Variaiton in the composition of small molecule compounds in the egg yolks of Asian Short-toed Larks between early and late broods
2024-01-22ShiyunDingNaZhuShupingZhang
Shiyun Ding, Na Zhu, Shuping Zhang
aKey Laboratory of Ecology and Environment in Minority Areas (National Ethnic Affairs Commission), Minzu University of China, Beijing, 100081, China
bCollege of Life and Environmental Sciences, Minzu University of China, Beijing, 100081, China
Keywords:Asian Short-toed Lark Egg laying date Small molecule compounds Variation Yolk
ABSTRACTThe egg yolks of birds contain most of the maternally derived materials required for embryo development and are an important factor influencing embryo development and offspring viability.Individual variation in egg-laying date frequently occurs in passerines inhabiting highly seasonal environments.Females laying in early and late stages of the breeding season encounter different environment temperatures and food conditions,which can affect the levels of metabolities in their bodies,thereby altering the transmission of these materials to the eggs.We test a hypothesis that yolk small molecule compounds of Asian Short-toed Lark(Alaudala cheleensis)could vary between early(mid-May)and late(mid-June)broods.Using the UHPLC-MS/MS method,683 compounds belonging to 21 compound groups are detected in the yolks.The contents of 18 compounds are significantly different between early and late broods.Ten differential compounds are significantly higher in the early laid eggs, among which γ-aminobutyric acid,creatine,prostaglandins,palmitoleic acid,linoleic acid,and trans linoleic acid are related to low environment temperature response.The eggs laid in late stage exhibit significantly higher levels of 5-L-glutamyl-L-alanine and γ-glutamate-leucine,1,3-dimethyluric acid and mannose,which may be attributed to females in the late group consuming more insects.We suggest conducting a comprehensive investigation to reveal the yolk small molecule compounds mediated maternal effects on offspring phenotypes under varying ecological conditions.
1.Introduction
In addition to providing the ideal environment for embryonic development, avian eggs contain all the maternally derived materials required for the developing embryo.Studies in wild birds have shown that the yolk contains a wide range of compounds,including hormones,lipids, and antioxidants (Speake et al., 1999; Gil et al., 2007; Hargitai et al., 2016;Mentesana et al.,2019), which play important roles in embryonic development and can affect the behavior and viability of offspring(Groothuis et al.,2005;Tobler et al.,2007;Catoni et al.,2008;Ho et al.,2011;Giraudeau et al.,2016a,2016b;Mentesana et al.,2021).For example,thyroid hormone promotes development in vertebrates and is necessary for the differentiation of the central nervous system,muscle,bone, intestine, and lung tissues (Anne McNabb, 2007).Additionally,testosterone has been shown to affect begging behavior and early growth and survival of nestlings (Andersson et al., 2004), saturated and polyunsaturated fatty acids provide the embryo with basic cell components and the energy needed to sustain development(Surai and Speake,2008;Kazak and Cohen,2020;Czerwonka et al.,2021),and antioxidants such as carotenoids,vitamin C(VC),and vitamin E(VE)can protect the biological molecules of embryos from oxidative damage and regulate immune cell function to improve immune defense (Saino et al., 2002;Parolini et al.,2017).
In highly seasonal environments, there is a significant within-year variation in the timing of breeding between individuls within passerine bird populations (Arnold, 1992; McCleery et al., 2004; Verhulst et al.,2010; Zhao et al., 2017a; Lv et al., 2019; Zhang et al., 2019).Females breeding in early spring often encounter low temperature and less abundant food, while the females breeding later may avoid these unfavorable conditions (Rotics et al., 2018; Shang et al., 2021).It has been known that environmental temperature and dietary components can affect the levels of hormones,polyunsaturated fatty acids,cholesterol and vitamins in mothers’ bodies during egg formation, thereby altering the levels of these materials transmitted from mothers to their eggs (Collin et al., 2003; Royle et al., 2003; DuRant et al., 2010; Ruuskanen et al.,2016; Panaite et al., 2021).For examples, thyroid hormone and corticosterone can be transmitted from female birds to their eggs in higher quantities in cold environment (DuRant et al., 2010; Ruuskanen et al.,2016), and the type of lipids in the diet of mothers can affect the composition of fatty acids in egg yolk, especially the content of polyunsaturated fatty acids (Panaite et al., 2021).Therefore, understanding the variation in the composition of yolk materials between early and late broods is essential for understanding maternal effects on the developmental plasticity of bird morphology, physiology, and behavior under varying ecological conditions.
