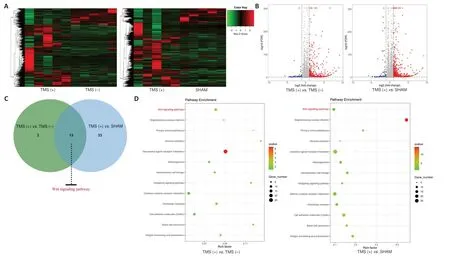
Figure 4 |The Wnt signaling pathway is highly associated with upregulated expression of mRNAs in the TMS (+) group.
In this study, we found that 1 and 10 Hz rTMS promoted the proliferation of NSCs culturedin vitro, with high-frequency stimulation having a better proliferative effect on NSCs.High-frequency rTMS is believed to promote the proliferation of NSCs for the following reasons.First, rTMS can promote the release of neurotrophic factors for NSC proliferation, such as BDNF and NGF(Zhao et al., 2019).Second, it can regulate the internal environment of NSCs,such as their signal transduction and gene expression profile, thus regulating the proliferation and differentiation of NSCs (Guo et al., 2014; Liu et al., 2015).Third, rTMS can activate cell proliferation-related pathways, such as ERK, AKT and other signaling pathways (Pinter and Brainin, 2013), which is consistent with our results.We also found that compared with low-frequency rTMS,high-frequency rTMS can promote BDNF release, which may be the reason for the different effects of the frequencies.
Our previous studies and those of others have shown that rTMS can effectively promote the proliferation of neural precursor cells or NSCs,with the underlying mechanism likely due to the induction of BDNF release and promotion of AKT phosphorylation (Zhao et al., 2019).However, it is not clear how rTMS can further promote NSC proliferation after inducing AKT phosphorylation.The Wnt signaling pathway is characterized by the phosphorylation and degradation of GSK3β as well as catenin entry into the nucleus, which is a key way to regulate cell proliferation and differentiation(MacDonald et al., 2009).
Several studies have shown that the canonical Wnt signaling pathway can regulate NSC proliferation (Penner et al., 1993; Li et al., 2020).Through transcriptome sequencing, we found that rTMS has a significant effect on the this signaling pathway, which can promote the proliferation of NSCs (Wexler et al., 2009).Further experiments revealed that rTMS does not directly promote the expression of Wnt family members, which act upstream of the Wnt/β-catenin pathway.Whereas inhibiting AKT phosphorylation inhibited the phosphorylation of GSK3β and β-catenin entry into the nucleus.These results indicate that rTMS can activate the GSK3β/β-catenin pathway by promoting the phosphorylation of AKT to upregulate expression of the cell cycle regulators, CDK2 and P21, and thus promote NSC proliferation.
In addition, BDNF is one of the key factors in promoting NSC proliferation.However, BDNF synthesis requires the activation of related signaling pathways that promote transcription, suggesting that BDNF may be an indirect effect of rTMS, and that rTMS affects other signaling pathways in NSCs.Related reports have noted that rTMS can promote the release of neurotransmitters from neurons by activating calcium channels, and thereby plays an important role in the recovery of nerve function (Grehl et al., 2015).However, whether rTMS can activate NSCs by activating calcium channels is currently unclear.In this study, we found that rTMS can promote Ca2+influx in NSCs by activating the P2 calcium channel/CaM pathway, thereby promoting AKT phosphorylation and activating the GSK3β/β-catenin pathway.As far as we know, our study is the first to report another putative mechanism for rTMS in the regulation of NSC proliferation, and therefore provides a new target and theoretical basis for the clinical development of rTMS therapy.

Figure 5 |Expression of phosphorylated-AKT (p-AKT), AKT, phosphorylated-GSK3β (p-GSK3β), GSK3β, and β-catenin in the nucleus of the ipsilateral hemisphere (excluding the infarct area).

Figure 6 | rTMS activates AKT through the Ca2+/P2 calcium channel/CaM pathway.
