Betulinic acid protects against ovarian impairment by decreasing F-2 toxin-induced oxidative stress and inf lammation associated with the downregulation of p38 expression in mice
2024-01-24LiKong,XinyuGao,LijuanZhu等
Keywords: Betulinic acid F-2 toxin Ovarian damage p38 MAPK signaling pathway
ABSTRACT F-2 toxin is an estrogenic mycotoxin that causes reproductive disorders in animals. Betulinic acid (BA)is a natural pentacyclic lupane-structure triterpenoid that has diverse pharmacological activities. In this study, the antioxidative and anti-inflammatory effects of BA and its underlying mechanism are explored in F-2 toxin-triggered mouse ovarian damage. We found that BA alleviated the F-2 toxin-induced ovarian impairment by stimulating follicle growth, reducing inflammatory cell infiltration, repairing damaged mitochondria and endoplasmic reticulum. Simultaneously, BA not only reversed F-2 toxin-induced reduction of follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels in the serum, but also restrained the protein expression of the estrogen receptors α (ERα) and ERβ. Moreover, BA restored the balance of F-2 toxin-induced ovarian redox system disorders. Subsequently, we found that 0.25 mg/kg BA played an anti-inflammatory role in the F-2 toxin-induced ovarian impairment by decreasing interleukin-1β (IL-1β),IL-6, and tumor necrosis factor-α (TNF-α) mRNA expression, as well as inhibiting p38 protein expression.These data demonstrated that BA exerts its protective effect on F-2 toxin-induced ovarian oxidative impairment and inf lammation by inhibiting p38 expression, which implies a natural product-based medicine to ameliorate F-2 toxin-caused female reproductive toxicity and provides a detoxifying method for food contaminated by mycotoxin.
1. Introduction
F-2 toxin, also called zearalenone, is a nonsteroidal estrogenic mycotoxin mainly produced by the fungi of the genusFusarium,which is widespread in moldy grains and food[1]. F-2 toxin contamination poses huge economic losses for many countries due to its presence in a wide range of crops[2]. Simultaneously, F-2 toxin gives rise to hyperestrogenic syndromes and threats human health[3]. The chemical structure of F-2 toxin is similar to that of natural estrogens such as estradiol[4]. Therefore, F-2 toxin has estrogen-like activity and competitively binds to the estrogen receptor(ER), resulting in reproductive disorders in livestock. Swine is the most F-2 toxin-sensitive species among domestic animals while birds are more resistant to F-2 toxin[3]. Previous evidence has shown that the toxic effects of F-2 toxin on mammals are ref lected in the damage of meiotic progression and the poor survival rate of fetal oocytes[5].In female pigs, the clinical symptoms induced by F-2 toxin mainly manifest as redness and swelling of the vulva, uterus enlargement,cyst formation, and enlarged mammary glands on the ovaries[6]. A recent study has shown that F-2 toxin has maternal and utero toxicity in mice through inducing fetal growth retardation and abortion[7].
The F-2 toxin harmful effects on the reproductive system are largely related to oxidative stress (OS)[8].In vitro, a study has verified that F-2 toxin elevates the levels of reactive oxygen species (ROS)and malondialdehyde (MDA) in immortalized murine ovarian granular KK-1 cells[8]. Moreover, many researchers found that F-2 toxin exposure induces OS resulting in apoptosis, DNA damage, and autophagy in porcine embryo[9]. F-2 toxin also damages the appearance of the ovaries while activating the OS-mediated mitochondrial apoptosis signaling pathway[10]. Mitogen-activated protein kinase(MAPK) cascades are universally conserved eukaryotic-signaling modules, which play important roles in the inflammatory response and redox balance[11]. Studies have found that F-2 toxin treatment not only activates the c-Jun N-terminal kinase (JNK) pathway to increase the expression and synthesis of pro-inflammatory substances in porcine spleen, but also up-regulates the MAPK signaling pathway to induce T-lymphocytes apoptosis[12-13]. These findings revealed that OS, inflammation, and reproductive disorders triggered by F-2 toxin seriously impairs animal health, causing considerable economic losses in livestock industry[2-3,5,8-10]. At the same time, F-2 toxin also threatens human health through the food chain[3]. Therefore, it is an important task for reducing economic losses and maintaining human health to find a plant-derived antioxidant forFusariumtoxin.
