Divergent responses of Picea crassifolia Kom.in different forest patches to climate change in the northeastern Tibetan Plateau
2024-01-22ZhongtongPngQingMoLingjunZhuQingoLuJiqingCiMingmingGuoKunXuYundongZhng
Zhongtong Png, Qing Mo, Lingjun Zhu, Qingo Lu, Jiqing Ci, Mingming Guo,Kun Xu, Yundong Zhng,*
a Key Laboratory of Forest Ecology and Environment of National Forestry and Grassland Administration, Ecology and Nature Conservation Institute, Chinese Academy of Forestry, Beijing, 100091, China
b Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China
c National Engineering Laboratory for Applied Technology of Forestry & Ecology in South China, College of Life Science and Technology, Central South University of Forestry and Technology, Changsha, 410004, China
d Swiss Federal Institute for Forest, Snow and Landscape Research WSL, Birmensdorf, 8903, Switzerland
e Environment and Sustainability Institute, University of Exeter, Penryn Campus, Penryn, Cornwall, TR10 9FE, UK
f College of Forestry, Hebei Agricultural University, Baoding, 071000, China
Keywords:Climate change Picea crassifolia Kom.Forest patches Tree growth Resilience
ABSTRACT Global climate changes have significantly affected tree growth and forest structures and functions in some arid and semi-arid regions,which are becoming warmer and wetter.Due to natural factors such as climate and terrain,some tree species may form different forest patches at the edges of their distribution areas.However, how forest patches of various sizes respond to climate change is unclear.In this study, we collected 203 tree cores from six different sizes of forest patches at the edge of the distribution area of Picea crassifolia Kom.in the northeast Tibetan Plateau.And we used the dendrochronology method to study the response of tree growth and resilience in different forest patches to climate change from 1961 to 2020.We simultaneously measured the contents of nonstructural carbohydrates (NSC), total nitrogen and total phosphorus of tree needles.Our results showed that the growth of trees in small- and medium-size forest patches (0.8-18.6 ha) has increased significantly.The early growing season (May-July) minimum temperature was the most important climate factor driving the growth of small- and medium-sized patch trees.The early growing season maximum temperature was the most important climate factor that inhibited the growth of trees in the largest patches(362.8 ha).The growth of individual trees in medium forest patches was better and the correlation with annual minimum temperature, maximum temperature, precipitation, actual evapotranspiration, and palmer drought severity index was stronger.The higher NSC content, stronger photosynthesis, and higher nitrogen utilization efficiency in leaves might be one of the reasons for the better growth of trees in moderate forest patches.In extreme drought years, as the forest patch area increased,the overall trend of tree growth resistance showed a unimodal pattern,with the highest at a forest patch area of 7.1 ha, while the overall trend of tree growth recovery was opposite.Therefore, we should strengthen the management of trees in large forest patches to cope with climate change.
1.Introduction
Drought caused by rapid global warming has forced the growth of some trees,even increasing the mortality rate of trees(Liang et al.,2016;Gentilesca et al.,2017;Jiao et al.,2019).The response of tree growth to environmental changes is a physiological and ecological process that will be simultaneously affected by environmental and genetic factors(Willis et al.,2018;Zhu et al.,2021).Climate warming can generally accelerate the growth of trees in cold regions (Holtmeier and Broll, 2005; Silva et al.,2016),and increased precipitation can also promote the growth of trees in arid and semi-arid areas (Siyum et al., 2019; Holdrege et al.,2021).In cold and arid regions, the response of tree growth to climate change shows a balance between water and heat required for tree growth(JevŠenak et al.,2021).
The Qilian Mountains are located in the northeast edge of the Tibetan Plateau, which are in arid and semi-arid regions and have a plateau continental climate.Recently, the climate is becoming warmer and wetter (Lin et al., 2017).Picea crassifolia is a widely distributed tree species in the Qilian Mountains.Its growth is often influenced by temperature and precipitation, and is more sensitive to climate changes(Song et al.,2020).In the natural distribution area of P.crassifolia,due to the impact of climate and terrain factors, P.crassifolia often forms a forest-grass ecotone in high-altitude areas and develops island distributions,that is,independent forest patches(Meng et al.,2007;Harsch and Bader, 2011; Wang et al., 2020).The forest patch area will affect the microclimate within the forest.The larger patch area always follows the lower soil temperature and evaporation, which will affect the effective soil moisture (Liu et al., 2012).The area of forest patches may change with climate change(Thompson et al.,1998),and forest landscapes with different degrees of fragmentation also have different responses to climate (Noss, 2001).In the semi-arid region of the Mongolian Plateau,Shi et al.(2021) also found differences in the tree growth-heatwave relationship among different forest patches through methods of dendrochronology.Therefore,in areas with patchy forests,the results of tree-ring studies conducted only in one forest have underestimated the accuracy of displaying the growth of trees in the entire region, and critical studies are needed to be conducted in different forest patches.
It is generally believed that the extreme climate has a significant impact on ecosystems, and this impact is expected to intensify in the future (Liu et al., 2013).In response to extreme events, trees exhibit strong resilience for their growth(Fang and Zhang,2019).The resilience of tree growth is regarded as the ability of trees to resist interference in the ecosystem, as well as to maintain and restore their structure and functions, including resistance and recovery (Walker et al., 2004).The resilience of tree growth can be calculated from tree-ring data,which can well reflect the interference pressure of tree growth (Fang et al., 2021).Trees can actively resist the impact of adverse interference, such as extreme climate events, and restore their growth status before the interference if the resilience threshold is not exceeded (Rahman et al.,2019).