Although there is a lot of informations about yolk materials, the overall composition of small molecule compounds in the yolks of wild birds remains uncertain.Some small molecule compounds found in the yolk of poultry eggs and other model animals have been shown to play critical roles in embryonic development.For example, arginine and threonine can promote cell proliferation in poultry lymphoid organs(Toghyani et al., 2018).Additionally, some organic acids have been shown to have important functions in the growth and development of chicks.For example, formic acid and propionic acid have antibacterial activity and have been shown to promote the development of the gastrointestinal tract and immune organs in poultry (Khan and Iqbal,2016),taurine promotes muscle and brain development(Zielinska et al.,2012; Mersman et al., 2020), and creatine serves to store energy for embryo growth(Zhao et al.,2017b).Therefore,it is necessary to identify the compound pool in the yolk of wild birds and determine how they vary between early and late broods.
The Asian Short-toed Lark(Alaudala cheleensis)is one of the dominant passerines in the Hulunbuir grassland in Inner Mongolia, China.The species breeds one time per year in Hulunbuir and exhibits significant individual variation in the timing of egg-laying,lasting from early May to mid-June (Zhang et al., 2017).During this period, the average ambient temperature in May is about 8◦C lower than in June(Shang et al.,2021),and the insect food resource is more abundant in June than in May(Zhang et al., 2019).Therefore, the Asian Short-toed Lark is a suitable case species of wild birds for investigating the differences of yolk small molecule compounds composition between early and late broods.
In this paper, we tested a hypothesis that yolk small molecule compounds of Asian Short-toed Lark(Alaudala cheleensis)could vary between early and late broods.We used UHPLC-MS/MS to detect the small molecule compounds in the yolks,comparing eggs sampled in early(mid-May) and late (mid-June) periods of the breeding season in 2021.We then analyzed the potential biological function of the compounds with significant difference between early and late broods using KEGG database.
2.Materials and methods
2.1.Study site
The study area was situated in the Hulun Lake National Nature Reserve (47◦45′50′′–49◦20′20′′N; 116◦50′10′′-118◦10′10′′E), in the northeastern portion of the Inner Mongolian Autonomous Region,China.This is a semiarid,steppe region where the mean annual temperature is-0.6◦C.The average daily temperature in January and July is-20.02◦C and 22.72◦C, respectively.The daily average temperature of the study site in May was significantly lower than that in June of 2021(Appendix Fig.S1).Because the diet of the larks changed from grass seeds in May to insects in June (Zhang et al., 2019), the insect abundance in early-May and early-June were surveyed at the study sites in 2021.Seven 5 m ×5 m square samples were randomly selected and insects were captured and counted using sweeping net method in these samples and cluster analysis showed that the insect abundance in May was significantly less than that in June(Appendix Fig.S2).
2.2.Egg sample collection
We collected one newly laid egg of the Asian Short-toed Lark from different nests in early stage(May 14 to May 19)and late stage(Jun 9 to June 14) of the breeding season of 2021, and totally 12 eggs were collected.After removal from the nest, the eggs were immediately put into liquid nitrogen for temporary storage and taken back to the laboratory where they were transferred to a -80◦C refrigerator for storage until required for analysis.
2.3.UHPLC-MS/MS analysis on yolk
2.3.1.Preparation of samples for analysis
The frozen eggs were taken out and thawed,and the yolks and whites quickly separated.Two hundred and fifty milligrams of yolk was placed into 100 mg liquid nitrogen for grinding.The samples were placed in 2 mL EP tubes and resuspended with prechilled 80% methanol using a vortex.The samples were then incubated on ice for 5 min and centrifuged at 15,000 g,4◦C for 20 min.LC-MS grade water was used to dilute 300 μL of supernatant to a final concentration containing 53% methanol and centrifuged at 15,000 g, 4◦C for 20 min.Finally, the supernatant was collected as an LC-MS analysis sample.
