Scaly-sided Merganser (Mergus squamatus) equalizes foraging costs with depth by switching foraging tactics
2024-01-22PeizhongLiuMeihanLiuDongyangXiaoYingHeRongFanCaiLuLiWenQingZengGuanghunLei
Peizhong Liu, Meihan Liu, Dongyang Xiao, Ying He, Rong Fan, Cai Lu, Li Wen,Qing Zeng,*, Guanghun Lei,**
aCentre for East Asian-Australasian Flyway Studies, Beijing Forestry University, Beijing, 100083, China
bSchool of Ecology and Nature Conservation, Beijing Forestry University, Beijing, 100083, China
cCollege of Forestry, Beijing Forestry University, Beijing, 100083, China
dThe People’s Government of Shangqing Town, Yingtan, 335004, China
eNSW Department of Planning, Industry and Environment, Science, Economics and Insights Division, Sydney, 2150, Australia
fDepartment of Earth and Environmental Sciences, Macquarie University, Sydney, 2109, Australia
Keywords:Foraging behavior adaption Foraging energetics In-stream habitats Migratory waterbird
ABSTRACTThroughout evolutionary history, animals are finely tuned to adjust their behaviors corresponding to environmental variations.Behavioral flexibility represents an important component of a species'adaptive capacity in the face of rapid anthropogenetic environmental change, and knowledge of animal behaviors is increasingly recognized in conservation biology.In aquatic ecosystem, variation of water depth is a key factor affecting the availability of food; thus, the foraging behaviors of many waterbirds, especially piscivores.In this study, we compared the foraging behaviors of the Scaly-sided Merganser (Mergus squamatus), an endangered migratory diving duck endemic to East Asia,in habitats with different water depths(Shallow waters:0–40 cm;Deep waters:40–300 cm),using video camera records obtained from the known wintering sites during three winters from 2018 to 2020.Further, the energy expenditure of foraging behavior profile and energy intake based on fish sizes were calculated to study the foraging energetics.In total, 200 effective video footages that contained 1086 min with 17,995 behaviors and 163 events of catching fish were recorded.Results showed that: 1) time length for fishing(including eye-submerging, head-dipping, diving and food handling) of M.squamatus in shallow waters was significantly more than in deep waters; 2) M.squamatuss spent significantly more time for preparing (including vigilance,preening and swimming)in deep waters than in shallow waters;3)the mean catch rate was 0.28 fish/min in shallow waters,which is significantly higher than the value of 0.13 fish/min in deep waters;4)despite the distinct foraging behavior profiles and energy intakes, M.squamatus showed similar energetics in shallow and deep waters.We concluded that M.squamatus is a good example of behavioral flexibility that aligns with expectations of optimal foraging theory, in that it behaves in accordance to resource availability in different environments, resulting in high foraging efficiency.
1.Introduction
Wild animals have been constantly subjected to environmental fluctuations during their evolutionary history, and have evolved to match physiology and behavior to the predictable environmental variations in their natural habitats (Schmidt-Nielsen, 1997).Adjusting behavior is often the first response when environmental conditions are altered(Snell-Rood, 2013).The ability of wildlife to adjust its behaviors appropriately in response to environmental changes (behavioral plasticity or flexibility)is crucial for their survival(Sih et al.,2011;Wong and Candolin,2015).Behavioral plasticity could allow species to adjust behavior to suit the conditions of its immediate environment and, in so doing, increase its fitness (Van Buskirk et al., 2012).Under the current conditions of intensified anthropogenic disturbance and accelerated climate change (Corlett, 2015; Riddell et al., 2018), organisms must respond quickly to these anthropogenic-induced changes to reduce the risk of extinction (Sih et al., 2011; Ducatez et al., 2020) and gain additional time for genetic adaptation(Pigliucci,2005).
There is a diverse range of behavioral adaptations,such as changes in the timing of and investment into breeding observed in Southern Rockhopper Penguins (Eudyptes chrysocome chrysocome) (Dehnhard et al.,2015), shifts in diets of Herring Gulls (Larus argentatus) (Bukacinska et al.,1996),and alternations of daily foraging home ranges according to food availability (Lei et al., 2019).Among all adaptive tactics, foraging behavior plasticity is among the first and most studied aspect of animal behaviors(Pyke et al.,1977;Pyke,1984;Perry and Pianka,1997),and is considered as one of key factors for animals to survive new or unusual challenges such as food shortages or extreme climatological events(Sih,2013).
Many birds,especially migratory waterbirds,exhibit great flexibility in foraging tactic(Erwin,1983;Stafford et al.,2014;Lei et al.,2019).For example, Great Knots (Calidris tenuirostris) extended their feeding time and adjusted their food selection to adapt the sudden decline of original main food(Zhang et al.,2019).Masked Boobies(Sula dactylatra)clearly increased dive bouts and a high feeding frequency from a nearby shallow shelf (“local habitat”) to distant deeper waters (“distant habitat”)(Sommerfeld et al., 2015).The optimal foraging theory (OFT) (Emlen,1966; MacArthur and Pianka, 1966), rooted in natural selection and focused on diet selection,habitat choice,time allocation,and movement patterns (Pyke et al., 1977), could provide the basis to predict how foraging animals interact with their environment and to understand how individuals may trade off alternative options in response to time- or energy-constraints(Baert et al.,2021).
To assess animals' foraging plasticity in a changing environment,habitat–behavior interactions within the wide array of environmental conditions are to be characterized (Gilmour et al., 2018).Flexible and specialized foraging strategies are dependent on the stability of available resources in habitats (West-Eberhard, 1989).For most piscivorous waterbirds, water depth might be the primary habitat quality parameter(Zeng et al.,2018a).Different water depths mean different species,sizes and quantities of food and therefore a key factor to affect their foraging behaviors and techniques (Harvey and Stewart, 1991; Giraldo et al.,2017).