Because of the differences in growth sensitivity, stomatal characteristics,and physiological characteristics,the growth resilience of different tree species to extreme climates is significant(Fang et al.,2020).Changes in the external environment such as the duration of drought also have important effects on tree growth resilience (Huang et al., 2018).For instance,Fang et al.(2021)found that the growth resistance of Juniperus przewalskii and J.tititica in humid areas of the Tibetan Plateau increased with the increasing frequency of drought,but those trees in humid areas decreased.Wang et al.(2019) found that the growth of J.przewalskii in the northeastern Tibetan Plateau was affected by extreme drought,especially trees at low altitudes with the narrowest rings, the lowest resistance value,and the highest percentage of missing rings in extreme drought years.The environment for high-altitude trees is relatively harsh.However,due to regional differences,its growth is often stressed by different climatic factors (Jiao et al., 2016; Zhang et al., 2018).Shi et al.(2023) found that both daytime and nighttime warming can significantly promote the renewal of the northern Hemisphere alpine treeline.While, Barros et al.(2017) found that intense and frequent droughts offset forest expansion driven by climate and land use change on the treeline of the French Alps.Therefore,it is very important to study the response of high-altitude trees to environmental changes, especially the impact of extreme events.
The contents and availability of non-structural carbohydrates(NSC),total nitrogen (TN), and total phosphorus (TP) in leaves reflect the available nutrient levels and environmental adaptability of plants, and have a significant impact on plant growth and development (Andersen et al.,2004;Li et al.,2008;Xie et al.,2018).For example,Xia et al.(2022)found that as the distance from the city center increased, the available phosphorus content in the soil increased,and the phosphorus content in the leaves of Sophora japonica also increased.When there are differences in temperature, soil moisture, and fertility environments, the growth patterns and resource allocation of trees at different altitudes will also differ (Qin et al., 2022).There is also great variation in the microenvironment within different sizes of forest patches (Liu et al., 2012), but there is still limited research on the physiological differences of trees to climate change in different forest patches.
In order to study the response patterns and physiological differences of trees in different forest patches to climate change, we randomly selected forest patches with clear boundaries, uniform distribution, and significant differences in size in the western edge of the P.crassifolia distribution area for stand investigation, tree-ring research and physiological index measurements of needles.The purpose of this study was to investigate (1) the trend of tree growth and its climatic driving factors,(2) the resilience of tree growth to extreme climate, and (3) the physiological indicators of trees in different forest patches.This study provides a reference for predicting tree growth dynamics and forest management in different forest patches in the northeastern Tibetan Plateau under climate change.
2.Materials and methods
2.1.Study area and meteorological data
The study area is located on the western edge of the distribution area of P.crassifolia, in the Qilian Mountains (northeastern Tibetan Plateau)and in the eastern part of the Qaidam Desert Basin(Fig.1).The majority of land use types are grasslands, followed by deserts and forests.The interior of the mountain area is broken,and there are many valleys.The soil type is mostly mountain brown coniferous forest soil and mountain grayish brown forest soil.The ecosystem is seriously fragile.
The gridded climate dataset with a spatial resolution of 2.5 min was obtained from TerraClimate product(Abatzoglou et al.,2018).Due to the close geographical location of sampling sites, the same grid(98°35′-98°45′E, 36°55′-37°05′N) climate data were used for all six forest patches.Climate data from 1961 to 2020, including monthly minimum temperature (MinT), maximum temperature (MaxT), precipitation(Pr),actual evapotranspiration(AE),and palmer drought severity index(PDSI)were used in this study.
The study area belongs to plateau continental climate, with drought and little rain throughout the year,and cold and long winter.From 1961 to 2020, the changes in monthly temperature (MinT and MaxT) and precipitation at the study site showed a single peak pattern, with all reaching their highest levels in July (Fig.2).Meanwhile, the precipitation in this region was mainly concentrated from May to September,with a total of 240.1 mm, accounting for 86.3% of the annual precipitation(Fig.2).Annual meteorological factors(MinT,Pr,AE,and PDSI)showed a significant upward trend over time from 1961 to 2020 (P <0.001),indicating that the area was warming and wetting(Fig.3).
The growth season of P.crassifolia is mainly from May to September,when the tree growth is sensitive to climatical factors, and the rapid growth period of the tree is mainly from May to July(Wan et al.,2020;Zeng et al., 2020).Therefore, in this study, we simultaneously divided and extracted the meteorological data of the early growing season (egs,May-July)and the late growing season(lgs, August-September).
2.2.Tree-ring sampling
In the western margin of P.crassifolia distribution area in Qilian Mountains (Tongpu Town, Ulan County, Qinghai Province), we randomly selected forest patches with clear boundaries, uniform distribution,and significant differences in size and area within the range of 20 km × 20 km for research.Forest patches with similar elevations but different areas were Forest patch 1(FP1,0.8 ha),Forest patch 2(FP2,4.4 ha), Forest patch 3 (FP3, 7.1 ha), Forest patch 4 (FP4, 18.6 ha), Forest patch 5 (FP5, 41.0 ha) and Forest patch 6 (FP6, 362.8 ha) (Fig.1 and Table 1).