Betulinic acid (BA) is a naturally occurring pentacyclic triterpenoid, widely distributed in food, medicinal herbs and plants,such as the bark of birch trees, sour jujube fruits and the roots ofTetracera potatoria[14-15]. This natural compound has a wide range of biological and pharmacological activities, such as antiviral,antitumor, anti-inflammatory, and anti-OS[15-16]. We previously showed that BA protects mouse thymus and spleen from oxidative damage caused by T-2 toxin via increasing the antioxidant enzyme activity, down-regulating the MAPK signaling pathway, and up-regulating the nuclear factor erythroid 2 (NF-E2)-related factor 2(Nrf2) signaling pathway[16-17]. Moreover, a study was found that BA exhibits significant antiestrogenic effects via the repression of mRNA and protein levels of ERα[18]. BA treatment also suppresses the ERβ signaling pathway and pro-inflammatory cytokine levels to inhibit endometriosis[19]. However, it is still unknown whether BA possesses a favorable effect against F-2 toxin -evoked ovarian injury.To verify our hypothesis, in the current study, an mouse ovarian oxidative damage model was established by orally administering F-2 toxin, and vitamin E (VE) was used for a positive control because of a classical antioxidant possessing anti-oxidative and anti-inflammatory properties[20]. The purpose of this research was to examine the protective potential and underlying mechanism of BA on F-2-triggered ovarian impairment, which could develop fresh approaches for using natural product-based medicines and provide detoxification methods for food contaminants.
2. Materials and methods
2.1 Reagents
F-2 toxin (MSS1023) was obtained from Jiangsu Hengrui Pharmaceutical Co., Ltd. (Lianyungang, Jiangshu, China). BA(855057) was acquired from Sigma (St Louis, MO, USA), and VE (T3251) was obtained from Sigma-Aldrich (Shanghai, China).Haematoxylin and eosin (H&E) staining solution (G1005),dihydroethidium (DHE) (GDP1018), 4’,6-diamidino-2-phenylindole(DAPI) (GDP1024), and the deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL) kit (G1501) were purchased from Servicebio (Wuhan, Hubei, China). Bicinchoninic acid (BCA) (A045-4-2), superoxide dismutase (SOD) (A001-3-2), glutathione peroxidase(GSH-Px) (A005-1-2), catalase (CAT) (A007-1-1), and MDA (A003-1-2) assay kits were acquired from Nanjing Jiancheng Biotech(Nanjing, Jiangsu, China). Mouse follicle-stimulating hormone (FSH)(CSB-E06871m), luteinizing hormone (LH) (CSB-E12770m) and enzyme-linked immunosorbent assay (ELISA) kits were provided by Cusabio Biotech Co., Ltd. (Wuhan, Hubei, China). An enhanced chemiluminescence (ECL) reagent (KGP1202) was obtained from Nanjing KeyGen Biotech Co., Ltd. Trizol (AG21102), PrimeSeript RTreagent Kit (AG11728), and SYBR Green I fluorescent (AG11701)were purchased from Accurate Biology (Changsha, China). The primary antibodies against ERβ (49757T),β-actin (3700S), p38(9212S), p-p38 (9211S), extracellular signal-regulated kinase(ERK) (9102S), p-ERK (9101S), JNK (9252S), p-JNK (9251S),Nrf2 (12721S), heme oxygenase-1 (HO-1) (82206S), and kelch-like erythroid cell-derived protein with CNC homology[ECH]-associated protein1 (Keap1) (8047S) were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.2 Animal experiments
Ninety healthy female Kunming mice (7 weeks) were raised in a climate-controlled (20–26 °C) animal room under relative humidity(50%–70%), with 12 h of light per day. All mice were allowed to adapt for 1 week, and then randomly separated into 6 groups(n= 15/group), the control group, 20 mg/kg F-2 toxin group, F-2 toxin + 0.25 mg/kg BA treatment group, F-2 toxin + 0.5 mg/kg BA treatment group, F-2 toxin + 100 mg/kg VE treatment group (positive control), and 0.5 mg/kg BA alone group. The drug dosages, oral administration time, and specific medication regimen have been described in previous studies[16-17,21-22]. The mice in the control group and 0.5 mg/kg BA alone group were orally given 5% ethanol solution while the other groups were continuously gavaged with 20 mg/kg F-2 toxin (dissolved in 5% ethanol) for 1 week to establish an ovarian injury model. Subsequently, 100 mg/kg VE or different doses of BA were suspended in 1% soluble starch and given orally daily to different groups for 2 weeks, while the control group and F-2 toxin group were orally administered an equal volume of 1% soluble starch.
Blood samples were obtained from the orbital veins of mice.Then, the mice were dissected and the ovaries were collected. One part of the ovaries was used for the observation of its morphology and ultrastructure, and the other part was used for the follow-up experiments, such as detecting antioxidative capacity, quantitative real-time PCR (qRT-PCR), and western blot analysis.