The Scaly-sided Merganser(Mergus squamatus)is endemic to East Asia and is listed as globally endangered(BirdLife International,2017).It is a habitat specialist, which occurs on clear, flowing rivers in the mountainous regions(Zeng et al.,2018a).It mainly breeds in Southeast Russia,and Northeast China, and winters in the central and southern China,especially middle and lower Yangtze River (Zhao and Pao, 1998; Zeng et al.,2018b).M.squamatus may be an opportunist piscivore who selects the most abundant food source (Zhao and Pao, 1998), such as fish and macroinvertebrates.It has long thin hooked bill with many serrations along the edges(Zhao and Pao,1998)and is adapted to active hunting of moving prey in water column.M.squamatus mainly takes two foraging modes:diving and head-dipping(Zhao and Pao,1998;Solovieva,2002).In breeding areas, M.squamatus usually immerses its head into shallow waters to pick aquatic insects and use diving techniques to catch fish in deep waters (Zhao and Pao, 1998; Solovieva, 2002).In its wintering habitats, water systems of Poyang Lake, it was reported that the piscivorous duck dove to fishes living in the middle and low layers of the river(Zhi and Shao,2020).To date,the foraging strategy and energy budgets of M.squamatus at different water habitats were largely unknown(Solovieva,2002).
The loss of lateral and longitudinal connectivity of free-flowing rivers through flow regulations such as damming presents a major threat to global freshwater biodiversity (Tonkin et al., 2018; Dudgeon, 2019;Barbarossa et al., 2020) including the obligated freshwater megafauna(He et al., 2019) and foragers such as waterbirds (Zeng et al., 2018a).Knowledge of animal behavior is fundamental in understanding and mitigating the effects of habitat loss and fragmentation.In this study,using three years(from 2018 to 2020)of video footages of M.squamatus foraging at the wintering habitats with different water depths,we aim to evaluate energy intake as dependent on water depth.We also compared the foraging energetics in different habitats by estimating the behavior energy costs,fish catch rates,and energy intakes.We use these findings to highlight the risk of the rapid anthropogenic environmental changes to habitat specialists(Sih et al.,2011).
2.Materials and methods
2.1.Study area
This study was conducted in a 5-km reach of the Shangqing River,a tributary of Poyang Lake in Jiangxi Province (Fig.1).The prevailing regional climate is humid subtropical with no dry season.The average annual temperature is 17.9 C and the average annual precipitation is 1878 mm (Zhang et al., 2021).The site is one of the major wintering grounds of M.squamatus, providing foraging habitat for a population of about 50 individuals every year (Wang et al., 2010).Both sides of the river section are fringed with intact riverine vegetation of dense forest with only occasional human presence.Since the onset of 10-year fishing ban in Yangtze River Basin,there is no fishing in the waterway and other human activities (such as transportation) are limited.We observed minimal human disturbance to the site during our 3-year study.There are many gravel bars and islands in this river reach,providing ideal habitats for M.squamatus to forage and roost(Zeng et al.,2015).
Multiyear surveillance reveals that the ducks mainly use six sites(Fig.1), and there is no physical barrier between them.Therefore, the wintering birds can move freely among these sites within a day.The water depth of the six locations varies from 0 to 3 m in winter(authors’own measurement).Considering the different foraging behaviors (i.e.,diving and head-dipping, see Fig.2 below), we divided the sites as shallow(average water depth about 25 cm,up to 40 cm)and deep(mean water depth about 130 cm,up to 300 cm)(Fig.1).
2.2.Behavior recordings and identification
Video recording was often used to monitor the foraging behavior in a natural complex environment (de Bruijn et al., 2021).In this study,Nikon P900S was used by researchers,who hid behind a camouflage net and/or vegetations about 50-m distance from the site to avoid any disturbance to the birds,to record the foraging activities of M.squamatus in three winters (2018–2020).Video footages would normally be taken in the entire daylight period from six fixed locations in clear days.Recording was initiated when the researchers noticed a bird starting foraging and the camera then followed the bird till it finished the foraging(i.e., start to rest)or disappeared from the range of view.Each foraging bout is a valid video footage and could contain several fishing events,which involves multiple foraging associated activities,including diving(head-dipping),vigilance, eye-submerging, food-handling,swimming,and preening(Table 1,Fig.2).The duration of foraging could last for 3–15 min.
We focused on the foraging behaviors of individuals, which were identified as eight activities from three related types: fishing, preparing and other social activities (Table 1).Here we distinguished two main fishing behaviors: diving and head-dipping (Fig.2).While diving is mainly performed in deep waters,head-dipping is the main fishing tactic in shallow waters.The length of time that a M.squamatus spent in each behavior was divided by the length of the foraging bout to give the percent of time spent in each behavior.
2.3.Video analysis
All videos were analyzed in Boris Video v7.9.1.BORIS (Behavioral Observation Research Interactive Software, University of Torino) is a free, open-source and multiplatform standalone program that allows a user-specific coding environment to be set for a computer-based review of previously recorded videos or live observations (Friard and Gamba,2016).The software has been used in study of behavior of human,farm animals, and wildlife (Aletta et al., 2016).In BORIS, once the coding process is completed,the program can extract a time-budget for single or grouped observation automatically and present as an at-a-glance summary of the main behavior features(Friard and Gamba,2016).Data of behavior duration and frequency was recorded and conversed to excel files.
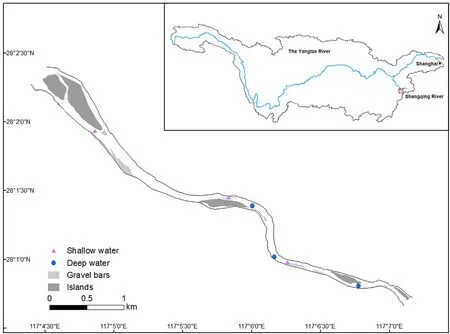
Fig.1.The study area in Shangqing River and its location in the Yangtze River Basin.
