Vagus nerve stimulation in cerebral stroke: biological mechanisms, therapeutic modalities, clinical applications, and future directions
2024-02-11LiDuXuanHeXiaoxingXiongXuZhangZhihongJianZhenxingYang
Li Du, Xuan He, Xiaoxing Xiong, Xu Zhang, Zhihong Jian, Zhenxing Yang,*
Abstract Strokeis amajor disorder of the central nervous system that poses a serious threat to human life and quality of life.Many stroke victims are left with long-term neurological dysfunction, which adversely affects the well-being of the individual and the broader socioeconomic impact.Currently, poststroke brain dysfunction is a major and difficult area of treatment.Vagus nerve stimulation is a Food and Drug Administration-approved exploratory treatment option for autism, refractory depression,epilepsy, and Alzheimer’s disease.It is expected to be a novel therapeutic technique for the treatment of stroke owing to its association with multiple mechanisms such as altering neurotransmitters and the plasticity of central neurons.In animal models of acute ischemic stroke, vagus nerve stimulation has been shown to reduce infarct size, reduce post-stroke neurological damage, and improve learning and memory capacity in rats with stroke by reducing the inflammatory response, regulating bloodbrain barrier permeability, and promoting angiogenesis and neurogenesis.At present, vagus nerve stimulation includes both invasive and non-invasive vagus nerve stimulation.Clinical studies have found that invasive vagus nerve stimulation combined with rehabilitation therapy is effective in improving upper limb motor and cognitive abilities in stroke patients.Further clinical studies have shown that non-invasive vagus nerve stimulation, including ear/cervical vagus nerve stimulation, can stimulate vagal projections to the central nervous system similarly to invasive vagus nerve stimulation and can have the same effect.In this paper, we first describe the multiple effects of vagus nerve stimulation in stroke, and then discuss in depth its neuroprotective mechanisms in ischemic stroke.We go on to outline the results of the current major clinical applications of invasive and non-invasive vagus nerve stimulation.Finally, we provide a more comprehensive evaluation of the advantages and disadvantages of different types of vagus nerve stimulation in the treatment of cerebral ischemia and provide an outlook on the developmental trends.We believe that vagus nerve stimulation, as an effective treatment for stroke, will be widely used in clinical practice to promote the recovery of stroke patients and reduce the incidence of disability.
Key Words: cerebral stroke; neuroplasticity; non-invasive vagus nerve stimulation; rehabilitation;vagus nerve stimulation
Introduction
Stroke remains the most common fatal and disabling disease in the world (Thayabaranathan et al., 2022).Current mainstays of treatment for acute ischemic stroke include intravenous thrombolysis and mechanical thrombectomy (Xiong et al., 2019; Hasan et al., 2021); however, < 10%patients are effectively managed owing to technical or time limitations(Kuriakose and Xiao, 2020), with approximately 60% patients achieving only some degree of recovery after 6 months (Lee et al., 2015) and residual sequelae mainly manifesting as hemiplegia, sensory impairment, dysphagia,blurred vision, and cognitive impairment (Thriftet al., 2017; Hurford et al.,2020).Hence, exploring a novel, simple, and accessible therapy without compromising the established protocols such as existing thrombolytic therapy(Berge et al., 2021) and/or mechanical thrombectomy (Mokin et al., 2019) is the current focus of research.
As an important component of the parasympathetic nervous system, the vagus nerve contains a complex neuroendocrine-immune network linking the central nervous system and multiple peripheral organs, and plays an important regulatory role in maintaining the body’s energy metabolism,as well as regulating physiological or pathological processes such as stroke(Mravec, 2010).As a carrier of input and output information, the vagus nerve connects multiple brain regions and provides a degree of integration and appropriate feedback to perceived information (Yuan and Silberstein, 2016a;Goadsby et al., 2018; Fonseca et al., 2019; Huffman et al., 2019).Vagus nerve stimulation (VNS) has been used with good efficacy in the treatment of multiple brain dysfunctions (Stefan et al., 2012; Howland, 2014; Rong et al., 2016; Liu et al., 2023).Based on this, VNS was approved by the Food and Drug Administration (FDA) for the treatment of refractory epilepsy, refractory depression, cluster headache, and migraine (Silberstein et al., 2016; Conway and Xiong, 2018; Tassorelli et al., 2018; Aaronson et al., 2021).In addition,VNS has been applied to explore other brain disorders (Pruitt et al., 2016;Welling et al., 2016; Mondal et al., 2019; Dawson et al., 2020).
Several basic and clinical studies have confirmed that invasive vagus nerve stimulation (iVNS) combined with rehabilitation significantly reduces the size of post-stroke cerebral infarction, reduces neurological symptoms, and significantly improves cognition and limb motor function (Dawson et al.,2016; Kimberley et al., 2018; Du et al., 2022).However, concurrent iVNS therapy is associated with complications, with patients prone to postoperative complications such as cardiac arrhythmias, peritracheal hematoma, vocal cord injury, and respiratory distress (Ben-Menachem et al., 2015; Ma et al., 2019;Zhao et al., 2019; Li et al., 2020b).Furthermore, the time requirements of thrombolytic therapy (Berge et al., 2021) and/or mechanical thrombectomy(Mokin et al., 2019) make implantation of vagus nerve stimulators in the acute phase of stroke patients impractical.Previous research has found that transcutaneous VNS is informative for patient management and can act similarly to VNS through non-invasive stimulation of the vagus nerve (nVNS),typically through ear/cervical transcutaneous vagus nerve branch stimulation(Yap et al., 2020).In terms of upper limb recovery, nVNS is as effective as iVNS (Yakunina et al., 2018; Zhang et al., 2021) and can be applied to other dysfunctions such as aphasia and limb weakness, and can reduce the unsafe nature of iVNS (Redgrave et al., 2018b).Hence, it is a new research hotspot in the field of VNS treatment.The purpose of this review is to summarize and analyze the available literature on the efficacy of VNS in stroke and explore the possible mechanisms of its neuroprotective and neuroplasticity-improving effects.The clinical efficacy of iVNS and nVNS have been analyzed to describe their potential for clinical treatment of stroke and to further evaluate their efficacy and mechanisms of action.Finally, the paper also provides a review of the applications of different types of VNS and an outlook on their application.
Retrieval Strategy
A computer-based online search of the PubMed database was performed to retrieve articles published up to June 30, 2023.A combination of the following text words (MeSH terms) was used to maximize search specificity and sensitivity: “cerebral stroke”; “brain stroke”; “vagus nerve stimulation”;“neuroprotection”; “neuroplasticity”; and “rehabilitation.” The results were further screened by title and abstract, and only those studies exploring the relationship between VNS and neuroprotection in stroke were included to investigate the neuroprotective characteristics of VNS on stroke.No language or study type restrictions were applied.Articles that only involved neuroprotection in stroke without VNS therapy were excluded from the study.
Types of Vagus Nerve Stimulation and Effects of Vagus Nerve Stimulation on Ischemic Stroke
The vagus nerve originates from a complex mixture of nerves in the medulla oblongata of the brainstem and reaches the thorax, abdomen, and eventually the colon via the cervical foramen, where the vagus nerve is closely connected to the peripheral organs via the central nucleus (Berthoud and Neuhuber, 2000; Wiles et al., 2007; Hammer et al., 2018; Claudino Dos Santos et al., 2023).The cervical and nodal vagal ganglia are located in the region of the cervical foramen, with the nodal ganglia containing mainly cell bodies of the VN afferent fibers (Ruffoli et al., 2011).The auricular branch of the vagus nerve, as the only afferent branch of the vagus nerve distributed on the surface of the body, mainly innervates the external and internal auditory canal and the skin near the auricle (Peuker and Filler, 2002;Kiyokawa et al., 2014; Buttet al., 2020).Depending on the need for surgical implantation of a stimulator, there are two clinical stimulation methods: iVNS and nVNS.In recent years, four different VNS modalities have been proposed by scholars: cervically implanted VNS (iVNS), transcutaneous cervical VNS(tcVNS), transcutaneous auricular VNS (taVNS), and percutaneous auricular VNS (Farmer et al., 2020; Figure 1).The iVNS is a novel pacemaker-like device that works by stimulating electrodes connected to the left cervical vagus nerve with wires connected to a pulse generator and implanted in the skin of the upper chest (Pruittet al., 2016; Dawson et al., 2021; Pigato et al., 2023).For stimulation of nVNS, afferent VNS electrodes are used, connected to an external stimulator distributed through the skin in the external ear (taVNS)or neck (tcVNS) (Straube et al., 2015; Gaul et al., 2016; Genheimer et al.,2017).After long-term studies, iVNS was approved by the FDA in 1997 for the treatment of intractable semi-epilepsy (Morris et al., 2013) and in 2005 for the treatment of recurrent mono- and biphasic depression (Young et al., 2020).iVNS has been approved as a novel therapy in Europe, where it is also approved for epilepsy and drug-resistant/non-drug-resistant depression(DeGiorgio and Krahl, 2013; Young et al., 2020).Furthermore, iVNS has been widely used in clinical settings around the world.