In addition to the yolk samples, quality control (QC) samples were also prepared by combining experimental samples of equal volume.These samples were then used to calibrate the chromatography-mass spectrometry system, monitor the condition of the instrument, and evaluate the stability of the system throughout the experiment (QC sample data are shown in Appendix Fig.S1).A blank sample was run simultaneously to remove background ions.
2.3.2.UHPLC-MS/MS analysis method
The SCIEX QTRAP®6500+mass spectrometry platform was used for UHPLC-MS/MS detection.Samples were injected onto an Xselect HSS T3(2.1 × 150 mm, 2.5 μm) at 50◦C and a flow rate of 0.4 mL/min.Using reagents with good inertness, low viscosity, and sample-specific solubility as eluent not only prevents damaging the stationary phase but also increases the sample diffusion coefficient and improves elution efficiency.The gradient elution method was used in this study with eluent A(0.1% formic acid-water) and eluent B (0.1% formic acid-acetonitrile)selected for the eluent solutions.During one analysis cycle, the partition ratio of eluent A to B varied with time and the change in solution polarity was able to elute the different components of the complex samples to the maximum extent.
The QTRAP® 6500+ mass spectrometer was operated in positive polarity mode under the following conditions: curtain gas 35 psi, collision gas at medium,IonSpray voltage 5500 V,temperature 550◦C,with the ion source gases 1 and 2 both having a flow rate of 60 mL/min.The QTRAP® 6500+ mass spectrometer was then operated in negative polarity mode under the following conditions:curtain gas 35 psi, collision gas at medium,IonSpray voltage-4500 V,temperature 550◦C with the ion source gases 1 and 2 both having flow rates of 60 mL/min.
2.4.Compound identification
Multiple Reaction Monitoring(MRM)was used to identify the names of the small molecular compounds based on the novo database (Novogene, Beijing).Q1 (parent ion), Q3 (daughter ion), RT (retention time),DP (declustering potential), and CE (collision energy) were used for metabolite identification,and Q3 was used for metabolite quantification.The data files generated by HPLC-MS/MS were processed using the SCIEX OS Version 1.4 to integrate and correct the peak.The main parameters were set as follows: minimum peak height, 500; signal/noise ratio,5;gaussian smooth width,1.The area of each peak represents the relative content of the corresponding substance.Finally, all the chromatographic peak area integral data were exported to obtain the qualitative and quantitative results of the compounds.

Fig.1.The compounds in the yolk of Asian Short-toed Larks annotated in the Novogene database.
2.5.Statistical methods
All compound data were imported into metaX, and partial leastsquares discriminant analysis(PLS-DA) was conducted to assess the differences of yolk compounds amongst the eggs sampled in May and June,and 7-fold cross validation and 200 permutation tests were used to verify the reliability of the model.Variable importance in the projection(VIP)in PLS-DA allowed the molecules likely to be most responsible for between-group differences to be identified (Wone et al., 2011).Compounds with VIP values>1.0 in the model were regarded as potentially important and tested for statistical significance.Independent T-test was used for inter group comparison,and compounds with P<0.05 and VIP>1,FC>1.2 or FC<0.833 were identified as differential compounds.Metabolic pathways for compounds were obtained from the Kyoto Encyclopedia of Genes and Genomes(KEGG)database.
3.Results
3.1.Small molecule compounds detected in the yolks of Asian Short-toed Larks
The compounds identified demonstrated distinct separation in both positive and negative ion modes.A comparison of the superimposed total ion chromatography(TIC)of three QC samples indicated a high degree of overlap(Appendix Fig.S3).The response strength and retention time of the QC samples were good and the separation effect between the peaks was distinct, indicating that the detection system was stable and the chromatographic and mass spectrometric conditions were suitable for the analysis of samples in this study.Based on novoDB, a total of 683 compounds were identified in the egg yolks of Asian Short-toed Larks (Appendix Table S1), belonging to 21 compound groups including amino acids,organic acids,nucleotides,saccharides,lipids,hormones,vitamins,and biogenic amines,to name a few(Fig.1).All these compounds were mainly enriched in metabolic function and organ system function annotated in KEGG database (Fig.2).In the “metabolism” function,compounds were mainly enriched in amino acid metabolism, lipid metabolism, glycerol phospholipid metabolism, nucleotide metabolism,and vitamin metabolism.In the “organismal systems” function, compounds were mainly enriched in the endocrine system and nervous system pathways.The specific metabolic pathways and the compounds enriched in them are detailed in Appendix Table S2.