All experimental procedures involving mice were performed according to protocols approved by the Animal Care Committee of Hunan Agricultural University and abided by the Animal Care and Use Guidelines of China (approval code: 43321911) with an approval date of March 11, 2019. The mice were treated with diethyl ether under light anaesthesia, and all efforts were made to minimize animal suffering.
2.3 Histopathological examination by H&E staining
Ovarian tissues were collected and fixed in 10% neutral-buffered paraformaldehyde. After rinsing with running water, the samples were successively dehydrated with different concentrations of alcohol,embedded in paraffin, cut into 5 μm sections and then dried in an oven overnight at 37 °C. Afterwards, the sections were dyed with H&E and a neutral gum seal[10]. Finally, the morphological changes of the ovary sections were observed using an optical microscope (Olympus,Tokyo, Japan).
2.4 Ultrastructural observation by transmission electron microscope (TEM)
The ovarian tissues were fixed in glutaraldehyde solution for 3 h, then rinsed in phosphate buffer saline (PBS) and dehydrated.Uranium-lead double staining ultrathin sections (60–80 nm) were placed at room temperature (22–25 °C) overnight. Subsequently, a TEM (HT7700, Tokyo, Japan) was used for observation and image analysis of sections[17].
2.5 ROS detection
Fresh ovarian tissues were immediately frozen in liquid nitrogen,and then made into frozen sections, stained using the oxidative fluorescent dye, DHE, to evaluate intracellular ROS levels. After the ovarian slices were washed with PBS and stained with DHE for 30 min at 37 °C in the dark, the DHE could freely enter living ovarian cells and form ethidium bromide, which binds to DNA in the nucleus and generates the red fluorescence[16,22]. Then, the ovarian cells with red fluorescence were observed by a fluorescence microscope (BA410,Motic) and analyzed with the Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
2.6 Antioxidant capacity detection
Blood samples, collected from the retro orbital veins of the anesthetized mice, were placed in 2 mL centrifuge tubes. After standing at room temperature for 2–4 h, the blood samples were centrifuged for 5 min at 1 000 ×g. Later, the supernatant was used to detect the serum CAT, MDA, SOD, and GSH-Px levels according to the manufacturers’ protocols[22].
Meanwhile, 0.1 g of ovarian tissue samples were placed in 2 mL centrifuge tubes filled with 0.9% normal saline according to the ratio of weight (mg):volume (μL) = 1:9. The supernatant was collected after the samples were homogenated and centrifuged. Then, the CAT,MDA, and SOD levels were measured with the corresponding assay kits purchased from Nanjing Jiancheng Biotechnology Company in accordance with the manufactures’ instructions[10,22].
2.7 Reproductive hormones detection in serum
The FSH and LH levels in the serum were determined by commercially available ELISA kits based on our previous published study[10].
2.8 Immunohistochemistry
The paraffin-embedded ovarian tissues were deparaffinized and rehydrated before performing antigen retrieval. After cooling and washing three times with PBS, the tissues were put in 3% hydrogen peroxide solution and washed with PBS again. Next, the tissues were blocked with bovine serum albumin and then incubated with the primary antibody overnight at 4 °C and washed with PBS three times.Following incubation with the secondary antibody and washing with PBS three times, the samples were counterstained with hematoxylin,dehydrated, and finally observed under a microscope[22].
2.9 qRT-PCR
The mRNA expression of interleukin-1β (IL-1β),IL-6,IL-10, and tumor necrosis factor-α (TNF-α) was detected by qRT-PCR. Total RNA was extracted according to the Trizol method and then the RNA in the samples was reverse transcribed to cDNA using the PrimeSeript RTreagent Kit. The amount of cDNA was amplified in a 20 μL PCR reaction system under the corresponding reaction conditions.Then, qRT-PCR was performed on the ABI Step One instrument using SYBR Green I fluorescence. The mRNA sequences ofβ-actin,IL-1β,IL-6,IL-10, andTNF-αwere synthesized by Sangon Biotech(Shanghai, China) and shown in Table 1. Values were normalized toβ-actin as an internal reference. Fold change was calculated by the 2-ΔΔCtmethod[23].