As a treatment strategy for brain injury, rTMS has shown encouraging therapeutic effects in all aspects of basic research and clinical trials(Wassermann and Zimmermann, 2012; Schoisswohl et al., 2019).Initially,research on rTMS mainly focused on the physiological, biochemical, and functional changes after local and remote cortical stimulation, with a focus on synaptic remodeling and neurotransmitter transmission.Increasingly, studies have shown that in addition to improving neuronal function through a series of electrochemical effects, rTMS also plays a significant regulatory role in other functional cells.Ueyama et al.(2011) highlighted that rTMS can activate endogenous NSCs in the hippocampus and subventricular zone.Hong et al.(2020) indicated that rTMS can inhibit neurotoxic astrocytic polarization in cerebral ischemic stroke.Clarke et al.(2017) reported that rTMS can affect microglia levels in the marginal area of injury.While Abbasnia et al.(2015)found that both 1 and 30 Hz rTMS treatment could increase NSC proliferation and neuronal differentiation.
Although the different intervention effects and modes of action of rTMS on brain injury are gradually being discovered, various effect parameters of rTMS in the treatment process need to be refined and standardized, and its internal mechanism of action needs to be further clarified.In short, in dealing with the clinical treatment of brain injury, more efforts are needed to discover its mechanism of action and optimize current rTMS treatment parameters for patient screening to obtain a better rehabilitation treatment effect.Moreover,our study has demonstrated that rTMS may promote neural recovery following stroke by promoting the proliferation of NSCs.However, it has also been reported that NSCs may not be an efficient therapeutic approach for major tissue damage in ischemic stroke (Janowski et al., 2015).The complex repair mechanisms after stroke makes it challenging to achieve ideal results solely by activating endogenous NSCs.Various other strategies can be used in combination, including the use of supportive biomaterials, the integration of neurorehabilitation strategies, pharmacotherapy, and cell transplantation(Boltze et al., 2019).In this study, we have chosen to use male rats to minimize the gender factor.This does not imply that our results are exclusive to male rats; future research on female rats will also be conducted.
In summary, we have demonstrated that high-frequency rTMS can promote the proliferation of NSCs and activate Ca2+influx mediated by the p-AKT/GSK3β/β-catenin signaling pathway.High-frequency rTMS treatment promotes NSC proliferation in the peri-infarct area and nerve function recovery in a rat model of ischemic brain injury.rTMS treatment may be an efficient and potential approach to support neurogenesis for further therapeutic applications, but also provide an effective platform for the expansion of NSCs.Although this study has identified a novel rTMS-related pathway that promotes NSC proliferation following cerebral infarction, it is not yet clear whether there are other crucial pathways involved.Additionally,gene knockout or overexpression methods were not used in this study to further investigate the mechanism, which is also a limitation that requires consideration in future experiments.
Author contributions:XH and LZ were responsible for the study design and manuscript drafting;JL,YF and ZH carried out most of the experimental work,including cell extraction and culturing,western blot assay,RNA purification,flow cytometry assay,CCK-8 assay and real-time-PCR;HZ conducted the MCAO model and immunostaining assay;MY conducted TMS stimulation and data analysis.All authors read and approved the final version of this manuscript.
Conflicts of interest:The authors declare that they have no conflicts of interests.
Data availability statement:The RNA-seq datasets generated in this study have been deposited in the CNCB National Genomics Data Center,with accession number PRJCA010295.Any other datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:Schematic diagram of the strategy to stimulate ischemic stroke rats.
Additional Figure 2:Effects of different frequencies of rTMS on NSCs apoptosis.
Additional Figure 3:Effects of different frequencies of rTMS on proliferation related markers.
Additional Figure 4:rTMS does not influence the expression on members of Wnt family.
Additional Figure 5:Regulation of cytosolic calcium ion concentration is found to be highly associated with the upregulated expressed mRNAs in TMS(+)group.
Additional Table 1:Primers used for the amplification of rat transcripts by qPCR
杂志排行
中国神经再生研究(英文版)的其它文章
- Glycolysis and glucose metabolism as a target for bioenergetic and neuronal protection in glaucoma
- MAP4K inhibition as a potential therapy for amyotrophic lateral sclerosis
- How do lateral septum projections to the ventral CA1 influence sociability?
- RNA sequencing of exosomes secreted by fibroblast and Schwann cells elucidates mechanisms underlying peripheral nerve regeneration
- Crosstalk among mitophagy, pyroptosis, ferroptosis,and necroptosis in central nervous system injuries
- Clustering of voltage-gated ion channels as an evolutionary trigger of myelin formation