3.2.Differences in yolk small molecule compounds between early and late broods
PLS-DA model analysis of 683 yolk compounds from 12 Asian Shorttoed Lark eggs was well established(R2Y(cum)>0.5)(Fig.3A).In the 7-fold cross validation and 200 permutation tests,R2 is greater than Q2 and the intercept between Q2 regression line and Y-axis is less than 0, indicating that PLS-DA model is not overfitting, and the prediction of this model is reliable (Fig.3B).The model showed that the composition of yolk compounds was significantly different between the early and late groups(Fig.3A),with 18 compounds differing significantly between the two groups(Fig.4).The contents of lipids(palmitoleic acid,linoleic acid,trans linoleic acid), hormones (prostaglandins B2, 16 α-hydroxytestosterone), vitamins(γ-tocopherols, retinol),amino acids(γ-aminobutyric acid), and organic acids (2-aminooctanoic acid, creatine) were significantly higher in the early group than the late group,and the contents of sugars (D-mannose), amino acids (5-L-glutamyl-L-alanine,γ-glutamic acid leucine,aspartic acid diglucoside),and organic acids(4-hydroxyphenylpyruvic acid, 1,3-dimethyluric acid, 2-aminoethyl phosphate,and 3-2-hydroxyphenyl propionic acid)were significantly lower in the early group than the late group(Fig.5).
3.3.Metabolic pathway enrichment analysis on the differential compounds
The differential compounds were enriched in the following metabolic pathways annotated in the KEGG database:ubiquinone and other terpene quinone biosynthesis (4-hydroxyphenylpyruvate, γ-tocopherol), arachidonic acid metabolism(prostaglandin B2),retinol metabolism(retinol),lysosomes (D-mannose), light transduction (retinol), phosphate and phosphonate metabolism (2-aminoethyl phosphate), linoleic acid metabolism(linoleic acid),serum ergic synapse(prostaglandin B2),fatty acid biosynthesis(palmitic acid),unsaturated fatty acid biosynthesis(linoleic acid), biosynthesis of monolactam (4-hydroxyphenylpyruvate), metabolism of fructose and mannose (D-mannose), biosynthesis of phenylalanine, tyrosine, and tryptophan (4-hydroxyphenylpyruvate), ABC transport (D-mannose, 2-aminoethyl phosphonate), catabolism of vitamins (retinol), metabolism of phenylalanine (3-2-hydroxyphenyl-propionic acid),tyrosine metabolism(4-hydroxyphenylpyruvate),metabolism of amino acid sugars and nucleotide sugars(D-mannose),metabolism of 2-oxo carboxylic acids (4-hydroxyphenylpyruvate), galactose metabolism (D-mannose), glycine, serine, and threonine metabolism (creatine), and arginine and proline metabolic pathways(creatine)(Fig.6).
4.Discussion
Previous studies have demonstrated that yolk material composition varies under different environment temperatures and food conditions(e.g., Aydin and Cook, 2009; Pitk et al., 2012; Lessells et al., 2016;Ruuskanen et al., 2016; Panaite et al., 2021).The concentration of testosterone, androstenedione and thyroxine in the yolk decreases with the increase of ambient temperature before egg laying (about 10 days)(Yoshida et al., 2011; Ruuskanen et al., 2016).A research on Great Tits(Parus major)has shown that temperature is negatively correlated with 5 α-dihydrotestosterone in both female's plasma and the yolk (Lessells et al.,2016).The type of fatty acids and the amount of carotenoid intake of female birds can affect the composition of fatty acids,cholesterol and vitamin content in their yolks (Aydin and Cook, 2009; Panaite et al.,2021).In this study, we found 683 small molecule compounds in the yolks of Asian Short-toed Larks.Most of these small molecule compounds have not been previously identified in wild bird eggs; therefore, understanding of the variation in yolk small molecule compounds under different environment conditions remains limited.Our results showed that 18 compounds differed significantly between early and late broods of Asian Short-toed Larks,which supports our hypothesis.Because these differences were discovered firstly in wild birds,there is little knowledge about the ecological signicance of these differences.We attempt to analyze the potential maternal environmental factors causing these differences and the potential effects of the compunds on embryo development according to available literatures on poultries and non-avian taxons,in order to facilitate further researches.