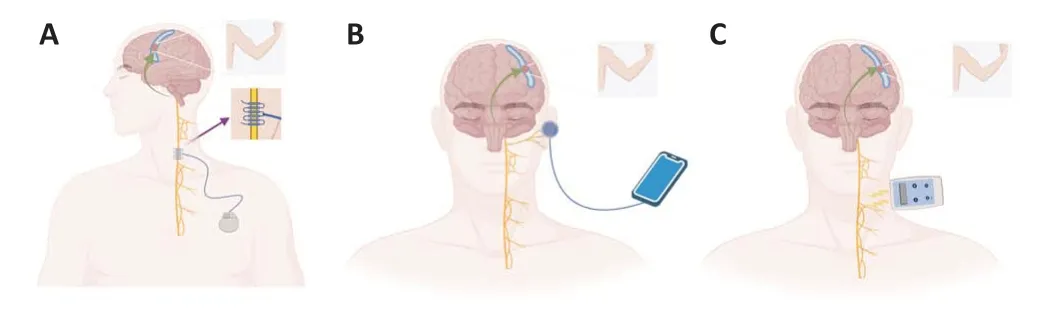
Figure 1 | Pattern for vagus nerve stimulation (VNS).
Given the invasive nature of iVNS and the multiple complexities of the treatment process, researchers have extensively explored a completely noninvasive mode of stimulation.nVNS has become a hot topic in clinical and translational medicine owing to its similar efficacy as iVNS, ease of handling,accessibility, and low adverse effects (Ben-Menachem et al., 2015; Frangos et al., 2015; Marin et al., 2018).nVNS entered clinical use in 1997 and has similar clinical outcomes and physiological effects to iVNS, with superior efficacy and physiological effects compared to iVNS (Redgrave et al., 2018b).tcVNS has also been approved by the FDA for the treatment of migraine(Martelletti et al., 2018) and cluster headache (Gaul et al., 2016; Marin et al., 2018).A number of controlled clinical trials have also demonstrated that combined rehabilitation with iVNS and nVNS can effectively improve motor and perceptual abilities in the upper limbs after stroke and offer new avenues for rehabilitation after stroke (Khodaparast et al., 2013; Capone et al., 2017; Dawson et al., 2021).In recent years, VNS has been widely used in the treatment of various neurological disorders such as epilepsy, migraine,and depression, but its main application has been in stroke.Here, we provide a systematic analysis of the basic and clinical rationale for the existing VNS therapies used to treat stroke, a comprehensive evaluation of the pros and cons of VNS for stroke, and an outlook on the clinical application of VNS therapies.
Effects of Vagus Nerve Stimulation on Ischemic Stroke
According to recent research results (Lindemann et al., 2020; Du et al., 2022;Zhao et al., 2022), VNS can reduce the volume of cerebral infarction, alleviate neurological dysfunction, improve forelimb motor function, and enhance memory and cognitive abilities in animal models of stroke (Table 1).
VNS can reduce infarct volume and neurological dysfunction
Numerous animal experiments have confirmed that VNS significantly reduces the size of brain infarcts and improves neurological function in rats (Ay et al.,2015; Zhao et al., 2019, 2022; Li et al., 2020b; Lindemann et al., 2020; Du et al., 2022).These studies found a 25–50% reduction in brain infarct size in the iVNS group compared to the control group in the same animal model of brain infarction, similar to previously reported findings with iVNS (Ay et al., 2011;Sun et al., 2012; Hays et al., 2016).Ay et al.(2016) used tcVNS intervention to observe its effects in rats after middle cerebral artery occlusion (MCAO)by varying the treatment window by plus/minus 1 hour at a time until a stimulation effect comparable to 30 minutes was achieved, and assessed its effects on brain tissue infarction and neurological recovery.It was found that the effect of tcVNS on reducing myocardial infarct size and improving neurocerebral function were consistent with stimulation starting within a 4-hour window after MCAO in rats.Furthermore, improvements in forelimb function were sustained even after cessation of VNS stimulation, a finding that was consistent with the results of Hays et al.(2016) who used iVNS combined with rehabilitation training to treat elderly rats with ischemic stroke.Preclinical experiments in animal models of stroke have found that in addition to the stand-alone use of VNS for stroke, VNS combined with rehabilitation not only improves cortical plasticity but also forelimb strength in rats with cerebral infarction (Khodaparast et al., 2013, 2016; Hays et al., 2014, 2016).In addition, several clinical rehabilitation samples have shown (Dawson et al.,2016; Capone et al., 2017; Redgrave et al., 2018b) that VNS combined with rehabilitation was effective in improving motor function in stroke patients and could improve patients’ limb movement scores.In addition, a study by Kilgard et al.(2018) also showed that VNS combined with tactile stimulation improved tactile thresholds, proprioception, and stereoscopic recognition in patients with chronic stroke.
VNS improves memory and cognition
VNS has also been shown to be effective in memory and cognitive dysfunction after stroke.Liu et al.showed that VNS improved learning ability in rats and facilitated brain recovery of spatial and fear memory within 30 min after ischemic stroke, where VNS was used as a single variable factor (Liu et al.,2016).Based on the results of this study, he proposed the hypothesis that VNS could mediate norepinephrine (NE) secretion through the locus coeruleus(LC) nucleus, and that elevated extracellular NE levels are an important mechanism by which VNS improves learning memory, mood, and neurorepair after stroke (Figure 2).In addition, the combined application of VNS and other rehabilitation therapies has the potential to improve memory.For example,repeatedly pairing a tone with a brief burst of VNS results in a reorganization of primary auditory cortex (Loerwald et al., 2018).VNS combined with training methods can improve learning and memory in rats (Sellaro et al., 2018), and affects people’s ability to recognize emotions based on eye regions and whole facial images and emotion recognition in human (Steenbergen et al., 2021).

Figure 2 | Proposed central mechanisms of vagus nerve stimulation.
The exact mechanism of action of cognitive impairment following VNS treatment is unclear.However, VNS may act through the thalamus-cortex(Shiramatsu et al., 2016) and the medial reticular formations of the medulla oblongata (Ogbonnaya and Kaliaperumal, 2013).VNS impairs stimulusspecific adaptation of the cerebral cortex without altering the thalamic response to it, suggesting that VNS may modulate inhibitory responses of the cerebral cortex, which suggests that VNS may modulate inhibitory responsesand thalamocortical connections, but not auditory peripheral-to-thalamic feedforward projections.The mechanism of action needs to be further investigated.