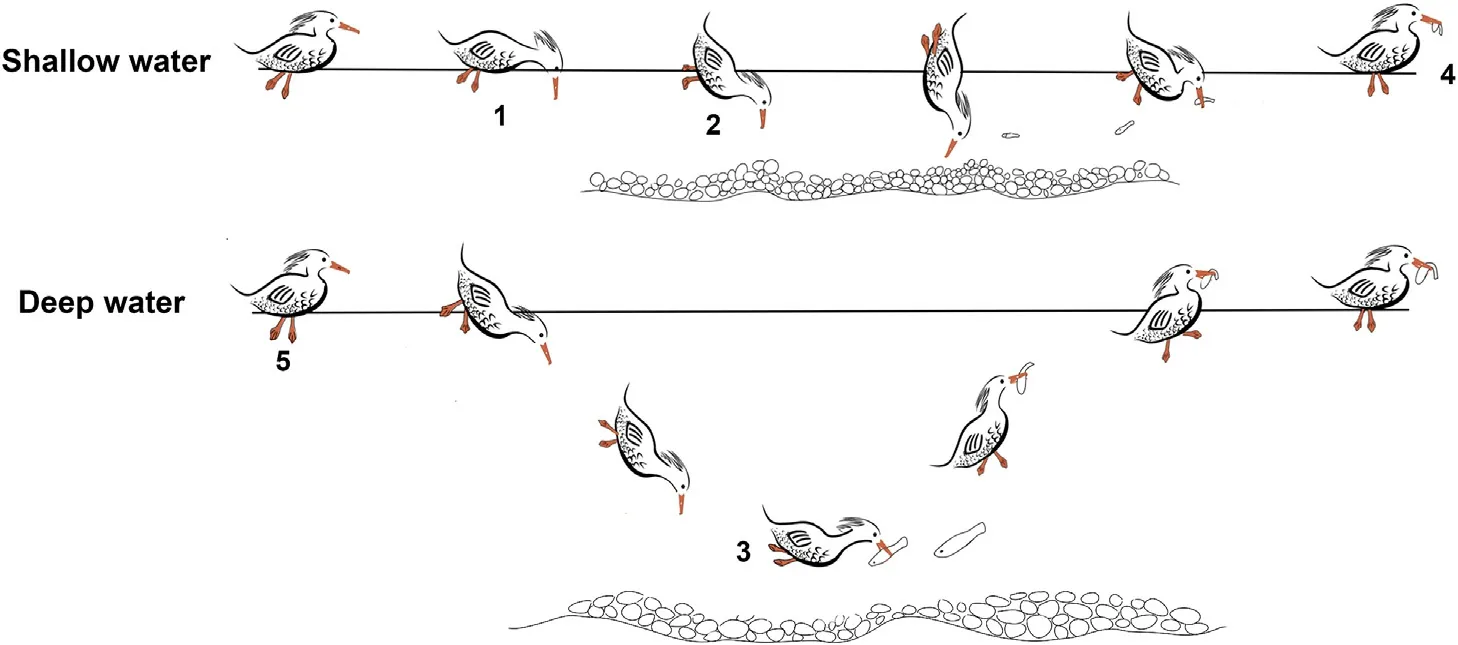
Fig.2.The identified foraging behaviors of M.squamatus at different water depths.

Table 1Description of identified behaviors of M.squamatus.
2.4.Energy budget
Energy gain:We estimated the energy gain of every successful fishing event (a foraging bout during which the focal bird caught one or more fish is counted as a successful fishing event).First, the prey size was determined through visual comparison of the captured fish with the beak volume(length:5.87 3.49 cm;width:1.35 0.15 cm;height:1.59 0.16 cm, n ¼ 10) of M.squamatus (NACRC, 2023), and was assigned to one of the five following classes:0.5(0.26–0.75)volume,1(0.76–1.25)volume, 1.5 (1.26–1.75) volume, 2 (1.76–2.25) volume, and 2.5(2.26–2.75)volume of beak(Fig.3).To determine the fish biomass,gill nets were used to sample fish abundance and richness in the Shangqing River prior to the implementation of the ten-year fishing ban policy in the Yangtze River Basin.A total of 17 fish species were recorded,and measurements of body length,body height,and weight were conducted.The most abundant species include Pseudohemiculter dispar, Sinibrama macrops and Sarcocheilichthys kiangsiensis.Based on the data obtained from this survey, we estimated that the average weight of fish with one beak volume to be 14.1 g.As the mean energy content of the fish is approximately 4.0 kJ/g(Gremillet et al.,1995;Newson and Hughes,1998)and the assimilation efficiency is assumed to be 80% (Feltham, 1995), the energy gain of having a fish with specific beak volume can then be calculated as:

Fig.3.Photos shows fish sizes in relation with the beak of M.squamatus.Fish size is used to estimate energy gain.
Energy expenditure: Energy expenditure was calculated using the observed foraging activity profile and the Basal Metabolic Rate (BMR).The BMR was determined to use the Aschoff-Pohl equation(Aschoff and Pohl, 1970):
The BMR coefficients of different behaviors were sourced from thepublished studies on Common Merganser (Mergus Merganser), Lesser Snow Goose(Anser caerulescens)and Black Duck(Anas rubripes)(Table 2).Because the BMR coefficient of other activities including socializing,preening and food handling varies greatly from literature, moreover,these activities account for only a small fraction of the total foraging time(less than 4.2% of the total, see Table 3 below), we excluded them in calculating energy balance.Based on the body mass (i.e., male 1125–1400 g, female 870–1100 g) of M.squamatus (Kear and Hulme,2005),the BMR of male and female was calculated as 365.00 kJ/day and 304.12 kJ/day,respectively.Out of our 200 valid video clips, we had a total of 97 males, 83 females, and 20 subadults.Considering that the subadult has body weight closer to that of female duck,we assumed that the mean BMR of M.squamatus was 334.56 kJ/day.The total energy expenditure was calculated as the sum of energy cost of each foraging behavior(i.e.,BMR of the behavior multiplied by the behavior time rate).