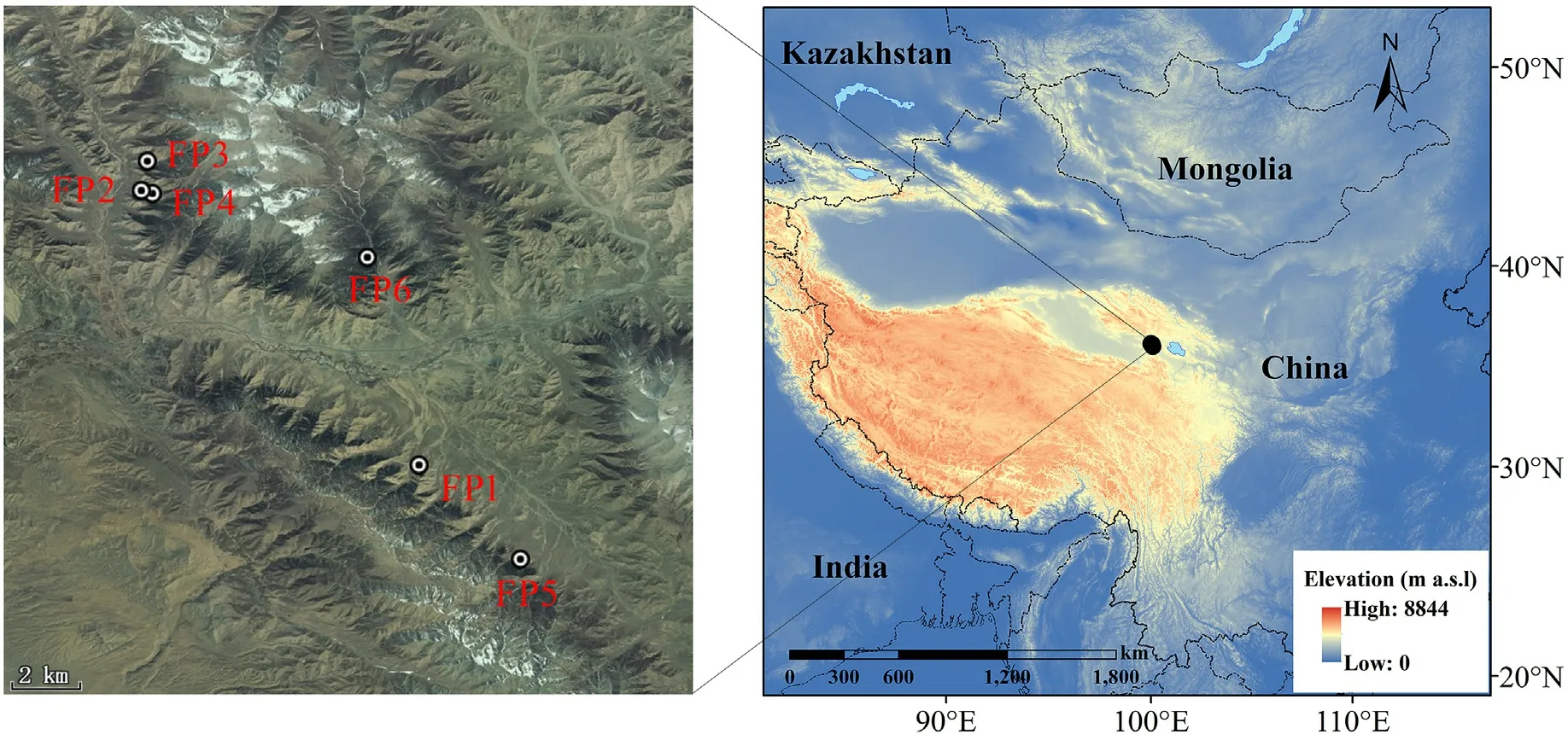
Fig.1.Locations of the sampling sites.

Fig.2.Monthly temperature and precipitation from 1961 to 2020.
At each site, more than 30 dominant trees were sampled for coring,and one core was taken from each tree in June 2022.According to the experimental requirements, some sample cores that do not meet conditions such as fracture or heart rot were removed,and a total of 203 tree cores were retained(Table 1).
We used standard dendrochronological techniques to air-dry,mount,fix, and cross-date all cores (Stokes and Smiley, 1968).The LINTAB 6.0 system(Frank Rinntech,Heidelberg,Germany)was used to measure the tree-ring width with an accuracy of 0.01 mm.And the computer program COFECHA(Holmes,1983)was used to check the quality of measurement and cross-dating, and the ARSTAN program was used to detrend the cross-dated regional/individual annual ring series using negative exponential or linear functions.After standardization, we established and obtained six tree-ring standard chronologies and a total of 203 individual tree-ring width series.Among them,the sample representativeness of six tree-ring standard chronologies was greater than 0.85,indicating that the tree-ring chronology was highly representative and could be used for dendroclimatological analysis(Table 2) (Wigley et al.,1984;Jiao et al.,2019).
2.3.Extreme drought years and tree growth resilience
We referred to the method of Fang and Zhang (2019) to select the years from 1961 to 2020 when the drought index of the main growing season(May to July)was 1.5 times standard deviation below the mean,namely the extreme dry year(1966, 1971 and 1995)(Fig.3f).We evaluate the response of forest patch trees of different sizes to extreme drought events by studying the resilience of individual trees in extreme drought years.
The resilience of tree growth usually demonstrates their ability to resist interference and recover to original state, so the stability of resistance (Rt) and recovery (Rc) can be used to represent the resilience of tree growth (Fang and Zhang, 2019; Zhu et al., 2023).The calculation formula for resistance and recovery is as follows(Lloret et al.,2011):
Among them,Dr represents the annual ring width index in the year of drought,while PreDr and PostDr represent the average annual ring width index during three years before and after the drought,respectively.At the same time, in order to remove a small portion of special tree growth causing significant deviation, in this study, individual tree cores with a recovery value of 5.0 were removed.
2.4.TN, TP, and NSC contents of leaves
In June 2022, we set up a 20 m × 20 m plot in the middle of each forest patch and randomly selected six healthy trees with similar diameter at breast height(DBH)and tree height from each plot.We use branch scissors to randomly cut off the four branches in different directions in the middle of each tree crown,number them,store them in a self-sealing bag,and air-dry them to avoid damage.
Take the branches back to the laboratory and clean them thoroughly.After wiping off the surface water droplets,place them in an oven and dry them to a constant weight.Afterward, remove the needles from the branches and grind and seal the needle samples for future use.The soluble sugars in spruce needles were extracted with an 80%ethanol solution, while starch was extracted using the perchloric acid method.The content of soluble sugars and starch was determined using the standard anthrone colorimetric method,and the NSC concentrations was defined as the sum of the soluble sugar concentrations and starch concentrations(Mo et al., 2020; Peng et al., 2021).We used the semi-microscopic Kjeldahl method to determine the TN content of needles (Shi et al.,2013),and after digestion with perchloric acid and sulfuric acid,the TP content was determined by colorimetry(Zhang and Shangguan,2018).