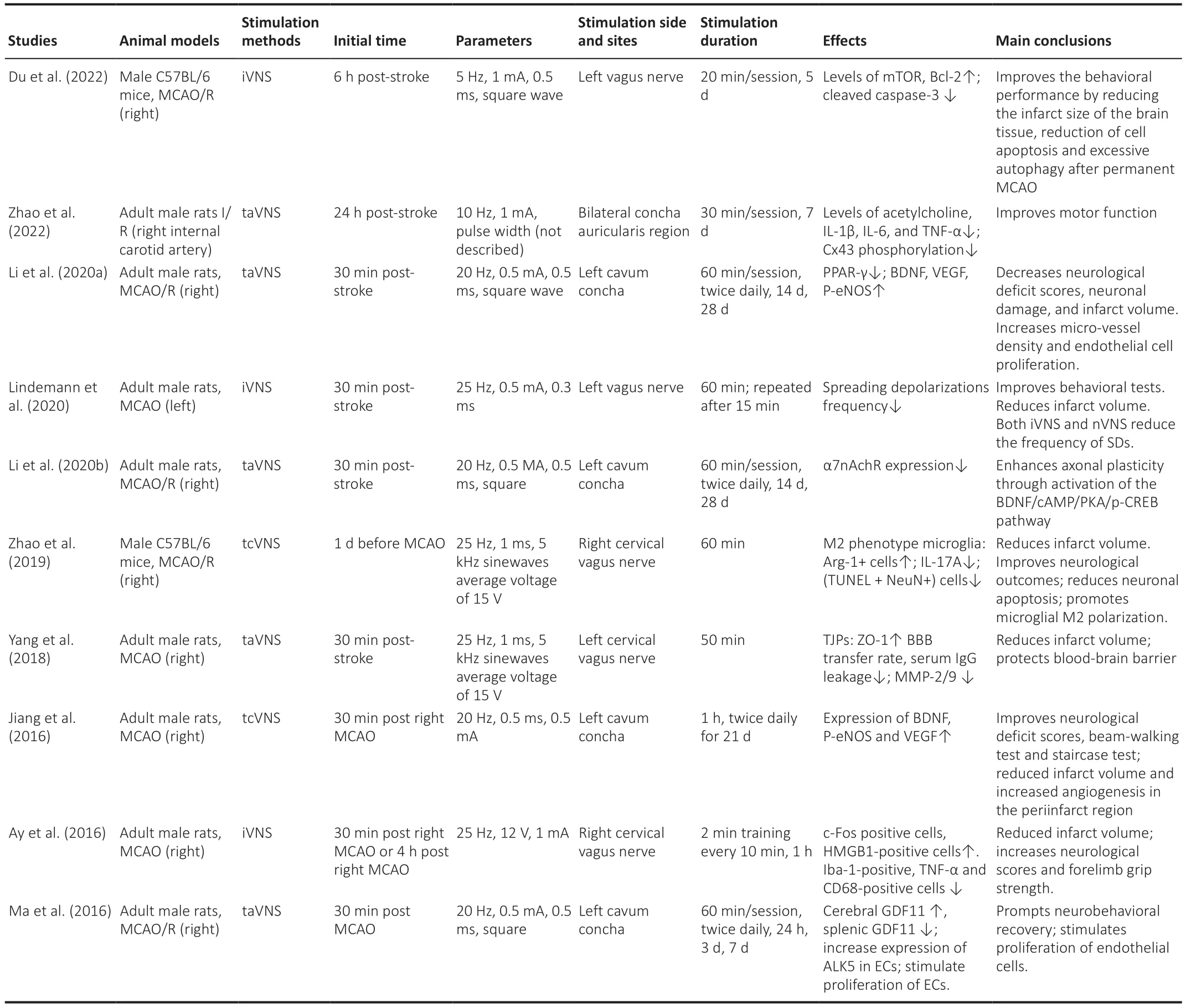
Table 1 |Main animal model studies of stroke receiving VNS in recent years
Mechanism of Action of Vagus Nerve Stimulation in Ischemic Stroke
Existing preclinical trials on VNS and stroke have mainly used VNS to establish rodent models of acute MCAO, originally designed by Ay et al.(2015) to provide a better understanding of the pathogenesis of VNS, given its better simulation of the clinical manifestations of MCAO and the different periods of treatment effect.In their study, Ay et al.(2016) used adult male Wistar rats as the study subjects and performed unilateral ligation of the middle cerebral artery to occlude the vessels for 1 hour.Rats were then subjected to leftventricular vagus nerve electrical stimulation with a pulse width of 0.5 ms,pulse frequency of 20 Hz, and pulse amplitude of 0.5 mA at 30 s/5 min.This study showed that in acute MCAO, VNS significantly reduced cerebral infarct volume compared to controls, and there was a significant improvement in neurological outcomes at 24 hours (Ay et al., 2015).Moreover, in this study, the effect of nVNS on infarct volume was found to be consistent when stimulation was initiated within 4 hours of MCAO, suggesting that the therapeutic effects of taVNS in acute stroke may involve changes in the signaling cascades and adaptations, with VNS treatment showing the same results.It was also found that nVNS treatment resulted in significantly higher levels of c-Fos-positive cell expression in the ipsilateral nucleus tractus solitarius (NTS) than in the contralateral side.In addition, nVNS down-regulated the expression of Iba-1,CD68, and tumor necrosis factor α (TNF-α) and up-regulated the expression of HMGB1.These results suggest that central brainstem function is activated and inhibits the production of residual inflammatory factor production after stroke,as seen in iVNS (Cunningham et al., 2008).Based on the results of existing VNS trials, we attribute the mechanisms by which VNS promotes functional improvement in ischemic stroke to the following: 1) VNS promotes cerebral neuroprotection in the acute phase of stroke and reduces neuronal apoptosis after stroke (Engineer et al., 2019); 2) VNS improves neural remodeling in the chronic phase of stroke, maximizing neuronal repair and compensation capacity, and reduces functional impairment (Xing and Bai, 2020).There is no absolute timing boundary between the effects by these two mechanisms, but they provide a concise and clear understanding of the main mechanisms of action of VNS at different times of ischemic stroke (Table 1).
VNS exerts its neuroprotective effects by enhancing the resistance of neurons to ischemic injury and promoting neural remodeling.Following cerebral ischemia, brain tissue undergoes a variety of damage such as excitotoxicity(Belov Kirdajova et al., 2020), oxidative stress (Chunchai et al., 2016),inflammatory response (Chamorro et al., 2016), apoptosis (Zhang et al.,2017), and mitochondrial dysfunction (Liu et al., 2018).Neuroprotection is designed to interrupt or reverse these adverse reactions.In addition to this, post-stroke can cause impaired microcirculation in the brain, impairing synaptic connections and neuronal processes (Chen et al., 2018).The main mechanisms of action of VNS to promote neural remodeling after stroke are by neural axon regeneration, synaptic enhancement (Meyers et al., 2018),neonatal neurons, and neovascularization (Murphy and Corbett, 2009).An in-depth exploration of VNS treatment in an acute MCAO rodent model is important to clarify the underlying mechanisms, temporal effects, and the strength of the therapeutic effects.By analyzing the results of existing experiments with iVNS and tVNS (Engineer et al., 2019) on the protection of cerebral neurological function after stroke, we found that they are both protective against post-ischemic injury in brain tissue, which may be because of an interaction of multiple underlying mechanisms.As tVNS has the same efficacy as iVNS as well as a higher safety (Burger and Verkuil, 2018; Engineer et al., 2019), we will detail the value of its clinical application in the following.Amongst others, the biological effects in animal models of stroke mainly include reduced systemic inflammation (Zhao et al., 2019), reduced diffusion depolarization (Lindemann et al., 2020), reduced blood-brain barrier (BBB)disruption (Yang et al., 2018), increased angiogenesis (Li et al., 2020b), and improved axonal regeneration and reorganization (Li et al., 2020b), among others (Figure 3).

Figure 3 |Potential neuroprotection mechanisms of vagus nerve stimulation (VNS).
Anti-inflammatory effects
The vagal nervous system inhibits the release of pro-inflammatory factors.Thus, it has been hypothesized that the VN maintains homeostasis through a complex network of neuro-endocrine-immune regulation that exists within it.The VN functions as a control center through interneural connections with brain regions that function to efficiently fuse information between different brain regions and to make adaptive adjustments and feedback.The VNS can affect a large range of vagal neurons that, in turn, regulate inflammatory responses (Yuan and Silberstein, 2016a), and its effects on a variety of inflammatory responses have been demonstrated.It has been reported that VNS inhibits the intestinal inflammatory response in rats with colitis and downregulates plasma expression of TNF-α, interleukin (IL)-1β,IL-6, and myeloperoxidase (Sun et al., 2012).tVNS was found by Hong et al.(2019) to activate vagal nucleus solitarius and dorsal motor nucleus neurons,downregulate intestinal cytokine expression, reduce the intestinal mucosal inflammatory response, decrease leukocyte recruitment to manipulated intestinal segments, and improve gastrointestinal transit after IM, thereby enhancing intestinal mucosal barrier function.In addition, tVNS was found to inhibit the secretion of inflammatory factors such as IL-6 and TNF-α.Lerman et al.(2016) showed that after 24 hours, the nVNS group’s non-LPSstimulated whole blood inflammatory factors such as IL-1β, TNF, chemokines,IL-8, macrophage inflammatory protein 1α, and monocyte chemoattractant protein-1 levels were significantly reduced.Meanwhile, Clancy et al.(2014) reported that tVNS increased the heart rate variability and inhibited sympathetic activity in normal subjects.