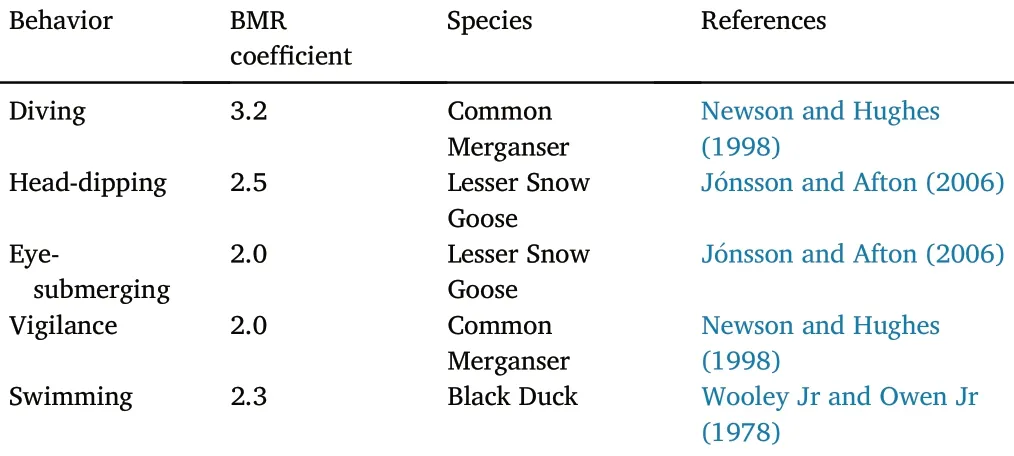
Table 2Published basal metabolic rate(BMR)coefficients of different foraging activities.
2.5.Statistical analyses
We compared the differences of activity profile and foraging energetics of the M.squamatus between shallow- and deep-water habitats using generalized linear mixed model (GLMM) within the Bayesian framework.As the recorded foraging bouts within sites were not independent(i.e., the same bird could be recorded multiple times), and this issue of pseudoreplications increases the likelihood of type I error(Gillies et al., 2006), we specified site as random effects in GLMM to explicitly account for the non-independence(Bolker et al.,2009;Barr et al.,2013;Davies and Gray, 2015).In addition, as not all variables are normally distributed, for example, the behavior variables were expressed as a percentage of the total length of foraging bout (i.e., bounded between 0 and 1), a beta distribution is more appropriate, we tested a range of distribution families, including Beta, Gamma, lognormal, Student-t,Weibull, skew normal, exponential, Gaussian, and exponentially modified Gaussian(exgaussian),and reported the best model selected by theLOO(leave-one-out cross-validation)procedure(Vehtari et al.,2017).
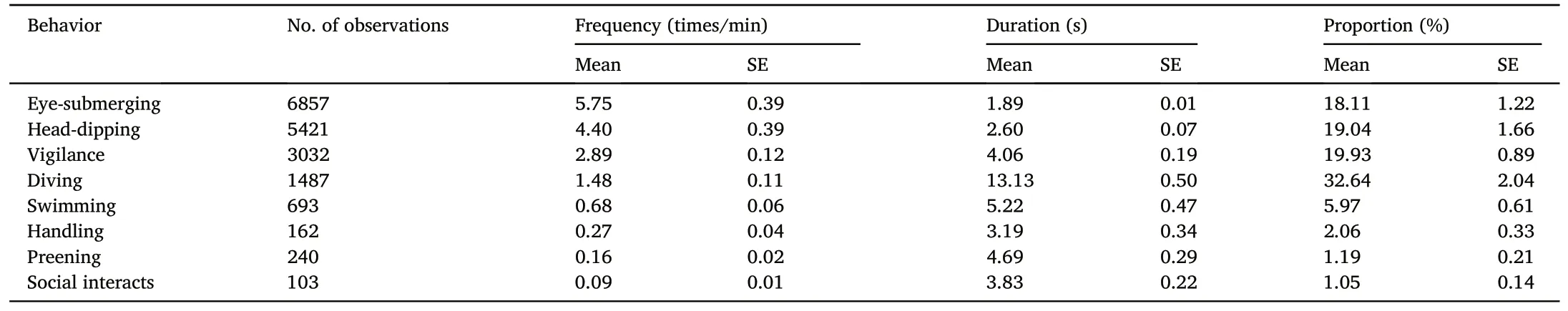
Table 3Summary of the foraging behaviors of M.squamatus during wintering in Shangqing River, China.
We also tested the effort of sex on fish catching through the comparison of two sets of models (i.e., with and without sex as covariates)using cross-validation for model selection(Piironen and Vehtari,2017).Because the difference of elpd loo(i.e.,the Bayesian LOO estimate of the expected log pointwise predictive density,Vehtari et al.,2017)was less than 4 for all paired models(Vehtari et al.,2019),we considered that the variable sex was not important for our estimation of the foraging energetics of M.squamatus.
To simplify the comparison of behavior profiles in shallow and deep habitat,the eight identified behaviors were group into three main classes(i.e.,fishing,searching,and resting,Table 1).
The Bayesian GLMMs were fitted using the“brms”package(Bürkner,2017) in R (R Development Core Team, 2019).The models were fitted with four Markov chains and each chain has 10,000 iterations including 5000 warmups.The posterior thinning rate was set to 20%,resulting in 4000 posterior samples for further analysis or visualization.We checked model convergence with Rhat scores (i.e., Rhat is close to 1 at convergence).In addition to the fitted mean value, we also reported the standard error of estimates from all models.
3.Results
During 90 days of the three winters, we obtained a total of 200 effective video footages (bouts), which contained 1086 min of 17,995 foraging activities (Table 3).Among these identified foraging bouts, 84 were in shallow-water habitats and 116 were in deep-water.A total of 98 successful fishing events was recorded,and collectively,the ducks caught a total of 163 fish (note that there were occasions we recorded the bird caught 2 or more fish in its beak).