Ten of the differential compounds are significantly higher in early laid eggs than late laid eggs.In the studies of rat or poultries, some of these compounds have been found to respond to low environment temperatures.γ-aminobutyric acid (GABA) accumulation rate increased in hypothalamus and corpus striatum of rats exposed to low environment temperatures (Biswas and Poddar, 1990).Cold stress can cause an increase in the expression of GABA transporters in rats(Odeon et al.,2010).GABAergic excitatory influences on the medullary raphe of chicks modulate thermogenesis under cold stress (Cristina-Silva et al., 2021).Creatine helps transport energy to muscles and nerve cells in vertebrates(Kazak and Cohen, 2020).It has been known that cold stress can cause creatine to transfer from muscle to body fluids in rats,which is related to the thermogenesis mediated by thyroid hormone (Kurahashi and Kuroshima,1978).In addition,creatine controls thermogenic respiration in adipose tissue of rats (Kazak and Cohen, 2020).Prostaglandins can activate thermogenesis in birds and mammals by inhibiting temperature-sensitive neurons in the hypothalamus (Tabarean, et al.,2004).Palmitoleic acid, linoleic acid, and trans linoleic acid are unsaturated fatty acids.A high proportion of unsaturated fatty acids in the cell membrane can increase ion permeability, increase the activity of ion pumps,maintain the electrochemical gradient across the membrane,and promote material transport inside and outside the membrane, thereby enhancing metabolic rate (Brand et al., 1994; Hulbert, 2005, 2008;Hulbert and Else,2005).The above available studies implicate that these differential compounds are related to low temperature response of mammals and birds.The average temperature of our study site in May is approximately 10 C,while it is approximately 20 C in June(Appendix Fig.S1).Lower temperatures in the early stage could induce higher levels of these compounds in female Asian Short-toed Larks,leading to a higher accumulation of these compounds in their eggs.