VN mediates the interaction of the central nervous and immune systems,with the cholinergic anti-inflammatory pathway being the mediating mechanism.Acetylcholine (ACh) stimulation of nicotinic receptors on splenic macrophages is responsible for this mechanism, which significantly attenuates the inflammatory response in stroke mice (Han et al., 2017; Hoover, 2017;Schuhmann et al., 2021).During cerebral ischemia, VNS induces the release of norepinephrine from splanchnic nerves and activates T cells by stimulating the cholinergic anti-inflammatory pathway that, in turn, generates ACH and acts on the α-7 nicotinic acetylcholine receptor (α-7nAChR), which is predominantly expressed on macrophages.Ultimately, the intact pathway inhibits the release of TNF and other cytokines (Bernik et al., 2002; Bonaz et al., 2013) into circulation.
Studies (Kalkman and Feuerbach, 2016; Hoover, 2017; Corsi-Zuelli et al.,2017) have shown that during stroke, the α-7nAChR is an important target for inhibiting the secretion of inflammatory factors by macrophages and dendritic cells.VNS causes macrophages and other microglia to produce large amounts of acetylcholine, which is activated in the α-7nAChR to suppress inflammatory responses (Kalkman and Feuerbach, 2016).Finally, TNF and other proinflammatory cytokines that play a role in inflammation are suppressed (Oke and Tracey, 2009).For mouse models of ischemic stroke, α-7nAChR agonists inhibit pro-inflammatory macrophage (M1) inflammatory responses and attenuate oxidative stress to reduce neurological damage in the brain after stroke (Han et al., 2014).A large body of literature reports that α7nAChRs can mediate taVNS, giving it a significant anti-inflammatory effect (Corsi-Zuelli et al., 2017; Zhao et al., 2022).
Microglia are an important cause of post-stroke neurological deficits, and blocking their activation can improve post-stroke neurological deficits and reduce brain damage (Sasaki, 2017).Recent studies (Sasaki, 2017; Zhao et al., 2019; Baig et al., 2022) have revealed that activated microglia play a key role in inhibiting neuronal apoptosis, increasing the number of new neurons and improving cognitive impairment after ischemic stroke.At the same time,the different polarization of microglia may explain the dual role of microglia in ischemia (Sasaki, 2017; Baig et al., 2022).Results fromin vitrocellular experiments suggest that following cerebral ischemia, ischemic neurons drive microglia to polarize towards the M1 phenotype that can exacerbate hypoxia and hypoxia-induced neurological damage (Hu et al., 2012).In contrast,under ischemic and hypoxic conditions, M2 phenotype polarization not only attenuates ischemic injury but also inhibits the post-ischemic inflammatory response (Hu et al., 2012) by producing anti-inflammatory cytokines such as IL-4 and IL-10 (Liu et al., 2016; Zhao et al., 2019) and promoting the repair of post-ischemic brain injury (Zhang, 2019).It was found that tVNS activates the α-7nAChR and is accompanied by M2-type polarization, suggesting that tVNS has certain anti-inflammatory effects (Zhang et al., 2017).tcVNS was shown by Zhao et al.(2019) to attenuate brain ischemic injury and reduce apoptosis by promoting M2 polarization in microglial neurons.Meanwhile, a study by Ay et al.(2016) found that tcVNS inhibited microglia activation and the number of cells containing TNF-α (expressed by M1 microglia) after MCAO.Similarly, tVNS was associated with upregulated expression of brain-derived neurotrophic factor (BDNF) (Jiang et al., 2016) and peroxisome proliferatoractivated receptor γ (Li et al., 2020a), both of which promoted the M2 microglial phenotype (Jiang et al., 2020), suggesting that nVNS may exert its role in ischemic neuroprotection by suppressing the inflammatory response.
Regulation of BBB penetration rate
Disruption of the BBB and resulting brain edema are important causes of secondary brain injury (Cai et al., 2014) and deteriorating neurological deficits in patients with ischemic stroke (Candelario-Jalil et al., 2022).The mechanism of occurrence primarily comprises pro-inflammatory cytokines such as TNF-α, which can induce matrix metalloproteinase activation (Candelario-Jalil et al., 2022).tcVNS was found to significantly inhibit BBB metastasis after stroke in the study by Yang et al.(2018) and serum IgG spillover in the lesion area of acutely ischemic rats, and correlated with brain infarct volume.In addition, VNS protected microvascular junctional proteins from destruction and significantly reduced matrix metalloproteinase-2/9 levels in mice braintissue after brain injury.At the same time, VNS reduced vascular permeability and AQP-4 expression in the cerebral cortex, delaying cortical damage and neuronal degeneration after TBI (Lopez et al., 2012).More interestingly,VNS significantly ameliorated cortical ischemia-reperfusion injury and attenuated microinfarction in mice, possibly by inhibiting BBB permeability,neuroinflammation, and oxidative stress (Chen et al., 2018).VNS treatment protected BBB integrity by limiting enhancement of the transcellular pathway in cortically dysplastic animals (Kaya et al., 2013), suggesting the potential clinical application of VNS for the treatment of BBB dysfunction after stroke.
Reducing excitotoxicity
The findings from Belov Kirdajova et al.(2020) suggest that after ischemic stroke, the oxidative phosphorylation process is interfered with by factors such as blood glucose and hypoxia, resulting in organismal failure and ion homeostasis, causing depolarization of cell membranes and the accumulation of large amounts of excitatory glutamate outside the cell, which in turn causes glutamate receptors to be chronically activated, resulting in increased Ca2+ions and disturbed glutamate secretion, thereby exacerbating the neuronal damage caused by ischemic stroke.Downstream Ca2+-dependent enzymes are thought to be key Ca2+-dependent signaling pathways, and their activation can cause the development of neuronal apoptosis, free radical formation, edema,and inflammation.Oxidative stress is the main cause of nerve cell damage after ischemic brain injury (Radak et al., 2014).A gerbil ischemia model was tested which showed that invasive VNS reduces glutamate secretion and increases hippocampal neuronal survival (Miyamoto et al., 2003).Following cerebral ischemia, hyperemia and excessive glutamate release cause massive production of reactive oxygen species in the brain tissue, which in turn exacerbates brain damage.Therefore, VNS has an effect on glutamatergic excitation and reperfusion damage to brain tissue following cerebral ischemia,and this effect is exerted via the afferent vagus nerve (Miyamoto et al., 2003).We tested a rat brain ischemia/reperfusion (I/R) model and showed that VNS modulates malondialdehyde, glutathione, and superoxide dismutase in rat cortical and subcortical brain tissue (Ekici et al., 2013), and reduces neuronal response to oxidative stress.In addition, in animal studies, VNS has been shown to alleviate cardiac damage while also maintaining the activity of antioxidant enzymes and reducing the production of reactive oxygen species and oxidative stress following cardiac ischemia (Kong et al., 2012; Chen et al., 2016a; Nuntaphum et al., 2018).VNS is considered to be a promising approach against cerebral ischemic diseases owing to its good cerebral protective effects and antioxidant properties.
Suppressing cell apoptosis
Following acute cerebral ischemia, apoptosis is a major contributor to neuronal death (Radak et al., 2017).Apoptosis affects a number of processes that include, among others, DNA breakage, degradation of the cytoskeleton and nuclear proteins, cross-linking of proteins, formation of apoptotic bodies, expression of phagocyte receptor ligands, and ultimately uptake by phagocytes (Xu et al., 2019).Previous findings (Jiang et al., 2014, 2015) have shown that in I/R rats, VNS treatment significantly reduces the expression of TdT-mediated dUTP-deficient markers, pro-inflammatory cytokines, and cleaved caspase 3 proteins.This suggests that VNS may play its important function in attenuating the inflammatory response and apoptosis in ischemic stroke.Furthermore, VNS reduces neuronal apoptosis, which is mainly achieved by upregulating the expression of lipocalin prostaglandin D2 synthase in ischemic stroke peripheral cortical neurons, as evidenced by decreased levels of Bax and cleaved caspase-3 and increased levels of Bcl-2 after brain I/R injury (Zhang et al., 2017).It has also been reported in the literature that VNS inhibits miRNA-215 expression, downregulates caspase-3,Bcl-2, and other related x-protein levels and attenuates cardiac apoptosis in the heart tissue of rats with chronic heart failure (Xuan et al., 2017).In a myocardial ischemia model, VNS also significantly reduced cardiomyocyte apoptosis in mice after heart infarction (Chen et al., 2016a; Xue et al., 2017;Nuntaphum et al., 2018).VNS-targeted inhibition of neuronal apoptosis will provide new ideas for the treatment of stroke.