3.1.General pattern of behavior profiles
Not surprisingly, the birds spent most time in activities relating to obtaining food including diving, head-dipping, eye-submerging, and vigilance,and food handling,preening and other social activities such as intraspecific interaction took only a fraction of a foraging bout(Table 3).
For each foraging activity,diving took the longest time with the mean duration of 13.13 0.50 s, followed by swimming (5.22 0.47 s),preening(4.69 0.29 s)and vigilance(4.06 0.19 s),socializing(3.83 0.22 s)and food handling(3.19 0.34 s).Despite M.squamatus having the highest rate of head-dipping and eye-submerging (4.40 0.39 and 5.75 0.39 times/min, respectively), their duration of each headdipping and eye-submerging were consistently the shortest (2.60 0.07 s and 1.89 0.01 s,respectively).Despite the relatively small SE in comparison with the mean, considering the large sample size, the birds showed highly variable behavior profiles.
3.2.Summary of Bayesian models
All final Bayesian models achieved convergence indicated by the values of Rhat(close to 1 for all cases).Most of the investigated variables,such as duration of bout, catches, total energy gain, proportion of foraging activities, were not normally distributed, and link functions using different families were specified in the GLMM to handle the nonnormality(Table 4).It is worth to note that Weibull distribution was the best for modelling extremes such as no of fish caught per minute, and Student-t is more robust for outliers.
The explanation powers of models varied from 11% (volume of fish caught per minute)to 65%(duration of bout)indicated by the Bayes-R2(Table 4).However, the interpretation of this should be cautious as the 95% creditable interval can be large (e.g., 0.01–0.40 for volume of fish caught per minute,Table 4).
3.3.Foraging behaviors at different habitats
The birds showed distinct behavior profiles in deep and shallow habitats.While the duration of a foraging bout was only slightly higher in shallow than in deep habitats (Fig.4A), the proportion of time spent in each activity classes differed significantly.Specifically, while the bird spent significantly more time in getting ready for fishing in deep waters(Fig.4B),the time for fishing was significantly greater in shallow waters(Fig.4C).In addition,time for social interaction was significantly higher in shallow waters(Fig.4D).
In both habitats,more time was spent for fishing than other activities.In deep waters,the bird spent an average of 62.8%,36.3%and 0.9%of the foraging time on fishing, preparing, and social interaction, respectively.In shallow waters, the values were 81.8%, 16.8%, and 1.4%.Fishing behaviors were mainly diving(87.3%)in deep waters and headdipping (53.7%) and eye submerging in shallow waters (43.1%).Preparing behaviors were mainly vigilance in both deep waters(88.6%)and shallow waters(75.5%).
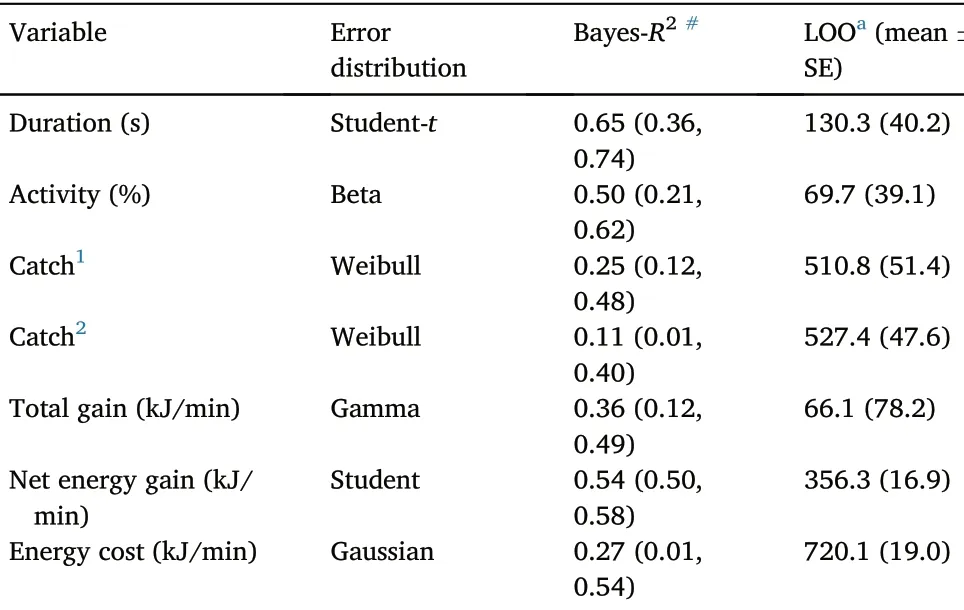
Table 4Summary of the final Bayesian models for M.squamatus foraging behaviors and energetics.