Fig.3.Change trend of meteorological data from 1961 to 2020.
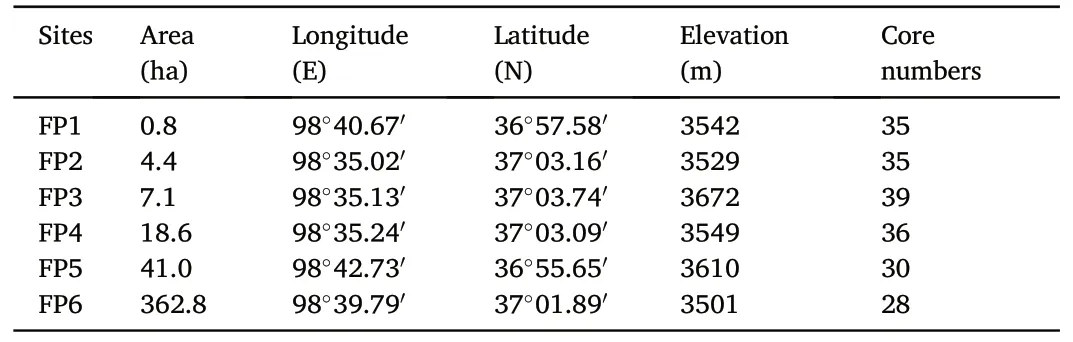
Table 1Sampling location and area of forest patches.
2.5.Statistical analysis
We calculated the Pearson correlation coefficient between climate factors using IBM SPSS Statistics 25.0,and those relatively unimportant climatic factors with high correlation(coefficient >0.7)were removed to reduce multicollinearity between meteorological factors.Based on the corrected Akaike information criterion(AICc),the model selection of tree ring standard chronology was analyzed by using the “glmulti” software package in R software (version 3.6.3) (Calcagno and de Mazancourt,2010; Du and Tang, 2022).A total of seven potential drivers were considered, including the minimum temperature (MinT.egs), maximum temperature (MaxT.egs), precipitation (Pr.egs) and palmer drought severity index(PDSI.egs)in the early growing season,and the minimum temperature (MinT.lgs), maximum temperature (MaxT.lgs) and actual evapotranspiration(AE.lgs)in the late growing season.
We estimated the relative importance of each climate variable by adding the Akaike weights of the model in which the variable appeared.A driving factor with a relative importance value exceeding 0.8 was considered important (Calcagno and de Mazancourt, 2010).In the final model,we chose the variance inflation factor(VIF <5 indicates weak or moderate collinearity) to evaluate the multicollinearity of the climate factors (Shrestha, 2020; Du and Tang, 2022).And we performed a separate conditional regression for each important factor while holding other variables constant.The variance explained by each climate variable was further estimated by averaging sequential sums of squares over all orderings of regressors(Gr¨omping,2006).The above regression analysis was conducted in R software(version 3.6.3).

Table 2Major statistic characteristics for chronologies of P.crassifolia.
To study the response differences to climate change among individual trees, we also calculated the Pearson correlation coefficients between individual tree-ring width series and annual climate factors from 1961 to 2020 using the IBM SPSS Statistics 25.0.At the same time, one-way ANOVA was used to detect the differences in the resilience and resistance of trees in different forest patches during extreme drought years,as well as the differences in TN, TP and NSC content during the growing season.
3.Results
3.1.Temporal variation of tree growth
From 1961 to 2020, the tree-ring chronology of small and mediumsized forest patches (FP1, FP2, FP3 and FP4) showed a significant upward trend over time(P <0.01),especially in FP3(Fig.4).The trend of tree-ring chronology changes of larger forest patches(FP5 and FP6)was not significant(Fig.4).
From 1961 to 2020,the proportion of trees with a significant upward trend in FP3 was the highest(74.4%),and the proportion of trees with a significant downward trend in growth was the lowest (2.6%) (Fig.5).Starting from the forest patch area of 7.1 ha(FP3),as the forest patch area increased, the proportion of trees with a significant upward trend in growth gradually decreased,while the proportion of trees with a significant downward trend in growth gradually increased (Fig.5).In the largest forest patch (FP6), the proportion of trees showing a significant upward trend in growth was about 14.3%,while the proportion of trees showing a significant downward trend in growth was about 32.1%(Fig.5).In addition,the proportion of trees with significantly increasing growth in smaller forest patches (FP1 and FP2) was higher than that in larger forest patches (FP5 and FP6), while the proportion of trees with significantly decreasing growth was the opposite(Fig.5).

Fig.5.Proportion of trees with a significantly positive, significantly negative,and not significant growth trend at six sites.
3.2.Relationship between tree growth and climate factors
The result showed that tree-ring chronology in FP1 was mainly explained by MinT.egs, MaxT.egs and MaxT.lgs (Fig.6a).Conditional regression analysis showed that tree-ring chronology in FP1 increased significantly with MinT.egs (variance explained 14.6%,P <0.001)and MaxT.lgs (variance explained 4.4%, P <0.05), while it decreased significantly with MaxT.egs (variance explained 5.5%, P < 0.05)(Table 3).The MinT.egs was also a main explanatory factor of the treering chronology in FP2, and conditional regression analysis also showed that tree-ring chronology increased significantly with MinT.egs(variance explained 16.8%,P <0.001)(Fig.6b and Table 3).