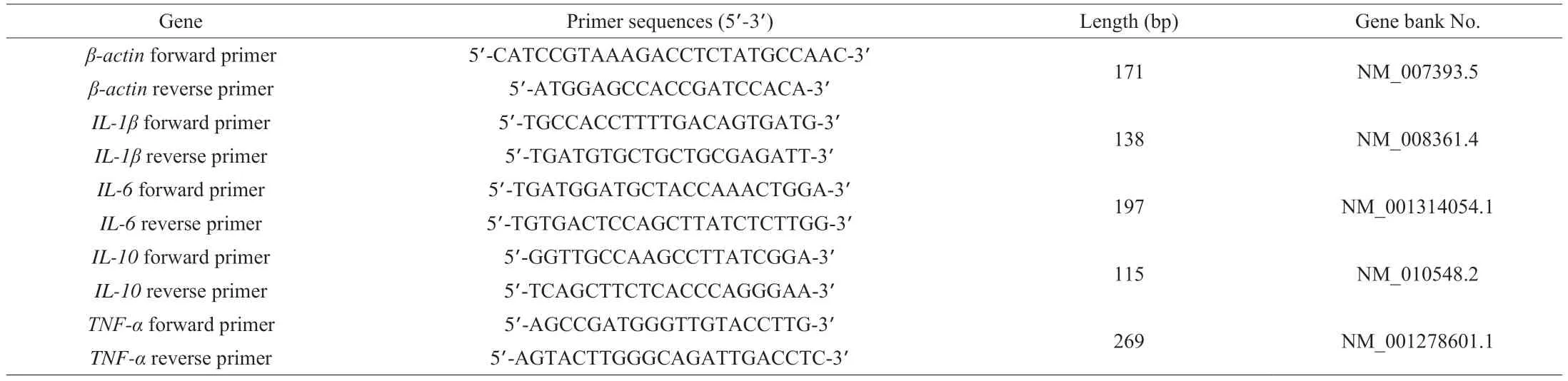
Table 1 RT-PCR primer sequences.
2.10 Western blot analyses
The ovarian tissues were prepared for western blot analysis as previously described[10,16-17]. Briefly, the samples were homogenized,lysed for protein extraction, and then quantified using a BCA protein assay kit. Proteins were electrophoretically transferred onto polyvinylidene fluoride membranes, blocked for 1 h, followed by incubation with the primary antibodies overnight at 4 °C. After washing three times for 10 min in tris-buffered saline with Tween-20(TBST), the membranes were incubated with the secondary antibody for 1 h and then washed with TBST again. Subsequently, protein bands on the membranes were visualized with an ECL reagent and quantified by Image-Pro Plus 6.0 software (Media Cybernetics, Inc.,Rockville, MD, USA).
2.11 Statistical analysis
All statistical analyses were performed using SPSS version 27.0 (SPSS Inc., Chicago, IL, USA). Data were analyzed by oneway analysis of variance (ANOVA) and the LSD’s post hoc test after verifying the normality and the homogeneity of variance with Shapiro-Wilk and Levene’s test, respectively. The error bars of all graphs were presented as the mean ± standard error of the mean(SEM). Significance thresholds were indicated in each figure legend.
3. Results
3.1 BA protected against F-2 toxin-induced ovarian damage in mice
To investigate the effects of BA on F-2 toxin-induced ovarian damage, H&E staining and ultrastructural observation were performed. As shown in Fig. 1A, compared with control group, the ovaries were dramatically congestive, and the number of growing follicles and corpora lutea were significantly decreased in the F-2 toxin group. Simultaneously, inflammatory cell infiltration occurred in F-2 toxin group. In contrast, the above changes were reversed by BA or VE treatment. Meanwhile, the ovarian granulosa cells were disorganized and not tightly attached to the follicle wall in the F-2 toxin group. In addition, the mitochondria were swollen, and some mitochondrial ridges broken. Endoplasmic reticulum expansion was more obvious than that of the control group in the ovarian cells.After BA treatment, the arrangement of ovarian granulosa cells,mitochondrial damage, and the endoplasmic reticulum expansion in the ovarian cells were improved (Fig. 1B).
3.2 BA regulated F-2 toxin-induced hormone secretion disorder
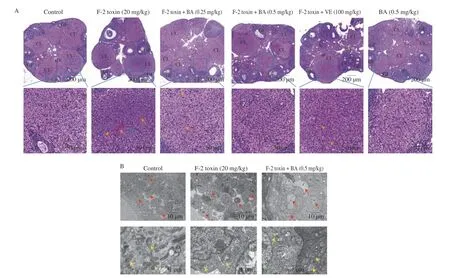
Fig.1 Protective effects of BA on ovarian damage in F-2 toxin-exposed mice. The H&E staining assay method was used to observe the histopathology of ovarian tissues (A). GF, growing follicle; CL, corpora lutea; BV, blood vessels; Triangle, primordial follicles; orange arrow, red blood cell; blue arrow, inflammatory cell; Scale bar, 200 μm and 50 μm, respectively. The ultrastructure of the ovary was observed by TEM to compare the pathological changes (B). Some remarkable changes were observed that F-2 toxin led to arrangement disorder of ovarian granulosa cells (red arrow), mitochondria (yellow arrow) swelling and ridges broken,as well as endoplasmic reticulum (green arrow) expansion. Scale bar, 10 μm and 1 μm, respectively.