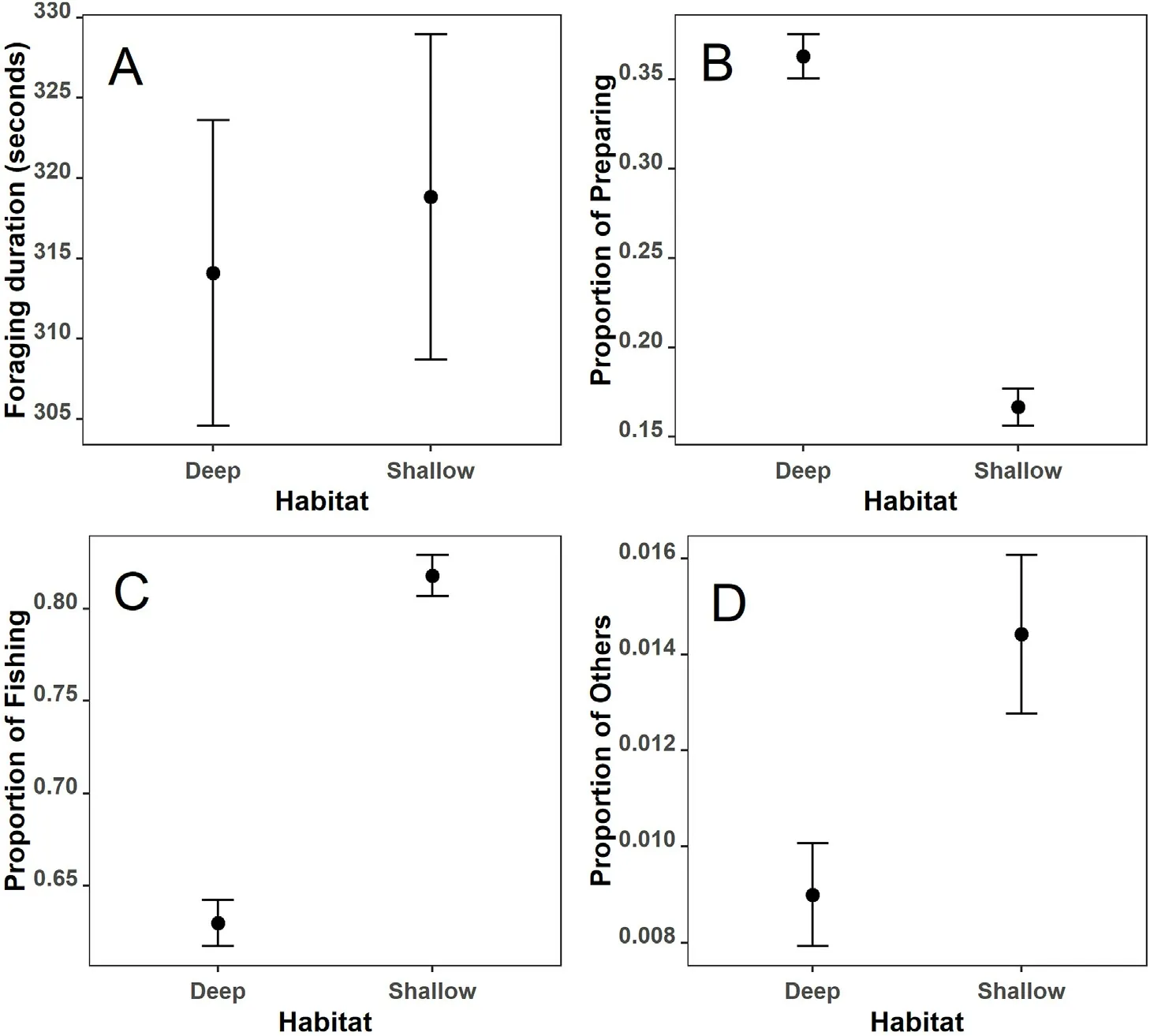
Fig.4.The difference in foraging behaviors of M.squamatus in deep and shallow habitats.The mean(dots)and the 95%credible intervals(vertical bars)are based on 4000 posterior draws of the fitted models.Note the different scales of Y-axis.(A)Foraging duration in seconds;(B)Proportion of preparing;(C)Proportion of fishing;and (D) Proportion of other activities.
3.4.Foraging gain at different water depths
As for the fish caught at different water depths, the mean fish catch rate was 0.28 fish/min in shallow waters, which is significantly higher than the value of 0.13 fish/min in deep waters(Fig.5A).However,as the prey in shallow waters was smaller(data not shown),the mean biomass of the fish captured was slightly greater in deep waters(Fig.5B).
3.5.Foraging energetics in shallow and deep waters

Fig.5.Fish catching rate (A) and biomass of captured fish (B) of the M.squamatus in deep and shallow habitats.The mean (dots) and the 95% credible intervals(vertical bars) are based on 4000 posterior draws of the fitted models.
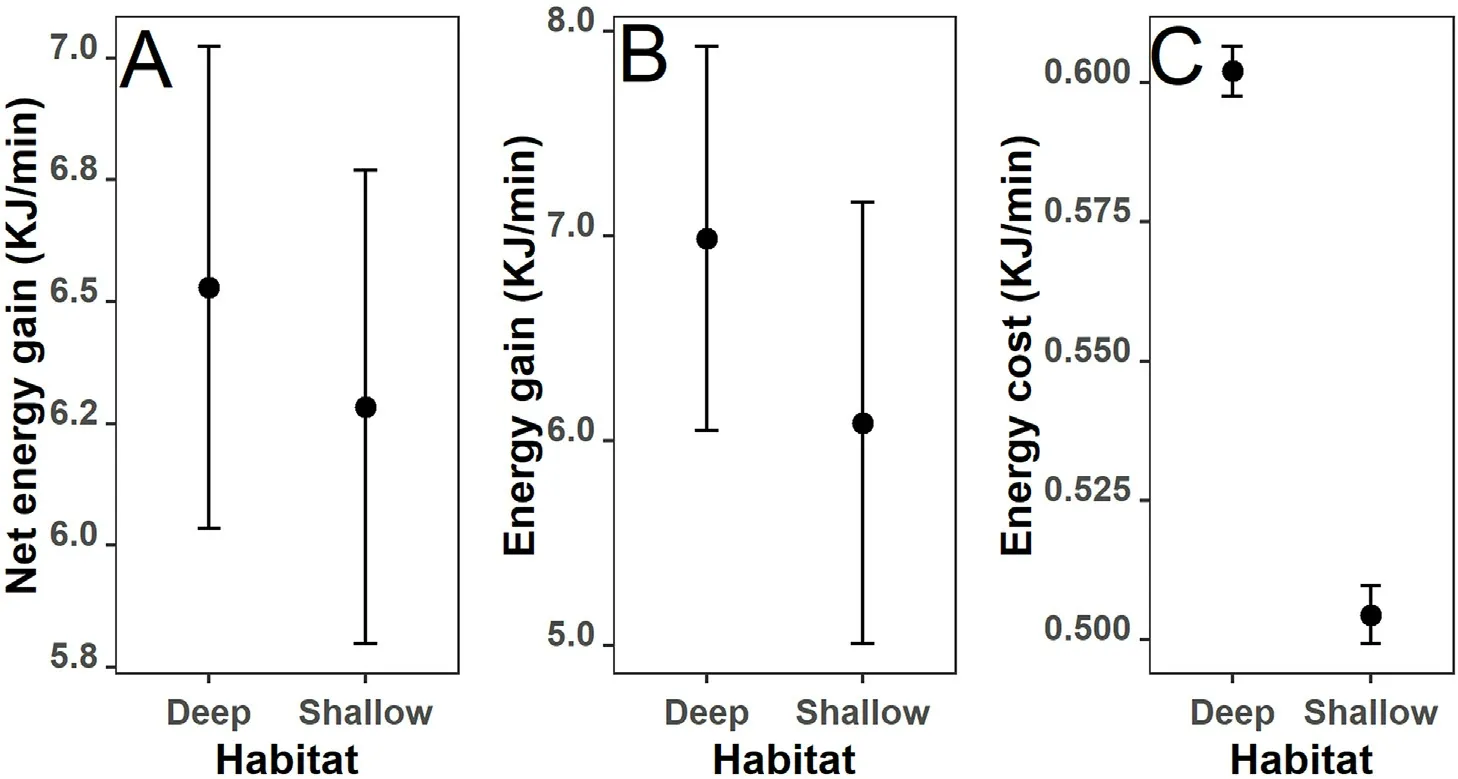
Fig.6.Foraging energetics of M.squamatus in deep and shallow habitats.(A)Net energy gain;(B)Energy gain from captured prey;and(C)Predation cost.The mean(dots) and the 95% credible intervals (vertical bars) are based on 10,000 posterior draws of the fitted models.Note the different scales of Y-axis.