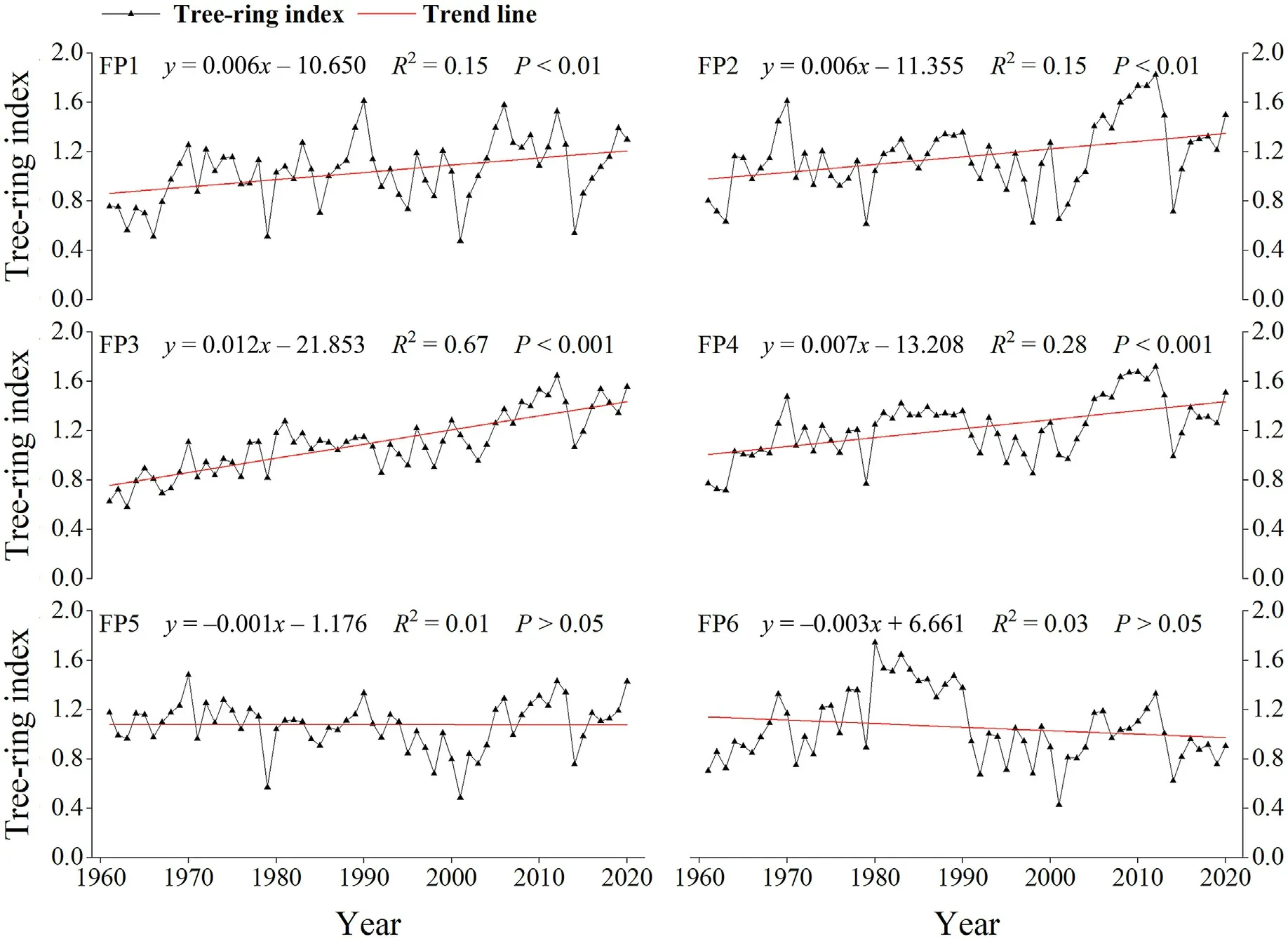
Fig.4.Standard chronologies in six forest patches from 1961 to 2020.
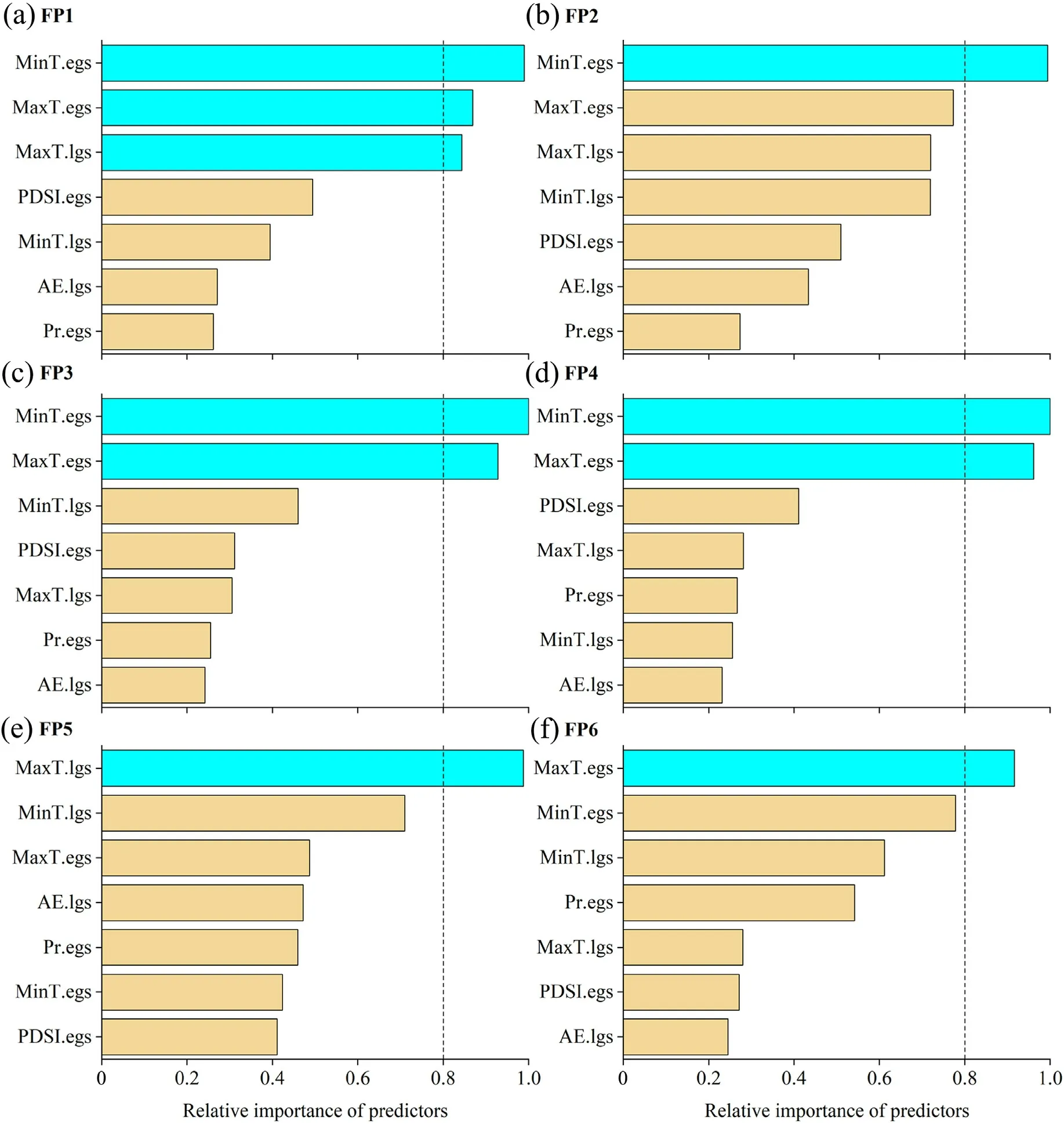
Fig.6.Relative importance of seven climatic variables that potentially regulate the temporal variation in tree-ring standard chronology during 1961-2020.