To explore the regulation of BA on the disordered hormone secretion caused by F-2 toxin, we detected the levels of reproductive hormones and the expression of ER in mice. Compared with the control group, F-2 toxin significantly reduced the levels of FSH and LH in the serum, and 0.5 mg/kg BA alone group decreased the level of FSH, while 0.25 mg/kg BA treatment reversed this phenomenon.In addition, VE also obviously alleviated the decreased level of LH induced by F-2 toxin (Figs. 2A and B). Subsequently, according to immunohistochemistry and western blot assays, BA or VE treatment inhibited the protein expression of ERα and ERβ that had been induced by F-2 toxin in the ovarian tissue. However, suppression of the ERβ protein was observed in the 0.5 mg/kg BA alone group relative to the control group (Figs. 2C-G). The above results indicated that BA effectively alleviated the hormone secretion disorder caused by F-2 toxin.
3.3 BA ameliorated the F-2 toxin-evoked oxidative stress in mice

Fig. 2 BA regulated the F-2 toxin-induced hormone secretion disorder. The contents of FSH (A) and LH (B) in the serum were measured by ELISA assays. An immunohistochemical method was used to detect the ovarian ERβ expression (C). The ERβ fluorescence density value was analyzed (D). The protein expression of ERα and ERβ was detected by western blot (E). The quantitative analysis of the protein bands was performed in ovarian tissue (F and G). Data were presented as mean ± SEM (n = 8). *P < 0.05 and **P < 0.01 vs. control group, #P < 0.05 and ##P < 0.01 vs. F-2 toxin group.
Based on our results that F-2 toxin led to OS in ovarian tissue, we next examined OS-related parameters in the ovarian tissue and serum to assess whether BA could relieve F-2 toxin-caused ovarian oxidative impairment[10]. Compared with the control group, we found that F-2 toxin significantly elevated the levels of ROS, MDA, and CAT(P< 0.05 andP< 0.01), as well as inhibited the SOD activity (P˃ 0.05)in the ovary. These effects were reversed by the BA or VE treatment(Figs. 3A-E). In addition, F-2 toxin resulted in a notable increase in MDA, CAT, and GSH-Px levels, and there was no a significant change of SOD activity in the serum compared with the observations in the control. Following BA or VE treatment, the levels of MDA,CAT, and GSH-Px were reversed. Simultaneously, 0.5 mg/kg BA treatment increased SOD activity in serum (Figs. 3F-I). Interestingly,0.5 mg/kg BA alone group showed that the CAT level in ovary and MDA level in serum were obviously reduced, but the levels of SOD and GSH-Px in serum were significantly elevated compared to the control group (Figs. 3D, F, H and I). As expected, BA ultimately reduced the accumulation of ROS and MDA and maintained the balance of the redox system in the mice.
3.4 Effects of BA on the mRNA expression of inflammatory cytokines in F-2 toxin saturated ovarian tissue
We investigated whether BA could regulate the mRNA expression ofTNF-α,IL-6,IL-1β, andIL-10induced by F-2 toxin in ovarian tissue (Fig. 4). We found that F-2 toxin dramatically increased the levels ofTNF-α,IL-6,IL-1β, andIL-10compared with the control group, while 0.25 mg/kg BA or VE treatment inhibited the rise of inflammatory cytokines. However, 0.5 mg/kg BA significantly promoted the increase ofIL-6andIL-1βlevels induced by F-2 toxin.In addition, the 0.5 mg/kg BA alone group significantly decreased the mRNA expression ofTNF-αandIL-10compared with the control group. Collectively, 0.25 mg/kg BA or VE ameliorated the inflammation by restraining the mRNA expression of proinflammatory cytokines.

Fig. 3 Effect of BA on redox system induced by F-2 toxin in ovarian tissue and serum. DHE was used to assess the ROS content in ovarian tissue (A), and the fluorescence was quantitatively analyzed (B). The levels of MDA (C), CAT (D), and SOD (E) in the ovarian tissue and the levels of MDA (F), CAT (G), SOD (H),and GSH-Px (I) in the serum were detected according to the manufacturer’s protocol. Data were presented as mean ± SEM (n = 8). *P < 0.05 and **P < 0.01 vs.the control group, #P < 0.05 and ##P < 0.01 vs. the F-2 toxin group.