Stimulating neurogenesis and enhancing neuronal plasticity
Neuronal regeneration and axonal remodeling are critical for neural repair in the days to weeks following ischemic stroke (Liu et al., 2015).After acute ischemic stroke, VNS leads to decreased neurofilament-L and myelin basic protein production, whereas glial fibrillary acidic protein and brain-derived neurotrophic factor are elevated 14 days after stroke (Bu et al., 2021).The subventricular zone of the lateral ventricular wall and the submedullary zone of the dentate gyrus are important sites of neuronal regeneration, and this process is regulated by multiple signaling pathways.Therefore, improving neural regeneration after stroke is an important strategy for stroke control (Lu et al., 2017).Studies suggest that VNS can induce the generation of available progenitor cells in the dentate gyrus of adult rats and that cell proliferation is the mechanism of action (Revesz et al., 2008; Ogbonnaya and Kaliaperumal,2013), suggesting that VNS can exert neuroprotective effects by inducing hippocampal plasticity.It has also been postulated that VNS has an activating effect on neuronal signaling pathways in the brain after TBI (Kumaria and Tolias, 2021; Srihagulang et al., 2022).Therefore, VNS promotes neuronal regeneration and is important in promoting post-stroke repair.
Biggio et al.(2009) showed that VNS induced an increase in bromodeoxyuridine (BrdU+) cells in the dentate gyrus.In addition, it consistently induced increased dual corticotropin (DCX) and BDNF immunoreactivity and upregulation of DCX+and BDNF+neurons in the hippocampus, and significantly upregulated BDNF/cAMP/PKA/P-CREB pathway protein expression after stroke, showing similar changes.Meyers et al.(2018) discovered that VNS increased plasticity in rat cortical and spinal motor neural circuits and increased their connections to neural circuits in the rat upper limb, suggesting that VNS promotes generalization, durable recovery, and structural plasticity in motor networks.Similarly, VNS promotes GDF11 expression in brain infarct tissues (Ma et al., 2016).Lu et al.(2018)showed that GDF11 improves cognitive function in stroke mice by regulating BDNF expression, promoting cell proliferation, and enhancing neuro/vascular regeneration after stroke.It was hypothesized that VNS could significantly increase axonal remodeling and improve neurological recovery after stroke (Li et al., 2020b).
Promoting angiogenesis
During stroke recovery, new arterial branches are gradually generated in the ischemic brain tissue, thereby improving local blood supply and facilitating the recovery of neurological function.Recent studies have found that neurological recovery after brain injury is accompanied by nerve regeneration and angiogenesis (Song et al., 2019; Alrafiah et al., 2021; Wang et al., 2021).Further findings have showed that VNS can increase the expression levels of BDNF, endothelial nitric oxide synthase, and vascular endothelial growth factor after stroke, thereby improving cognitive performance after stroke (Jiang et al., 2016; Ma et al., 2016; Zhang et al., 2017).Results from another study showed that VNS, in turn, increased GDF11 expression in brain tissue and decreased splenic GDF11 levels after stroke, thereby improving neurobehavior after stroke, suggesting a brain-spleen interconnection during stroke (Ma et al., 2018).In addition, VNS also upregulates the expression of ALK5 on ECs and enhances the proliferative capacity of ECs.Therefore, we hypothesize that GDF11 promotes post-stroke revascularization through redistribution and that GDF11/ALK5 may be a new target for stroke prevention and treatment.Taken together, VNS can increase GDF-11 levels after stroke, which in turn promotes angiogenesis and improves individual functions after stroke (Ma et al., 2016).
Ameliorating mitochondrial dysfunction and reducing the spread of depolarizations
Several studies have demonstrated that VNS is mitochondria-mediated and that VNS enhances mitochondrial function, maintains its dynamic homeostasis, protects the heart after myocardial I/R injury and shifts cardiac fatty acid metabolism towards β-oxidation following injury (Chen et al.,2016a; Nuntaphum et al., 2018).In addition, it has also been shown that VNS attenuates mitochondrial communal energy abnormalities in mouse cardiomyocytes after scalding (Nuntaphum et al., 2018).At the same time,VNS can reduce mitochondrial volume in cardiac muscle tissue, decrease ATP production, and regulate apoptosis-related signal transduction, thus inhibiting apoptosis (Shinlapawittayatorn et al., 2014).The above studies suggest that mitochondrial dynamics targeting VNS are expected to be a new idea for the prevention and treatment of acute myocardial infarction.
Following stroke, brain tissue undergoes diffuse depolarization, which is caused by failure of the sodium pump in the penumbra and can result in reduced blood flow within brain tissue, cytotoxic edema, apoptosis, and brain tissue damage following stroke (Dreier et al., 2018; Baig et al., 2022).Lindemann et al.(2020) showed that both nVNS and iVNS decreased SD frequency in the rat cortical area, while having no significant effect on blood flow, blood pressure, heart rate, or respiration during MCAO in rats after cerebral ischemia.They suggested that VNS is both a safe and efficient method to reduce the clinical burden of SD waveforms in stroke and other conditions.
Clinical Studies of Vagus Nerve Stimulation Treatment after Stroke
VNS has been approved by the EU in 1994 and by the FDA in 1997 as an adjunctive treatment for drug-resistant epilepsy in adults and adolescents over the age of 12 years (Aaronson et al., 2021).VNS is now being used increasingly in clinical practice and is considered an effective treatment for a range of neurological disorders such as depression, anxiety, and Alzheimer’s disease (Khodaparast et al., 2013; Hays et al., 2014, 2016; Dawson et al.,2016, 2022; Pruittet al., 2021).In addition, findings from clinical trials have found that VNS can directly improve neuromodulation in stroke patients and have targeted synaptic plasticity in central networks to improve brain injury recovery outcomes.Therefore, we have summarized the national and international studies related to the clinical treatment of stroke (Table 2).
Effect of VNS combined with rehabilitation exercises on upper limb function in stroke patients
The use of vagal electrophysiology techniques combined with exercise training can improve upper limb function in patients after stroke (Dawson et al., 2016, 2021; Kimberley et al., 2018).Dawson et al.(2016) first reported on the potential efficacy of invasive VNS in patients with chronic stroke.They selected 21 patients who had ischemic stroke under 6 months ago and moderate-to-severe upper limb injury to be treated with VNS combined with rehabilitation therapy or rehabilitation therapy alone.Treatment lasted 6 weeks and was performed at a frequency of 2 h/session and 3 sessions/week with > 400 exercise trials each time.The study results showed a significant increase in the Fugl-Meyer assessment-upper limb score in the VNS group with no side effects of the device (Dawson et al., 2020).According to Dawson’s recent study at 19 stroke treatment centers across the UK and USA,108 patients with moderate or severe upper limb dysfunction had suffered a stroke more than 9 months ago and had undergone 3 months of VNS treatment.Of these, 47% patients in the VNS group had clinically significant FMA-UE score outcomes, compared to only 24% in the control group (Dawson et al., 2021).
Similar findings were found by Redgrave et al.(2018b).In their study, 13 patients with upper limb disability who had had a stroke more than 90 days ago were tested using a combination of VNS and upper limb rehabilitation techniques at a frequency of 1 hour/session, 18 sessions/6 weeks, 30–50 sessions/8–10 arm movements (combined with VNS), with a significant increase in Upper Limb Fugl-Meyer (UFM) score of 17.1 ± 7.8 points after treatment.Capone et al.(2017) found that taVNS combined with robotic arm training significantly improved upper limb motor performance and longterm outcome in stroke patients.Morrison et al.also demonstrated that VNS significantly increased synaptic plasticity in cortico-brainstem circuits that regulate upper limb movements and enlarged cortical areas (Morrison et al.,2020, 2021).VNS combined with rehabilitation therapy can enhance synaptic remodeling in cortical upper limb circuits, providing new ideas for early intervention of moderate and severe upper limb dysfunction after stroke.
As we discussed earlier, previous studies have mostly focused on chronic stroke, and the efficacy of VNS for acute and subacute cerebral infarction and its mechanism and timing for promoting neuroplasticity after brain injury need to be further explored.Wu et al.(2020) found that after 15 days to 3 months of VNS treatment in patients with subacute cerebral infarction, the VNS group showed significant improvement in FMA-UE scores at followup, suggesting that VNS also has beneficial effects on the recovery of upper limb motor function in patients with subacute ischemic stroke.Therefore,in this paper, a more detailed study of the intermediate stage of stroke was conducted to further investigate the facilitative effect of VNS on functional remodeling of brain regions after stroke and its mechanisms.