Tree-ring chronologies in FP3 and FP4 were mainly explained by MinT.egs and MaxT.egs (Fig.6c and d).Conditional regression analysis showed that tree-ring chronologise in FP3 and FP4 increased significantly with MinT.egs (variance explained 31.6% and 22.1%, respectively, P <0.001) but decreased significantly with MaxT.egs (variance explained 4.7%and 5.1%,respectively, P <0.05)(Table 3).
Tree-ring chronology in FP5 was mainly explained by MaxT.lgs(Fig.6e).Conditional regression analysis showed that tree-ring chronology in FP5 increased significantly with MaxT.lgs(variance explained 16.0%, P <0.001) (Table 3).While tree-ring chronology in FP6 was mainly explained by MaxT.egs(Fig.6e).Conditional regression analysis showed that it decreased significantly with MaxT.egs(variance explained 10.2%,P <0.05)(Table 3).
In small and medium-sized forest patches (FP1-FP4), the increase of the MinT.egs is an important factor driving the positive growth of trees,especially in FP3(Fig.6 and Table 3).However,the increase of MaxT.egs inhibited the growth of trees in most forest patches, especially in the largest forest patches(Fig.6 and Table 3).
From 1961 to 2020, among different forest patches, FP3 had the highest proportion of trees(66.7%)with a significant positive correlation between individual tree-ring width series and annual minimum temperature,while FP5 had the highest proportion of trees(20.0%) with a significant negative correlation between individual tree-ring width series and annual minimum temperature (Table 4).Meanwhile, from the area of 0.8 ha (FP1) to 18.6 ha (FP4), there was a significant positive correlation between the growth of over 35% of the trees and the annual minimum temperature, but the proportion of trees with a significant negative correlation does not exceed 10% (Table 4).The proportion of trees in all forest patches showed a significant positive correlation between individual tree-ring width series and annual maximumtemperature was less than 10% (Table 4).Only in FP6, 32.1% of trees showed a significant negative correlation between individual tree-ring width series and mean maximum temperature (Table 4).

Table 3Conditional regression eigenvalues of important climatic driving factors.
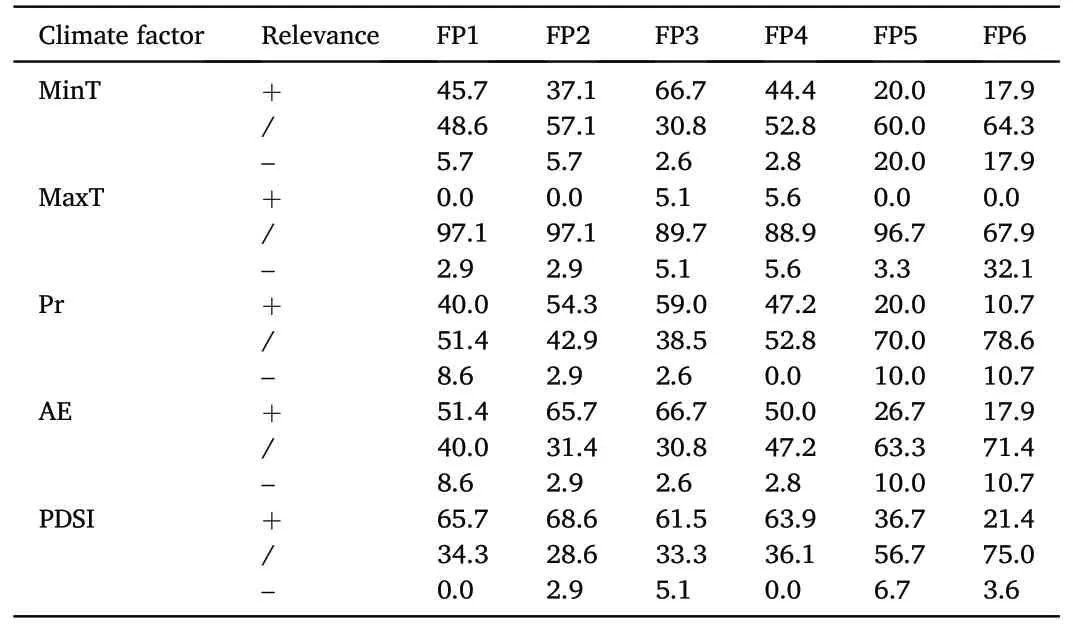
Table 4The proportional contributions of the significant correlations between individual tree-ring width series and annual climate factors from 1961 to 2020 (%).