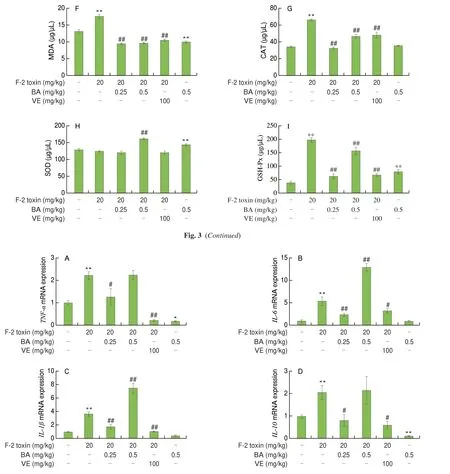
Fig. 4 Effects of BA on the mRNA expression of inflammatory factors in F-2 toxin-saturated ovarian tissue. Levels of TNF-α (A), IL-6 (B), IL-1β (C), and IL-10 (D)were detected by qRT-PCR. Data were presented as mean ± SEM (n = 3). *P < 0.05 and **P < 0.01 vs. the control group, #P < 0.05 and ##P < 0.01 vs. the F-2 toxin group.
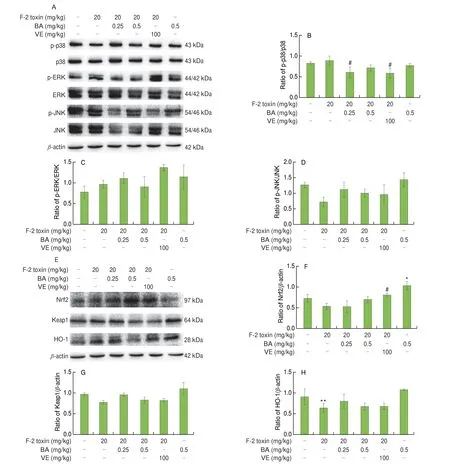
Fig. 5 Effects of BA on the protein expression of the MAPK/Nrf2 signaling pathways in ovarian tissue induced by F-2 toxin. The protein expression and phosphorylation of p38, ERK, and JNK were evaluated using Western blot (A). The ratios of p-p38/p38 (B), p-ERK/ERK (C), and p-JNK/JNK (D) were presented in the graph. Moreover, the expression of Nrf2, Keap1, and HO-1 was also detected by western blot analysis (E). We then used Image-Pro Plus 6.0 software to analyze the density value of the protein bands in Nrf2/β-actin (F), Keap1/β-actin (G), and HO-1/β-actin (H). Data were presented as mean ± SEM (n = 3).*P < 0.05 and **P < 0.01 vs. the control group, #P < 0.05 and ##P < 0.01 vs. the F-2 toxin group.
3.5 Effects of BA on the F-2 toxin induced protein expression of the MAPK/Nrf2 signaling pathways in ovarian tissue
The inflammation and OS induced by F-2 toxin regulated reproductive disease-related signaling pathways, such as the MAPK pathway and Nrf2 pathway[24]. Western blot assay was used to examine whether BA protected against ovarian damage caused by F-2 toxin by regulating the MAPK/Nrf2 signaling pathways. The results proved that 0.25 mg/kg BA or VE treatment significantly reduced the ratio of p-p38/p38, but not on the ratio of p-ERK/ERK and p-JNK/JNK compared with the F-2 toxin group (Figs. 5A-D). Furthermore,F-2 toxin not only restrained the Nrf2 protein expression compared with the control group although there was no significant difference,but also dramatically decreased the HO-1 protein expression. On the contrary, the Nrf2 protein expression was markedly enhanced in the 0.5 mg/kg BA alone group and VE treatment group (Figs. 5E-H). In conclusion, 0.25 mg/kg BA down-regulated the p38 expression to inhibit OS and inflammation in ovarian tissue.
4. Discussion
Mycotoxins and toxic fungal metabolites often contaminate feed and grains; this has already been considered a serious problem in agriculture, animal husbandry, and public health[3]. The ingestion of contaminated feed affects the production performance of livestock and gives rise to economic losses. At the same time, mycotoxins accumulated in meat products can also pose a threat to human health through the food chain[25]. F-2 toxin as a commonFusariumtoxin is found frequently during plant maturity and the processing of grains causing several reproductive disorders[4]. According to some researches, F-2 toxin-induced reproductive disorders are linked to OS and inflammation[5,8]. BA, a natural lupane-type triterpenoid,is widely presented in many fruits, vegetables, and plants with various pharmacological activities[14-15]. Simultaneously, it has been demonstrated that BA reduces the toxicity of mycotoxins avoiding harm to the reproductive system in male mice[21]. However, there is no report whether BA is protective against F-2 toxin-induced ovarian impairment. Thus, specific roles of BA in F-2 toxin-induced ovarian impairment were explored.