VNS in stroke patients improves perceptual function
Many types of neurological damage, such as stroke and nerve injury, can lead to abnormal sensory function in patients.Clinical case reports have shown that the use of VNS with tactile training significantly improves limb perception and results in specific, sustained plasticity in the cerebral cortex.Baig et al.(2022) found that taVNS with repetitive motor task training improved perceptual function in patients with chronic stroke.Twelve patients with ischemic stroke and residual upper limb weakness and sensory deficits lasting more than 3 months post-stroke received an average of 18 sessionsof VNS combined with rehabilitation exercises (1 hour each) over a 6-week period.The UFM score was used to evaluate the patients’ limb contact and proprioception before and after treatment.Seven of the 11 patients (64%)with perceptual impairment at baseline showed some improvement in perceptual impairment after treatment.Moreover, in UFM, those patients who had higher improvements in sensory impairment had better mobility improvements.Another long-term follow-up study reported that VNS combined with tactile therapy improved perception in the left hand and arm after stroke and proposed the idea that VNS may enhance synaptic connections to the muscle tissue of the rehabilitated upper limb by facilitating remodeling of the cortical-spinal motor nerve circuit (Meyers et al., 2018).

Table 2 |Main clinical studies of VNS in stroke in recent years
In some stroke patients, cortical reorganization was accompanied by phantom limb pain, which has a similar pathological mechanism to that of stroke.We have therefore proposed the hypothesis that established principles of stroke rehabilitation also apply to the rehabilitation of phantom limb pain (Acerra et al., 2007).Chronic pain after partial stroke is closely associated with abnormal cortical remodeling (Rasche et al., 2006; Michielsen et al., 2011), and poor plasticity of the central nervous system is thought to be responsible for some forms of sensory disturbance and chronic pain.Taken together, the authors speculate that combining VNS with sensory stimulation may be a novel means of promoting neuroplasticity and improving cognitive function in stroke patients with chronic stroke.
VNS therapy to treat post-stroke motor speech disorders and dysphagia
Because of the proximity of the two brain regions that control limb and speech movements and the common mechanism of action between them and rehabilitation, it has been suggested that the motor learning and rehabilitation principles commonly used in upper limb therapy could also be applied to the treatment of speech motor control (Grimme et al., 2011).Recent research has found that VNS significantly improves the function of muscle and brain circuits in the brain, increases plasticity in corticospinal upper limb circuits, and significantly enhances synaptic plasticity in corticospinal circuits that mediate mouth-lip movements (Morrison et al.,2021).Because VNS increases the plasticity of the cortical upper limb circuits and thus improves the recovery of upper limb function, motor speech disorders such as aphasia and dysarthria in stroke can be improved by a similar mechanism of action.
Swallowing disorders are one of the most common conditions following stroke (Barrittand Smithard, 2009; Stipancic et al., 2019), and approximately 40% of all stroke patients experience persistent swallowing disorders that are highly susceptible to respiratory tract damage and consequent lung infections(Terre and Mearin, 2009).Similar to the recovery process of motor speech impairment, plasticity in the oropharyngeal motor area in the cerebral cortex plays an important role in the recovery process after stroke.Owing to the high rate of comorbidity between motor speech dysfunction and the neurological control centers of post-stroke swallowing disorders, similar pathological mechanisms between the two have been observed, and the crossover between the two has potential clinical applications.Hence, VNS is expected to be a new training method to assist in improving speech function.
VNS treat post-stroke sleep-disordered breathing and other psychiatric disorders after chronic stroke
There is now a growing body of evidence that sleep-disordered breathing has a negative impact on cardiovascular risk, cognitive function, and quality of life, typified by chronic stroke (Duss et al., 2018).Numerous studies have confirmed that sleep-disordered breathing is an independent risk factor for poor prognosis in cerebral ischemia, and there is growing interest in its role in improving prognosis in cerebral ischemia.VNS has been shown to be effective for depression and primary insomnia (Liu et al., 2020).A variety of psychiatric disorders such as anxiety, depression, and drug use that arise after stroke are associated with maladaptive plasticity in the brain’s nociceptors (Brunoni et al., 2008; Lozano, 2011).Because of the interconnections between the basal ganglia and the frontal-thalamus, reduced functional connectivity between the two causes abnormalities in the affective circuitry, and after VNS stimulation, functional connectivity occurs in the emotion-related posterior cingulate cortex and basal ganglia.This suggests that VNS, together with appropriate behavioral or cognitive therapy, may be an effective intervention to improve mood and sleep abnormalities after stroke.
A clinical study to assess the efficacy and safety of VNS in all stages of stroke
Surgical complications as well as late complications related to the device and to stimulation of the VN may occur during VNS treatment (Giordano et al.,2017).The main early postoperative complications that occur include cardiac constriction due to excessive intraoperative exercise, peritracheal hematoma,infection, ventricular septal defect, hoarseness, dyspnea, and dysphagia.Delayed cardiac arrhythmias because of vagal excitation, laryngopharyngeal hypofunction, and vocal cord damage are common late and serious clinical sequelae (Ekmekci and Kaptan, 2017).The toxicities of VNS devices are mostly mild-to-moderate and can be adjusted without disassembly of the device,making them safe and reliable (Ekmekci and Kaptan, 2017).
At present, a large number of clinical trials have been conducted in China and abroad for the chronic recovery phase after stroke, but there is a lack of human clinical studies on the efficacy and safety of VNS treatment for the acute phase of stroke.Moreover, because of the limitations of administering acute invasive VNS treatment to patients with hyperacute cerebral ischemia,a registry study of acute nVNS is of great need.The Dutch NOVIS trial(NCT04050501) is the first large international clinical trial reporting nVNS after stroke (van der Meij et al., 2020).Briefly, 150 patients with anterior circulatory acute ischemic stroke will be randomly assigned to a maximum of 5 days of nVNS given through the GammacoreTMdevice or other optimal medical treatment.The investigators will perform CT perfusion on day 3 to assess the area of cerebral infarction, MRI on day 5 to determine the final infarct volume, and neurological function on day 5.The aim of this study was to determine the neurological efficacy and mechanism of nVNS in patients after acute stroke.Moreover, the similarity of the protective effect of nVNS to the findings in animal trials, mediated in part by the integrity of the BBB,was determined by CT perfusion scans to determine BBB leakage in patients(Yang et al., 2018).In addition, they will also conduct the TR-VENUS study(NCT03733431) study in Turkey; a trial that also uses different stimulation parameter protocols for nVNS in acute stroke to observe the therapeutic effects of nVNS in patients with ischemic stroke.
Randomized controlled trials are available for patients with subacute and chronic stroke (Table 2).A trial (ChiCTR1800019635) by Wu et al.(2020)investigated the role and effects of taVNS in patients with subacute cerebral ischemia (onset 15–90 days) by examining upper limb mobility.In this trial,21 patients with upper limb mobility abnormality were randomly grouped for a 15-day treatment using general rehabilitation with either true or pseudotaVNS.After 12 weeks, the taVNS group’s FMA-U, WMFT, and FIM scores were significantly improved compared to pre-treatment, and all measures were significantly improved compared to the pseudo-taVNS group.This study showed that taVNS improved upper limb mobility after subacute cerebral infarction with no significant side effects.However, there are no large-scale,multicenter clinical studies of taVNS for stroke.
VNS combined with rehabilitation therapy has good efficacy, but the invasive nature of the therapy is its main drawback.nVNS is safer, more tolerable, and has fewer side effects than conventional stimulation (Capone et al., 2017;Redgrave et al., 2018b; Wu et al., 2020).nVNS has the advantages of being easy to perform, rapid, cost-effective, and highly targeted owing to its noninvasive nature, and can be used for acute stroke treatment (Ben-Menachem et al., 2015).Overall, we see potential advantages for clinical translation of nVNS.As an adjunctive therapy for stroke and as a route to endogenous neuroprotection, nVNS is easily combined with thrombolytic/antiplatelet therapy (Ay et al., 2015).
Clinical Application Prospects for Vagus Nerve Stimulation Therapy
Despite the good animal and clinical evidence for targeted plasticity therapy with VNS in various neurological diseases and brain injuries, the large-scale clinical use of VNS still faces many difficulties and challenges owing to the individual variability of clinical patients and the diversity of comorbidities.