The proportion of trees with a significant positive correlation between individual tree-ring width series and precipitation and actual evapotranspiration increased with the increase of forest patch area,until FP3 reached its highest, with 59.0% and 66.7%, respectively (Table 4).Subsequently, as the area of forest patches increased, the proportion of trees with a significant positive correlation between individual tree-ring width series and precipitation and actual evapotranspiration decreased until FP 6 reached its lowest, with 10.7% and 17.9%, respectively(Table 4).From the area of 0.8 ha (FP1) to 18.6 ha (FP4), there was a significant positive correlation between over 60%of the tree-ring width series and the annual palmer drought severity index,but the proportion of trees with a significant negative correlation did not exceed 10%(Table 4).
3.3.Tree growth resilience in different forest patches
In extreme drought years,the growth resistance of trees in most forest patches was the highest in 1966 and significantly higher than the values in 1971 and 1995.Meanwhile,in different extreme drought years,as the forest patch area increased, the overall trend of tree growth resistance showed a unimodal pattern,with the highest in FP3(Fig.7).It indicated that under extreme drought, trees in medium-sized forest patches had relatively strong resistance to drought compared to larger or smaller forest patches(Fig.7).
In extreme drought years,the tree growth recovery of FP1,FP2,and FP5 was significantly higher in 1966 than in 1995,while the tree growth recovery of FP3 was significantly higher in 1995 than in 1966(Fig.7).In different extreme drought years, as the forest patch area increases, the overall change in tree growth resilience showed a “U” shape, and the overall trend of change is opposite to the resistance of tree growth(Fig.7).
3.4.Differences in N, P, and NSC content in needles of tree in different forest patches
The starch content in the needles of FP3 was the highest (36.97 mg·g-1)and significantly higher than that in other patches(20.51-27.37 mg·g-1)(P <0.05)(Fig.8).The NSC content was also the highest in the needles of FP3 (137.66 mg·g-1), and significantly higher than FP1(112.49 mg·g-1), FP2 (115.85 mg·g-1), and FP6 (115.97 mg·g-1) (P <0.05) (Fig.8).In addition, we found that the TN/TP value of needles remained the highest in FP3 (37.75), and was significantly higher than those in FP2(25.62),FP5(24.79),and FP6(24.58)(P <0.05).
4.Discussion
4.1.Effects of warming and wetting on the growth of trees in different size forest patches
The growth of trees in small and medium-sized forest patches(FP1-FP4) showed a significant upward trend, and the increase of minimum temperature in the early growing season (rapid growth period) was an important driving factor in promoting the growth of these trees.Tree growth in cold regions is usually positively correlated with temperature(Holtmeier and Broll,2005;Silva et al.,2016).When the temperature is within the threshold range, an increase in temperature is beneficial for breaking the dormant state of plants, improving photosynthesis and carbohydrate accumulation,promoting the growth of the xylem,and thus promoting the radial growth of trees (Jiang et al., 2017; Wen et al.,2022).Wan et al.(2020) found that P.crassifolia in the central Qilian Mountains began to grow rapidly from May to June and was mainly affected by temperature.At the same time, it stopped growing from August to September and was mainly affected by soil moisture.Tian et al.(2017) also reported that the main growth period of P.crassifolia in the Qilian Mountains began in mid May, and soil temperature was a key factor affecting the onset of tree growth.This indicates that trees are mainly limited by low temperatures during the rapid growth period.
At the same time, the increase of the maximum temperature in the early growing season inhibited the growth of trees in this area, and the effect was mainly in the larger forest patches.As also located in arid and semi-arid regions, tree growth was also limited by water availability,excessively high temperatures may exacerbate drought by exacerbating water deficits in high-altitude forests(Jiao et al.,2016;Peng et al.,2023).
The growth of individual trees in medium forest patches was better and the correlation with meteorological factors was stronger.Smaller forest patches may be more affected by edge effects (Laurance et al.,2006).Edge effects can affect plant lighting conditions and water conditions (Matlack, 1993), and have a certain correlation with soil moisture, N, P, potassium (K), and pH (Wekesa et al., 2018).However, the environment with smaller patches may not be the most conducive environment for tree growth.Nascimento and Laurance (2004) found that in Amazon forests, carbon loss was more likely to occur in smaller forest patches.
The larger forest patch area may result in the lower soil temperature but the higher effective soil moisture (Liu et al., 2012).Meanwhile, the promotion of tree growth by warming varies at different stages (Jiao et al.,2021),and an increase in temperature can also affect the water use efficiency of trees(Wu et al.,2015).Therefore,if there are differences in temperature among different forest patches of different sizes, then the same heating conditions also have different effects on the growth of trees in different patches.However, with the increase of precipitation and temperature in some regions,the response sensitivity of some trees may decrease and even have side effects (Wang et al., 2005; Zhang et al.,2013;Jiao et al.,2015).
The NSC contents and TN/TP of needles also exhibited a similar pattern in different patches as the proportion pattern of trees with a significant upward trend in growth.According to the carbon limitation hypothesis,due to a decrease in photosynthetic carbon assimilation,the NSC contents in leaves are expected to decrease with a decrease in temperature (Stevens and Fox, 1991; Wardle, 1993).Hence, when the temperature rises, plants are more adaptable to the environment for growth.The stronger photosynthesis may contribute to the higher NSC contents and then the faster growth rates.In addition,leaf photosynthetic capacity and NSC synthesis are closely related to leaf N and P concentrations (Xie et al., 2018).The higher N:P ratios of leaves in moderate forest patches may reflect the better adaptability of trees to the environment and higher N utilization(Güsewell,2004;Xie et al.,2018).