Despite the distinct behavioral profiles in different habitats (Fig.4),the M.squamatus showed similar foraging energetics(Fig.6),suggesting that the birds can fish equally efficiently in both deep and shallow waters.The estimates of mean net energy gains were 6.53 kJ/min and 6.28 kJ/min in deep and shallow habitats, respectively, and the standardized errors (SE) were comparably small (less than 0.50 in both habitats,Fig.6A).Energy gains through food intake were slightly higher in deep waters(mean estimates were 6.99 kJ/min and 6.08 kJ/min in deep and shallow waters,respectively,Fig.6B).Again,SE was small in comparison with the mean.The small SE suggested that the bird performed stably during the three winters in both habitats.Although the foraging energy costs were significantly higher in deep waters, this difference did not affect the comparison of energy balance as they were only a fraction of energy gain through food intake(generally less than 10%,Fig.6C).
4.Discussion
Animal behavioral traits often show wide and consistent variation among individuals (Wolf and Weissing, 2012; Dingemanse and Wolf,2013)and this variation has often been suggested to facilitate population persistence in novel and variable environments(Sih et al.,2011).In the context of rapid human-induced environmental change, such as habitat loss and fragmentation, it is urgent to integrate animal behavior and wildlife conservation studies for maximizing outcomes (Sutherland,1998;Greggor et al.,2016;Berger-Tal et al.,2019).
We found that the predatory waterbird had similar energetics despite distinct foraging methods, behavior profiles, and foraging efficiency in shallow- and deep-water habitats.These results suggest that M.squamatus is a good example of behavioral flexibility that aligns with expectations of optimal foraging theory (OFT) (Pyke, 1984), in that the duck behaves in accordance to resource availability in different environments,resulting in high foraging efficiency.The behavioral flexibility could be related to evolutionary adaptation(Snell-Rood,2013).
4.1.Behavior profiles are distinct
Waterfowl often use specialized feeding methods when foraging in different habitats (Guillemain et al., 2000), especially at areas with different water depths.For example,Bewick’s Swan(Cygnus columbianus bewickii) uses head-dipping in shallow waters and upending in deeper areas (Nolet et al., 2006).We observed that M.squamatus used mainly head-dipping in shallow waters and frequent diving in deep waters.On average,M.squamatus spent 44%of their foraging time in head-dipping at shallow waters and the proportion of diving was 55%in deep waters.Similarly, Common Merganser immerses their heads to search for prey under the shallow waters and dives repeatedly in deep waters(Anderson et al.,1974).
In both environments,majority of the foraging time was allocated to fishing (62.8% and 81.8% in deep waters and shallow waters, Fig.4).Nevertheless, the bird spent significantly more time fishing in shallow waters than in deep waters,which may contribute to the observed higher catch rates (Norberg, 1977).Diving was the main activity of fishing behaviors in deep water (87.3%) and mean diving time was less than the previous studies in Yihuang section, Fuhe River (23.6 6.3 s) and in Wuyuan section, Rao River (18.8 6.0 s) (Shao et al., 2014; Shao and Chen, 2017).The difference of mean diving time might be due to the depth of the river (Wanless et al., 1993; Carbone and Houston, 1994).Distinct with foraging in deep water,the durations of head-dipping and eye-submerging activities of fishing behaviors in shallow water were high(53.7%and 43.1%).In contrast,more time was spent on preparing in deep waters than in shallow waters.For preparing,vigilance was the main activity in both deep waters(88.6%)and shallow waters(75.5%),and the bird spent significantly more time on vigilance in deep waters than in shallow waters.Most wild animals would allocate more time to maintain high vigilance and reduce other behaviors when encountering disturbances (Fortin et al., 2004; Robinson and Merrill, 2013).As the bird could be more uncertain about the situation surrounding on water surface after diving, more time is required on vigilance in deep waters areas.Previous studied also found that watching-out duration was positively correlated to the previous diving and next diving (Ydenberg and Forbes,1988;Shao et al.,2014).