Differences between animal models and clinical application
An idealized wire embolization transient MCAO model for acute ischemic stroke has now been established in many animal studies.Although more stroke patients have been able to achieve emergency recanalization following intravenous or endovascular thrombolytic intervention, a significant number of patients still fail to seek timely medical attention (Faiz et al., 2013).Therefore, it is of great importance to construct an animal model that can simulate the type of stroke with permanent vascular occlusion.Moreover, the animal models used all simulate middle cerebral artery trunk occlusion injury,but the mechanism of action on deep penetrating vessels and small cortical vessels and posterior circulation infarction are still unclear.Furthermore,the existing studies on acute stroke have focused on young adults, but the majority of stroke patients are predominantly middle-aged and older adults,and it remains to be explored whether the efficacy of VNS is consistent with the results of animal studies (Schmidt-Pogoda et al., 2020).Therefore, studies in a variety of models, including aged mice, hypertensive rats, rats with experimentally induced diabetes or coronary artery disease, and rat models with other concurrent medications are warranted.
Timing and clinical effectiveness of VNS treatment interventions
For acute stroke, a majority of animal trials that have been conducted have shown that VNS was given immediately within approximately 30 minutes; we have analyzed its therapeutic effects (Table 1).Researchers have summarized the effects of VNS intervention at different times after ischemia, and a study by Ay et al.(2016) showed that VNS treatment given up to 4 hours after stroke still provided neuroprotection and reduced infarct size, but its effect on stroke was not significant when administered after 5 hours.The evaluation of the effect of VNS treatment at different moments after stroke is particularly necessary in clinical trials owing to the delay in hospitalization and diagnosis of stroke patients, which makes it difficult to obtain similar emergency measures as in animal studies (Faiz et al., 2013).At the same time,based on the large-scale study of nVNS conducted by NOVIS (van der Meij et al., 2020), researchers have analyzed the correlation between the timing of nVNS initiation and later neurological recovery and imaging performance to provide a basis for the clinical development of VNS protocols.If VNS can be successfully applied clinically and determined to be safe and reliable, it can be used in remote ischemic brain injury (Blauenfeldt et al., 2020).
Clinical studies and registered multicenter clinical trials of VNS (Table 2) have been conducted nationally and internationally on stroke patients recovering from a stroke more than 6 months later, and the results of clinical studies of VNS have shown that it improves neurological function primarily by promoting neuroplasticity in combination with rehabilitation training.The results of existing trials on VNS and stroke rehabilitation have shown that “pairing” VNS with specific tasks can improve neural remodeling in individuals on specific tasks (Dawson et al., 2020).However, the effects of VNS on post-stroke inflammatory responses, neuronal apoptosis, and cytotoxicity make VNS a less than ideal therapeutic window in the acute phase of stroke.Therefore, a combination of intensive thrombolysis, interventions, and medications from a large number of randomized controlled trials in the acute post-stroke period could help to clarify the significance of providing VNS treatment early on to improve clinical outcomes.
Choice of clinical use of iVNS versus nVNS
Traditional VNS is delivered by surgically implanting electrodes and stimulators in the neck to directly stimulate the VN in the neck (Figure 1),and it has demonstrated good neuroprotection in clinical studies for epilepsy,migraine, and even chronic stroke.However, it is not feasible in the context of thrombolysis and anticoagulation in patients with acute stroke, as it requires surgical intervention.Newer nVNS delivery devices that do not require surgery to deliver electrical stimulation directly to the auricle or neck skin, may add a therapeutic strategy for patients in the acute phase of sudden ischemic stroke.nVNS is feasible and well-tolerated at the currently tested doses, with adverse events comprising mainly skin irritation (18.2%) and headache (3.6%),which are mostly eliminated after adjustment of the device parameters(Redgrave et al., 2018a).The specific stimulation locations of the nVNS device include two main types of studies using tcVNS in the cervical region of the VN (cervical branch of the VN) and studies using taVNS in the external ear stimulation (auricular branch of the VN) with similar specific mechanisms (Yuan and Silberstein, 2016b; Redgrave et al., 2018a).
In ischemic stroke, for the neuroprotective effects of tcVNS and taVNS, Ay et al.(2016) validated them in an animal model of stroke, revealing the protective mechanism of VNS against stroke.nVNS significantly reduced the inflammatory response after cerebral ischemia in rats, inhibited immune activation, and attenuated damage to the organism, while in the vagal cortical region of the external ear, it activated brainstem afferent vagal nuclei by electrical stimulation and reduced the incidence of cerebral infarction (Ay et al., 2016).The results of this study are expected to provide new ideas for the development of treatments that target neuroprotective pathways in the brain.Furthermore, Nonis recorded somatosensory-evoked potentials vSEPs in 12 normal subjects after receiving cervical nVNS stimulation and observed that cervical nVNS activated vagal afferent nerves in rats and was similar to nVNS and non-invasive auricular potentials (Nonis et al., 2017).In addition, nVNS induced microglial M2-type polarization and was involved in the regulation of inflammatory responses after stroke (Zhao et al., 2019).This suggests that tcVNS holds promise as a potential alternative to implantable VNS.With the help of the MCAO animal model, Ay et al.(2015) demonstrated that tVNS effectively activates the brainstem afferent vagal nucleus in acutely ischemic rats, and has similar neuroprosthetic effects to implantable VNS.A study applying functional magnetic resonance imaging (fMRI) found that stimulation of the tVNS by the inner earlobe or cymba conchae resulted in activation of the locus coeruleus and solitary tract nuclei in the brainstem (Yakunina et al., 2018).The auricular branchial vagus nerve is a specific structure located in the external ear and is currently the only type of vagus nerve located on the surface of the body (Murray et al., 2016).Frangos et al.(2015) found significant activation of “classical” central vagal projections in the bilateral NTS, bilateral trigeminal nuclei, dorsal Raphe, ventricles, contralateral parietal areas, amygdala, Arkansas nucleus, and amygdala by fMRI (Figure 2).It was also found that the paracentral lobule was activated bilaterally (Frangos et al.,2015).Furthermore, taVNS significantly inhibited the sympathetic activity in normal subjects (Clancy et al., 2014).These results suggest that nVNS shares the same pathway or mechanism of action as conventional VNS.
Owing to the same mechanism and effectiveness as iVNS, taVNS is simpler,more portable, and safer, which gives it the potential advantage of easier translation to clinical use in a wide range of applications (Ben-Menachem et al., 2015).taVNS can be combined simultaneously with pharmacologic thrombolysis and antiplatelet therapy as a viable adjunctive therapy in the acute phase of ischemic stroke, making it a useful complementary strategy before the “stroke unit”(Jung et al., 2015).
Optimal parameter optimization for VNS
The ideal stimulation parameters for VNS are directly related to the clinical outcome; therefore, how to set the optimal stimulation conditions for VNS has become an important issue in the clinical application of VNS.Morrison et al.(2021) found that VNS intensity correlated with neural remodeling in the cerebral cortex in an inverted U-shape, i.e., medium-intensity VNS stimulation enhanced neural remodeling in the cerebral cortex, whereas low/high-intensity VNS had no such effect.This study suggests that different neurostimulation parameters have different effects on neuroplasticity and may influence the effect of VNS on neurological function.Further studies have found that iVNS stimulation location, electrode type, waveform settings,continuous or pulsed synchronous stimulation, current amplitude and frequency, and stimulation on/offtime can all have significant effects on the clinical outcome of VNS (De Ferrari and Schwartz, 2011).The duration and frequency of VNS treatment also play a critical role in treatment efficacy(Meyers et al., 2018; Nuntaphum et al., 2018).
It is difficult to determine the ideal stimulation site for a particular disease, as the clinical conditions, stimulation parameters, and other subject conditions vary within the subject.As the stimulation parameters required for afferent vagal activation vary between the ear and neck regions, a range of different stimulation factors must be investigated to compare taVNS with tcVNS.Further insights into the neurophysiological mechanisms used in taVNS treatment are needed in the future.Although there is no agreement on the optimal parameters, researchers studying nVNS have used parameters similar to those of the VNS analogues implanted in the neck in human clinical trials, and the following are some of the nVNS-specific stimulation parameters used in the experiments (Table 2).The stimulation current that is usually set depends on the sensitivity of the subject, or is just below their pain threshold.In the experiments, the stimulation intensity was increased by 0.1 mA/stimulation until the highest value reported by the participants was reached, with the intensity of the stimulation ranging between 0.5 and 6 mA.Another experiment regulated the intensity of this stimulus to a level above the detection threshold and below the pain threshold.This stimulus amplitude fluctuated between 0.5 mA (Jongkees et al., 2018) and 12 mA (Trevizol et al.,2016), similar to the range reported in other literature.