Moreover,a significant proportion of tree growth in forest patches has been affected by drought.In relatively arid areas, the formation of smaller forest patches may be related to soil moisture content (drought degree) (Birhanu et al., 2021).In semi-arid regions, stem flow, soil and litter moisture content were directly proportional to patch area(Barbosa et al.,2010).Although the warming and wetting trend of the climate in the research area has become increasingly evident in recent decades,warming has led to an increase in evaporation demand,extreme climate events have a greater trend in higher altitude areas and the wetting characteristics are not yet sufficient to change the arid climate(Shi et al.,2006;Lin et al.,2017).Therefore,most of the trees in forest patches will still be affected by drought for a certain period of time.
4.2.Differences in the growth resilience of trees in different size forest patches under extreme drought
During extreme drought years,the growth resistance of trees in most forest patches showed a significant decrease and then slowly increased over time.Extreme climate has a significant impact on ecosystem structure and functions and will intensify with future global warming (Chen et al.,2019).The generation of low resistance in trees is often influenced by extreme weather conditions (Bose et al., 2021).The decrease in tree resistance caused by drought stress may be due to increased temperature accelerating the transpiration rate of trees, as well as affecting photosynthesis,carbon balance,water and nutrient utilization in alpine forests,resulting in changes in the internal regulatory mechanisms of trees(Balster and Marshall,2000; Farooq et al.,2009).At the same time, the resilience of regional trees is a dynamic spatial pattern that can change under the influence of climate change.In northeastern Spain,due to the influence of tree size and drought intensity,the growth resilience of trees gradually decreases over time(Serra-Maluquer et al.,2018).In addition,Jiao et al.(2021) found that under climate warming, the resistance of Larix sibirica in Tianshan Mountains and Altai Mountains to extreme climate gradually decreased while the recovery increased.Lucash et al.(2017)found that climate change can reduce the tree growth resilience of forests in central northern Minnesota.

Fig.8.Differences in non-structural carbohydrate (NSC), total nitrogen (TN) and total phosphorus (TP) content in leaves of trees in different forest patches.Note: Different capital letters indicate significant differences between different sites.
Regional climate differences often limit tree growth to different climatic factors(Shen et al.,2020).Trees in high-altitude areas are affected by various stress factors such as drought, low temperature, and strong ultraviolet radiation,while trees can enhance their resistance or recovery ability by adjusting their physiological conditions, and thus adapt to stress (Helman et al.,2017;Rahman et al., 2019).Hence, trees begin to adapt to extreme drought events by adjusting themselves, but they will likely continue to be damaged by extreme drought for some time.We need to pay more attention to the occurrence of extreme drought events.
In this study, under conditions where the climate was relatively favorable for tree growth, trees with moderate patches were more resistant to extreme drought events.This may be related to the higher nitrogen storage in the branches of moderate forest patches being more resistant to water deficit (He et al., 2022).Additionally, the high non-structural carbohydrate content in the leaves of moderate patch forests also reflected a higher amount of plant carbohydrates that can be used to resist external adverse environments (Richardson et al., 2015,Hartmann and Trumbore,2016).
Under adverse climate conditions,the growth of trees with moderate patches may also be more affected.Shi et al.(2021)found that with the increase of the frequency of heatwaves,the growth of Larix sibirica in the northern Mongolian Plateau significantly decreased,the growth of trees in large patches (>10 ha) decreased by 8.4%, the growth of trees in medium patches(3-10 ha)decreased by 48.1%,and the growth of trees in small patches (<3 ha) decreased by 39.2%.At the same time, the overall pattern of changes in the recovery of different forest patches under extreme events was opposite to the resistance.The changes in resistance and recovery in ecosystems generally exhibit an opposite relationship, where an increase in resistance leads to a decrease in resilience,and vice versa(Fang and Zhang,2019).
5.Conclusion
As the regional climate gets warmer and wetter,the growth of trees in small and medium-sized forest patches (FP1-FP4) showed a significant upward trend.The increase of minimum temperature in the early growing season was the most important driving factor to promote the growth of trees, especially in medium-sized forest patches.While the increase of the maximum temperature in the early growing season inhibited the growth of trees in this area,especially in the largest forest patches(FP6).
The proportion of trees with a significant upward trend in growth and with a significant positive correlation with annual meteorological factors showed an unimodal change overall.The trend of NSC and N/P content changes in needles also showed a single peak pattern with the change of area, indicating that trees in moderate forest patches had stronger photosynthetic capacity.
In extreme drought years, as the forest patch area increased, the overall trend of tree growth resistance showed a unimodal pattern,while the overall trend of tree growth recovery was opposite.This indicates that medium-sized forest patch trees have been more resistant to extreme events, while larger and smaller forest patch trees have weaker resistance.We should pay more attention to the growth of trees in large-scale forest patches in the region to cope with future climate change,especially the occurrence of extreme droughts.
Funding
This work was supported by the National Natural Science Foundation of China(Nos.31971460 and 32271646s).
Data availability
The authors do not have permission to share data.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Zhongtong Peng:Conceptualization,Formal analysis,Investigation,Writing - original draft.Qifeng Mo:Resources, Writing - review &editing.Liangjun Zhu:Writing - review & editing.Qingao Lu:Investigation.Jiaqing Cai:Formal analysis.Mingming Guo:Writing-review& editing.Kun Xu:Investigation.Yuandong Zhang:Funding acquisition, Project administration, Resources, Supervision, Writing - original draft, Writing-review&editing.
杂志排行
Forest Ecosystems的其它文章
- Tree-based ecosystem services supply and multifunctionality of church forests and their agricultural matrix near Lake Tana, Ethiopia
- Influence of climate fluctuations on Pinus palustris growth and drought resilience
- Nutrient retranslocation strategies associated with dieback of Pinus species in a semiarid sandy region of Northeast China
- Book review “Continuous Cover Forestry - Theories, Concepts, and Implementation” by Arne Pommerening
- Impact of black cherry on pedunculate oak vitality in mixed forests:Balancing benefits and concerns
- Sensitivity of forest phenology in China varies with proximity to forest edges