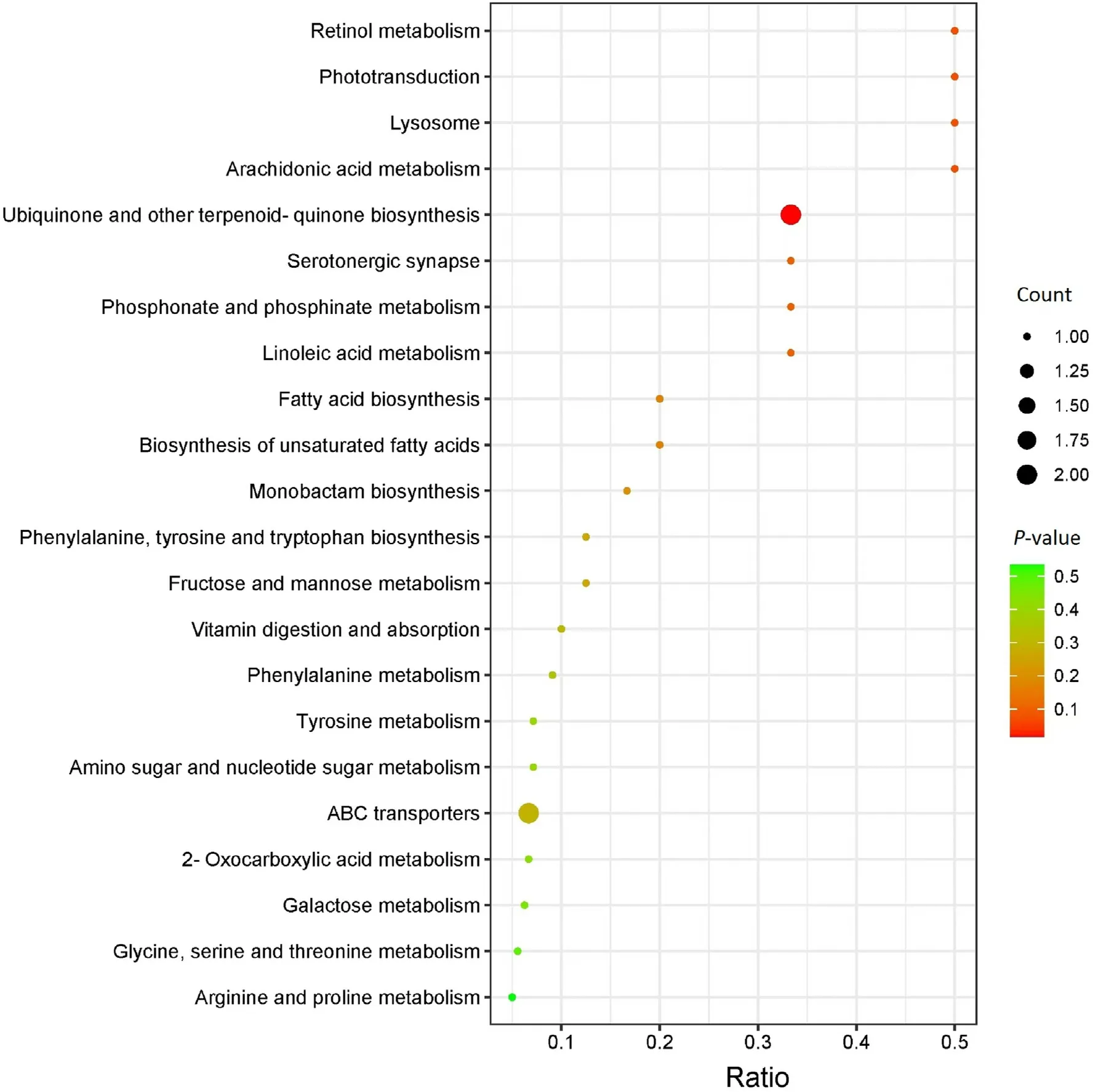
Fig.6.Metabolic pathways of differential compounds in yolk of Asian Short-toed Lark enriched in KEGG.The dots indicate the number of compound,and the color of the dots indicates the P-value of geometric test.(For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Variation in dietary components may be related to the differences in other compounds.γ-tocopherol and retinol were significantly higher in the early group compared to the late group.Animals obtain tocopherol,also known as VE,primarily from the seeds of plants(Park et al.,2004).Compared to June,insect abundance at the study site is relatively low in May (Zhang et al., 2019; Appendix Fig.S2); hence, plant seeds are the primary food source for the larks and female birds may obtain higher amounts of plant-sourced VE.Plants are the main source of vitamin A(VA)which is converted into retinol via intestinal absorption(Bonakdar et al., 2022).Therefore, the plant-rich diet in May results in females consuming more VA.The late group exhibited significantly higher levels of 5-L-glutamyl-L-alanine and γ-glutamate-leucine,1,3-dimethyluric acid and mannose, which could be attributed to females in the late group consuming more insects.Studies on the food and blood metabolites of young Short-toed Larks found that increased insect consumption leads to a significant increase in blood amino acid and mannose levels (Zhang et al., 2019).1,3-dimethyluric acid is a byproduct of amino acid metabolism(Van Milgen,2021).