Although in many animal experiments, researchers have identified stimulation parameters that work well and achieve good results, it is difficult to identify individualized ideal stimulation sites and parameters because participants in clinical studies have different physical conditions and tolerances (Gaul et al., 2016; Goadsby et al., 2018; Martellettiet al., 2018).Furthermore, as the parameters required for afferent vagal nerve activation in the ear and neck differ from those required for implantable vagal nerve activation, different stimulations are required to screen for optimal parameters for taVNS and tcVNS and iVNS before assessing the efficacy.The cymba concha and inner ear were considered to be the best locations for stimulation of the auditory vagus nerve (Butt et al., 2020), while the carotid bifurcation was the most effective area, so further determination of the location of taVNS stimulation is needed.Although there is no definitive answer to the optimal parameters,researchers have done similar clinical trials in humans with traumatic VNS in the neck as a reference.Specific stimulation parameters for nVNS (Table 2)have been widely adopted in some studies, with the current commonly used approach being to set the nVNS current just below its nociceptive threshold(Frangos et al., 2015; Lerman et al., 2016; Sclocco et al., 2020; Yakunina and Nam, 2021).Redgrave et al.(2018a) gradually increased the current from 0.1 mA to a maximum value, showing currents in the range of 0.5–6 mA.Another study set the stimulation current as: vagal response threshold < current intensity < pain threshold (Capone et al., 2017) and showed a large variation in current intensity between 0.5 and 12 mA because of inter-individual variability (Trevizol et al., 2016; Jongkees et al., 2018), so the setting of clinical stimulation parameters may need to be set individually based on individual differences and tolerance.
Although the efficacy and safety of VNS in animals and humans has been well established so far, further clinical trials are needed to clarify the stimulation conditions to achieve optimal efficacy of VNS (Kong et al., 2018; Mertens et al., 2018).
Clinical and neurophysiological biomarkers
Clinical and neurophysiological markers are key to evaluating the degree of vagal stimulation, and validated biomarkers can alter decisions about clinical treatment after stroke (Wu et al., 2015; Boyd et al., 2017).First, it helps to differentiate between responding and non-responding subjects to VNS stimulation, which in turn determines the appropriate configuration of medical therapy.Second, it assists clinicians in the preferential selection of VNS stimulation parameters and treatment duration, and in the individual design of VNS stimulation protocols at the patient level.Third, to verify whether tVNS can produce the same intensity of neuroprotective effect by the same route as invasive VNS and thus facilitate the clinical dissemination of nVNS for stroke.Fourth, to elucidate the effects and molecular mechanisms of VNS on acute and chronic cerebral ischemia, to assist in the determination of the temporal effects of neuroprotection, and to provide new ideas for clinical treatment.
Biological markers of VNS can be divided into two categories: markers that reflect the level of vagal activity and markers that reflect efficacy.These markers are mainly in the form of physiological, hematological,neurophysiological, or imaging to indicate the response to VNS stimulation.Burger et al.(2020) found that heart rate variability, pupillary response,salivary alpha-amylase, and P300 event-related potentials, through observation of normal subjects, were important indicators of VNS efficacy.In addition, they suggested a possible mechanism to identify biomarkers,including blood tests for pro- and anti-inflammatory cytokines associated with cholinergic anti-inflammatory pathways.Moreover, EEG measures of motor cortical connectivity, particularly ipsilateral M1 and ipsilateral premotor connectivity, are strongly associated with motor deficits and their improvement in post-stroke treatment are expected to be biological markers of cortical neural activity after cerebral ischemia.These could provide a basis for biological studies to identify subgroups of post-stroke patients.On this basis, a comprehensive biomarker evaluation of the effects of stimulation parameters (e.g., intensity, frequency, and pulse length) will be performed by high-field functional fMRI imaging of the brainstem nuclei, PET-MRI of microglia activation, and MRI imaging of post-stroke angiogenesis for the purpose of optimizing post-stroke neuroplasticity and neurological function.
Individual patient differences
Owing to individual differences between patients, a uniform combination of parameter pattern settings cannot be afforded to different patients (De Ferrari and Schwartz, 2011).In the absence of clear biomarkers to test treatment efficacy, further studies are needed to rule out confounding factors that reduce responsiveness to tVNS, which may include factors related to patient gender, age, physical health status, comorbidities and medication use, or stroke location, stroke extent, and stroke duration.As hypertension and hyperlipidemia are often comorbidities in stroke patients and can lead to degenerative infarction of the cerebral cortex microvasculature, does it reduce the effectiveness of VNS treatment.Diabetes is also a common complication in stroke patients (Chen et al., 2016b), and diabetes can exacerbate cerebrovascular infarction and inadequate blood supply, leading to the development of cerebral infarction and central nervous system dysfunction.Moreover, diabetes leads to dysfunction of the vegetative nerves,including the vagus nerve, which inhibits the favorable outcome of VNS.The use of drugs that affect central norepinephrine activity, such as β-blockers and tricyclic antidepressants, have also been reported to affect the vagus nerve response to VNS.Similarly, nicotine acts as an agonist of α7nAChR(de Jonge and Ulloa, 2007), and the effect of nicotine on the downstream signaling pathways of the VNS should be considered in the analysis of the results of clinical trials in patients who smoke.Given the various organismal and individual differences due to comorbidities and medications taken that may affect vagal function, it is important to take into account the influence of these factors in the setting of VNS treatment regimens and optimize different individualized parameters (Cai et al., 2014) to serve individual patients.
Limitations
This review has some limitations.Here, we review the mechanism and effect of vagus nerve stimulation on brain protection from the point of view of animal experiments and clinical trials according to the existing retrieved relevant literature, but the specific theoretical mechanisms need to be studied in depth, and some of the research conclusions need to be further validated.At the same time, comparison of the effects of different vagus nerve stimulation methods lack long-term large-sample statistical conclusions and the same clinical research background, so the validity of the conclusions of the article needs to be further confirmed.
Conclusion and Future Direction
We summarize and review the current animal experimental and clinical research results on VNS to improve stroke prognosis, report the current types of VNS stimulation and stimulation parameters, analyze the possible mechanisms and pathways of VNS action, and clarify the neuroprotective effects and side effects of VNS in clinical use.VNS has become an effective FDA-approved method to improve the dysfunction of various neurological diseases and enhance the rehabilitation effect.Therefore, we propose that VNS can be combined with a variety of acute and chronic phase cerebral ischemia therapies and conventional rehabilitation therapies to better improve neurological function after brain injury.We have summarized the clinical effects of combining VNS with rehabilitation therapies and found that VNS can promote the functional recovery of patients by inducing plasticity in the motor system.Based on this, we believe that the application of VNS in clinical studies of stroke is important to improve the quality of survival of stroke patients.
However, there are still many questions about the optimal choice of VNS and individualized parameter settings that need to be addressed in our ongoing,well-designed, pre-clinical and clinical studies.Although there are a large number of clinical trials that have demonstrated a beneficial effect of nVNS on the recovery of stroke patients, their studies have focused on limb motor function improvement.In fact, VNS also has important clinical research implications in the neuroprotection of post-stroke cognitive impairment,swallowing disorders, aphasia, and sleep-wake disorders.Taken together,we should improve VNS as a whole for the specific conditions of the patient and further optimize the stimulation parameters to achieve better results.In addition, established stimulation results should be analyzed to determine better treatment frequency, duration of stimulation per day, and threshold for treatment.Therefore, more efforts need to be made to focus on these issues in future studies on VNS.
Author contributions:LD and ZY wrote the manuscript.All authors participated in data collection,discussion of the manuscript and provided critical revisions,and approved the final version of the manuscript for submission.
Conflicts of interest:The authors declared no potential conflicts of interest with respect to this article.
Data availability statement:Not applicable.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Glycolysis and glucose metabolism as a target for bioenergetic and neuronal protection in glaucoma
- MAP4K inhibition as a potential therapy for amyotrophic lateral sclerosis
- How do lateral septum projections to the ventral CA1 influence sociability?
- RNA sequencing of exosomes secreted by fibroblast and Schwann cells elucidates mechanisms underlying peripheral nerve regeneration
- Crosstalk among mitophagy, pyroptosis, ferroptosis,and necroptosis in central nervous system injuries
- Clustering of voltage-gated ion channels as an evolutionary trigger of myelin formation