F-2 toxin can lead to morphological changes in reproductive organs and hence to mediate various reproductive problems[26]. Some studies have indicated that F-2 toxin not only induces the degeneration of growing follicles, but also results in the follicular cells losing the normal number and structure of mitochondria and endoplasmic reticula in pre-pubertal female dogs[27-28]. Furthermore, our previous research has proved that F-2 toxin increases the number of atretic follicles and the apoptosis of ovarian cells but decreases the number of the growing follicles in mice[10]. These findings were similar to the results caused by F-2 toxin in the present study. However, BA and VE treatment significantly ameliorated the pathomorphological changes of the ovaries, which indicated that BA and VE were able to protect against F-2 toxin-induced ovarian impairment. Interestingly, BA had a more significant improvement than VE in histopathological changes of the ovaries caused by F-2 toxin.
Generally, F-2 toxin has lower acute toxicity to animals when restricted to low concentrations, but 1 mg/kg F-2 toxin is enough to cause hyper-estrogenic clinical signs in pigs[1]. This is mainly because F-2 toxin has an estrogen-like effect and participated in estrogen negative feedback regulation[29-30]. Increasing numbers of studies have reported that the synthesis and secretion of FSH and LH, which affect sex hormones synthesis, are suppressed by F-2 toxin in different animals[29,31-32], these findings are consistent with the results in the present study. Furthermore, the F-2 toxin directly binds to ER (including ERα and ERβ) to interfere with estrogen function[30]. According to a study, F-2 toxin belongs to a selective ER modulator and has different affinities for ERα and ERβ under certain conditions[27]. The levels of ERα and ERβ in ovarian tissue were elevated by F-2 toxin in this study, which may be related to its estrogen-like effect. This finding was also consistent with our previous results[10]. Recently, several studies have proved that BA is a specific suppression agent of ERα and ERβ, which could repress the estrogen signaling pathway[18-19,33-34]. Further investigations reported that BA treatment not only inhibits the mRNA and protein levels of ERα, but also restrains ERβ expression by changing the epigenetics of the ERβ promoter[18-19]. In the present study, the increased levels of ERα and ERβ induced by F-2 toxin were reduced following BA and VE treatment. In addition, treatment with BA reversed the reduction of FSH and LH, proving that BA could ameliorate F-2 toxin-induced hormonal disturbances better than VE. A previous study has testified that the secretions of pituitary gonadotropins (including FSH and LH) are regulated by negative and positive feedback influences of ovarian steroids[35]. BA has a significant antiestrogenic effect, thereby decreasing the level of estrogenin vivo[18], which most likely to promote the secretions of FSH and LH in turn.
The physiological level of ROS plays an important regulatory role in folliculogenesis, oocyte maturation, the endometrial cycle,and luteolysis, which is closely related with reproductive events[36].However, excessive accumulation of ROS results in ovarian damage caused by OS[24]. In a previousin vitroexperiment, the F-2 toxin restrains the activities of CAT, SOD, and GSH-Px and then induces OS in porcine granulosa cells[37]. In anin vivoexperiment, F-2 toxin also leads to oxidative impairment in ovarian tissue by elevating the levels of ROS and MDA[10]. The data from our study showed that F-2 toxin significantly up-regulates the levels of ROS, MDA, CAT,and GSH-Px compared to the control group, indicating that F-2 toxin successfully causes ovarian OS and redox imbalance. It has been demonstrated that BA, as a plant-derived antioxidant, protects against oxidative damage triggered by F-2 toxin or T-2 toxin in the testis via increasing the antioxidative capacity and reducing the MDA content[21-22]. In addition, BA exhibites antioxidative effect in the immune system by inhibiting ROS accumulation and raising the levels of CAT, GSH-Px, and SOD[16-17]. In the present study, BA and VE treatment significantly decreased the levels of MDA, CAT, and GSHPx in the ovaries and serum, and promoted the SOD activity in the serum, as well as reduced the total ROS content in the ovaries. It is worth noting that BA has better antioxidative property than VE. This result indicated that BA repaired the F-2 toxin-triggered imbalance of redox system by modulating ROS generation and antioxidant enzyme system in ovary. Furthermore, the 0.5 mg/kg BA alone group significantly reduced the level of MDA in serum and the activity of CAT in ovary, while promoted the activities of SOD and GSH-Px in serum. These results indicated that 0.5 mg/kg BA alone relieved the peroxidation reaction and regulated the redox system in the normal physiological process to protect the ovarian tissue.