4.2.Food gain and energy balance is similar
The two feeding strategies (i.e., head-dipping in shallow waters and pursuit diving in deep waters) resulted in significantly different catch rates,and apparently the duck was more efficient in shallow waters with a catch rate of 0.28 fish/min(in comparison,the average catch rate was 0.13 fish/min in deep waters, Fig.5A).This is in consistency with studies on other piscivorous waterbirds.For example,European Shag(Phalacrocorax aristotelis) has higher prey–capture performance in shallower locations(Wanless et al., 1993).The difference in foraging catch rates could be linked to prey abundance and vulnerability,which is strongly affected by water depth (Monaghan et al., 1994; Schekkerman and Beintema, 2007;Lantz et al.,2011).As the key components of habitat complexity for stream fish, water depth has an important effect on the distributions and size of fish(Lonzarich and Quinn, 1995; Rose,2000) and macroinvertebrates as well (Lantz et al., 2011).Small and juvenile fish intended to inhabit shallow waters to avoid predatory fish(Blaber,1980;Sheaves,2006).
Although more fish were caught in shallow waters,the total gains in terms of biomass at different habitats were similar due to the larger size of fish in deep waters.That is to say, the higher prey quality offset the lower prey quantity in deep waters(Brodmann et al.,1997),resulting in slightly higher energy gains (6.99 0.94, and 6.08 1.08 kJ/min in deep waters and shallow waters, respectively, Fig.6B).Macroinvertebrates were not included in this study.Although studies from breeding grounds suggest that M.squamatus may feed on macroinvertebrates in shallow habitats (Zhao and Pao, 1998), macroinvertebrates consumed was much fewer at wintering ground based on our observation.As M.squamatus tends to bring its prey, including the small-sized macroinvertebrates(e.g.,larva of Acanthacorydalis sp.),to the water surface for feeding, the likelihood of M.squamatus foraging on macroinvertebrates without being detected is low.Therefore, the contribution of macroinvertebrates to the diets of M.squamatus in the wintering season in this study is negligible.Different foraging patterns imply highly different energy costs(Godfrey and Bryant,2000).In deep waters, the bird spent significantly more time in diving that has high basal metabolic rate(Table 1 and de Leeuw,1996).The frequent pursuit diving resulted in significantly higher energy cost in deeper waters(Fig.6C).However, as the forging energy consumption of M.squamatus was only a fraction of energy intake(less than 5%),it had little influence on the foraging energetics: the net energy gain was slightly higher in deep waters but the difference was not statistically significant(Fig.6C).
4.3.Optimal foraging theory
Predatory animals may have a series of alternative foraging modes(Kuwae et al.,2010).The optimal foraging theory(Pyke,1984)predicts that decisions on foraging strategy, including what, where and how to hunt, are based on a maximization of currencies (Bautista et al., 2001).Therefore, in their natural habitat, predators are expected to take different foraging strategies in response to both prey abundance and vulnerability such that they attain the highest energy intake rate.
As predicted by OFT (Pyke, 1984), M.squamatus showed high behavioral flexibility by adjusting its foraging methods and behavior profiles in shallow and deep areas.In deep waters,where prey abundance(Li and Gelwick,2005)and vulnerability(Linehan et al.,2001)are lower,M.squamatus allocated most of the time to repeated pursuit diving to minimize the foraging energy cost.This strategy was awarded with bigger gains and resulting in comparable energy gain as in shallow waters, where smaller preys are more abundant and easier to catch.The wintering grounds of M.squamatus are mainly mountainous streams(Zhao and Pao, 1998), where resources are patchily distributed (Taylor and Warren Jr, 2001).The behavioral flexibility is critical for predators foraging in heterogeneous environment (Abrahms et al., 2020), where prey is patchily distributed and may be unpredictable for diving birds(Tessier and Bost,2020).
5.Management implications and conclusions
Overall, our findings highlight that the wintering M.squamatus has high behavioral flexibility adapted to the heterogeneous foraging environment.However, its population is decreasing continuously in recent decades and is listed as globally endangered by the IUCN (BirdLife International,2017).This gives an indication that the rapid anthropogenic environmental changes may be excessing or even have excessed the adaptive capacity of the species(Sih et al.,2011).M.squamatus prefers to forage in clear, flowing river reaches with relatively shallow waters depth (less than 10 m), which provide diverse microhabitats, such as pools, glides, riffles, sand/gravel bars, and islands, for fish and benthic macroinvertebrates (Aadland, 1993).M.squamatus is morphologically specialized in hunting moving prey in such environment (Kondratyev,1999).The evolutionary adaptation could lose its competitive advantages as human pressures on existing habitats intensified(Lei et al.,2019)with increasing water resource developments and other activities such as sand mining and fishing.These human pressures could dramatically change the river landscape and decrease prey abundance.For example,river regulation by damming increases water depth and reduces habitat heterogeneity(Swales,2018;Zeng et al., 2018a;Lei et al.,2019).
Funding
This research was supported by the Fundamental Research Funds for the Central Universities (BLX202147) and the Joint Fund for Regional Innovation and Development of NSFC(U22A20563).
Ethics statement
All the field observations conducted in this study were performed in accordance with the current laws of China.Observers were distant from the birds and no disturbance to the animals was caused.
Authors' contributions
Conceptualization: P.L., Q.Z.and G.L.; Methodology: Q.Z.and L.W.;Investigation: P.L., M.L., D.X., Y.H.and R.F.; Writing—original draft preparation:P.L.and M.L.;Writing—review and editing: Q.Z.and L.W.;Supervision:Q.Z.,G.L.and C.L.;Project administration:C.L.and G.L.All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank The People’s Government of Shangqing Town and Forestry Bureau of Yingtan City.
杂志排行
Avian Research的其它文章
- Selecting the best: Interspecific and age-related diet differences among sympatric steppe passerines
- Morphology and morphometry of two hybridizing buntings at their hybrid zone in northern Iran reveal intermediate and transgressive morphotypes
- Quiet in the nest: The nest environment attenuates song in a grassland songbird
- Characteristics of cross transmission of gut fungal pathogens between wintering Hooded Cranes and sympatric Domestic Geese
- Fecal DNA metabarcoding reveals the dietary composition of wintering Red-crowned Cranes (Grus japonensis)
- Short-term night lighting disrupts lipid and glucose metabolism in Zebra Finches: Implication for urban stopover birds