The available studies in rats and poutries implicate that some differential compounds can influence the embryo development.Injecting GABA into eggs increased chick body weight at hatching (Goel et al.,2022).Mannose is associated with morphological changes in the embryonic notochord and plays a key role in the induction of tissue differentiation (Hassanzadeh Taheri, 2016).Prostaglandins can regulate embryonic stem cell differentiation and promote vascular and intestinal development (Pierzchalska et al., 2012; Ugwuagbo et al., 2019).Testosterone can promote muscle development and early bone growth in embryos(Navara et al.,2005).A higher level of unsaturated fatty acids in egg yolk promotes the organ formation,enhances immune capacity,and increases the metabolic rate of embryos(Hulbert,2005;Hall et al.,2007;Surai and Speake, 2008; Hulbert and Abbott, 2012).Tocopherol and retinol are important antioxidant that can protect embryos from oxidative damage caused by rapid metabolism during development (Hong et al.,2013;Tavakkoli et al.,2021).Retinol also aids in the development of embryonic visual organs and liver (Tanumihardjo, 2004; Weber,2009).
However,the effects of these differential compounds on the offspring phenotypes of wild birds remain unclear.Further comprehensive investigations are needed to understand the potential effects of these compounds on individual viability.Avian embryos exhibit remarkable tolerance to extremely low temperatures (reviewed in Ahmad and Li,2023).For examples,the eggs of Great Tit can tolerate sudden cold snaps of 2–3 C(Gladalski et al.,2020),and the eggs of the European Blackbird(Turdus merula) can be hatched after being exposed to 9 C for 8 h(Magrath,1988).Therefore,the functions of yolk GABA and unsaturated fatty acids in regulating the cold tolerance of bird embryos are worthy to be investigated.Within population phenotypic variations in morphology,physiology and behavior of nestlings have been observed in many birds(e.g.,Lipar,2001;Daisley et al.,2005;Duckworth et al.,2015;Criscuolo et al., 2020; Shang et al., 2021; Lu et al., 2023).For examples, the nestlings of Asian Short-toed Lark demonstrated different immunity in different birth stages(Lu et al.,2023);thus,the relationship between the immunity of nestlings and immune related yolk unsaturated fatty acids should be studied.The strength of nestling begging behavior often shows significant individual variation (Lipar, 2001; Daisley et al., 2005); thus,the function of yolk 16 α-hydroxytestosterone,creatine and unsaturated fatty acids in regulating muscle development, energy provision and metabolic rate of nestlings should be investigated to understand whether the begging behavior difference is a maternal effect.
5.Conclusion
In this study, 18 small molecule compounds are found to be significantly different in the yolks of Asian Short-toed Larks between early and late broods.Ten differential compounds are significantly higher in the early laid eggs, among which γ-aminobutyric acid, creatine, prostaglandins,palmitoleic acid,linoleic acid,and trans linoleic acid are related to low temperature response.The eggs laid in late stage exhibit significantly higher levels of 5-L-glutamyl-L-alanine and γ-glutamate-leucine, 1,3-dimethyluric acid and mannose, which could be attributed to females in the late group consuming more insects.Our findings provide a basis for further studies on the yolk compounds mediated maternal effects in wild birds.
Funding
This research was supported by the National Natural Science Foundation of China (No.32071515 to SZ) and Graduate Research and Practice Projects of Minzu University of China(BZKY2022042).
Authors’contributions
SZ conceived and designed the study.SD and NZ conducted the experiments in the field.SD analyzed the data.SD and NZ wrote the original manuscript, NZ revised the manuscript.All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no competing interests.
Ethics statement
The present study complies with the current laws of China and was approved by the Biological and Medical Committee of Minzu University of China(Permission No.ECMUC2020021AA).
Acknowledgements
We thank the Hulun Lake National Nature Reserve for the supports with fieldwork and two anonymous reviewers for their helpful comments on the manuscript.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.avrs.2023.100136.
杂志排行
Avian Research的其它文章
- Selecting the best: Interspecific and age-related diet differences among sympatric steppe passerines
- Morphology and morphometry of two hybridizing buntings at their hybrid zone in northern Iran reveal intermediate and transgressive morphotypes
- Quiet in the nest: The nest environment attenuates song in a grassland songbird
- Characteristics of cross transmission of gut fungal pathogens between wintering Hooded Cranes and sympatric Domestic Geese
- Fecal DNA metabarcoding reveals the dietary composition of wintering Red-crowned Cranes (Grus japonensis)
- Short-term night lighting disrupts lipid and glucose metabolism in Zebra Finches: Implication for urban stopover birds