Nrf2 is a crucial molecule regulating antioxidant defense and maintaining cellular homeostasis[38]. Normally, Nrf2 binds to Keap1 in the cytoplasm and is subsequently degraded by a proteasome pathway.Following activation, the Nrf2 protein disassociates with Keap1 and diverts to the nucleus to activate HO-1, the antioxidant gene[24]. A recent study emphasized the effectiveness of Nrf2 suppression of OS in fetal endothelial cells in utero[39]. Another study showed that F-2 toxin significantly down-regulates the Nrf2/Keap1 signaling pathway to induce OS in IPEC-J2 cells[40]. Moreover, F-2 toxin-induced liver tissue damage is associated with a decrease of Nrf2 expression[41].As mentioned above, our experimental data showed that F-2 toxin dramatically restrains HO-1 protein expression in the ovaries, reducing the antioxidative capacity of ovarian cells. These results indicated that F-2 toxin most likely down-regulated the HO-1 protein expression to cause ovarian oxidative damage. Previous studies have found BA alleviates T-2 toxin-induced spleen and thymus oxidative damage by activating Nrf2 signaling pathway[16-17]. Interestingly, the protein expression of Nrf2 signaling pathway has no significant changes in F-2 toxin-induced ovarian tissues followed by BA treatment, which indicates that BA may repair the redox system imbalance induced by F-2 toxin via other signaling pathways. However, 0.5 mg/kg BA alone group promoted Nrf2 protein expression, which may be associated with its high antioxidative capacity in this study.
Previous studies showed that reproductive OS could activate MAPK signaling pathway which involves three types of protein kinases (ERK, JNK, and p38) and caused inflammation[24,42-43].Simultaneously, inflammation contributes to ROS accumulation in turn[24]. Based on some researches, F-2 toxin regulates the release of inflammatory cytokines (IL-6, IL-1β, IL-10, and TNF-α,) and MAPK signaling pathway to mediate inflammation in spleen and liver[12,44].Furthermore, F-2 toxin also can cause intestinal inflammation through promoting the secretion of IL-1β and IL-18 in mice[45]. In this study,we found that F-2 toxin significantly elevates the mRNA expression of IL-6, IL-1β, and TNF-α, thereby mediating ovarian inflammation.However, the mRNA expression of the anti-inflammatory factorIL-10was promoted by F-2 toxin in the ovarian tissue. This result was in agreement with a previous study showing that T-2 toxin produced byFusariumalso up-regulated the mRNA expression ofIL-10during skin inflammation in mice[46]. Therefore, the increase ofIL-10induced by F-2 toxin may be a protective compensatory response of the body itself. Moreover, many studies have reported that BA exhibits antiinflammatory effects in some animal models[15-17,23]. For instance,BA plays a protective role in cyclophosphamide-induced intestinal damage and T-2 toxin-induced immune system via repressing MAPK signaling pathway in mice[16-17,23]. In the present study, 0.25 mg/kg BA and VE significantly inhibited the increased mRNA expression of inflammatory factors and the activation of p38 protein expression induced by F-2 toxin, alleviating inflammation in the ovaries. In contrast, 0.5 mg/kg BA elevatedIL-6andIL-1βmRNA expression compared to F-2 toxin group. However, the 0.5 mg/kg BA alone group prominently restrained the mRNA expression ofIL-6andTNF-α, which indicated that 0.5 mg/kg BA was harmless to the animals but not effective to inhibit F-2 toxin-induced inflammatory factors elevation. This part of experiment explained that 0.25 mg/kg BA has a therapeutic effect on the inflammation induced by F-2 toxin.Summarily, the above results proved that BA restrains p38 protein expression to protect against F-2 toxin-induced ovarian oxidative impairment and inflammation.
5. Conclusion
In our study, we demonstrated that BA treatment ameliorated the histopathological and ultrastructural changes of the ovaries induced by F-2 toxin, and prevented the estrogen-like effect of F-2 toxin.Moreover, BA repaired the redox system balance and inhibited the mRNA expression of inflammatory factors to exert antioxidant and anti-inflammatory effects. Further in-depth studies also proved that BA played a protective role against F-2 toxin-induced ovarian impairment via the down-regulation of p38 protein expression. These results revealed a theoretical basis for BA treatment of F-2 toxininduced ovarian impairment, which might be offer a novel therapeutic potential for food contaminated with mycotoxin.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (32273084), the Special Funds for Construction of Innovative Provinces in Hunan Province, China (2020NK2032), the Natural Science Foundation of Hunan Province, China (2020JJ4368),Innovation Foundation for Postgraduate of Hunan Province, China(CX20220670) and Innovation Foundation for Postgraduate of Hunan Agricultural University, China (2022XC010).
杂志排行
食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango