“Treat and repair” therapy for giant patent ductus arteriosus complicated with pulmonary embolism
2024-01-13JianMingWANGLiLiMENGJiaWangXIAOZhongChaoWANGJingSongGENGQiGuangWANG
Jian-Ming WANG, Li-Li MENG, Jia-Wang XIAO, Zhong-Chao WANG, Jing-Song GENG,Qi-Guang WANG✉
Department of Congenital Heart Disease, General Hospital of Northern Theater Command, Shenyang, China
Patent ductus arteriosus (PDA) is one of the most common congenital heart defects, accounting for 5%–10% of congenital heart diseases.[1]Its combination with pulmonary embolism (PE) is extremely rare, and there are few relevant reports.Especially giant PDA complicated with PE is prone to pulmonary hypertension (PH), which will significantly increase the right heart load, right ventricle hypertrophy, and result in clinical escape diagnosis or misdiagnosis.The adult patients with giant PDA often had the hemodynamic status of the Eisenmenger syndrome, which was once considered to be a surgical or interventional treatment contraindication.[2]If combined with PE, it would further lead to increased pulmonary vascular resistance and right heart failure, with high disability and mortality, which could make management extremely difficult.[3]With the advent of PH targeted drugs and the progress of standardized diagnosis and treatment of PE, we reported a case of patient with giant PDA complicated with PE who successfully received interventional treatment following “treat and repair” strategies.
A 57-year-old woman complained of chest tightness and shortness of breath after activity for more than ten years, and aggravated her condition accompanied by abdominal distension, lower limb edema, awakening at night and dyspnea, and decreased activity tolerance for more than ten days.Her blood pressure was 127/62 mmHg and heart rate was 110 beats/min.On examination, thick respiratory sounds on auscultation in both lungs, and slight moist rales can be heard on both lungs.Cardiovascular physical examination revealed the heart boundary expands to the lower left with grade 1/6 systolic murmur along the 2ndto 3rdribs at the left edge of sternum,2/6 grade systolic murmur was detected at auscultation area of tricuspid valve, and the pulmonary valve second sound was slightly stronger, with peripheral vascular sign, severe edema in both lower limbs, and peripheral venous pressure of 24 cm H2O.There was no jugular venous distention.Echocardiography from local hospital showed bicuspid aortic valve with severe aortic regurgitation.
Laboratory examination showed N-terminal pro-B-type natriuretic peptide (NT-proBNP) was 5091 pg/mL,cardiac troponin T was 10 ng/L and the D-dimer was 0.63 mg/L.The upper limb oxygen saturation was 92%,whereas lower limb oxygen saturation was 88%.The screening of rheumatic, immune, and connective tissue disease was negative.The results of tumor markers test for female were negative.The electrocardiogram showed atrial fibrillation.The transthoracic echocardiography(TTE) revealed that PDA (1.5 cm) with PH (systolic pulmonary artery pressure: 113 mmHg), mild aortic stenosis and mild to moderate aortic regurgitation, mild mitral regurgitation, and mild to moderate pulmonary valve regurgitation.The tricuspid annular plane systolic excursion (TAPSE) was 14 mm, and the ratio of the right to left ventricular diameters (RV/LV) was 33/40.The computed tomography (CT) angiography showed that the PDA,and aortic stenosis with valve neoplasm formation to be excluded (Figure 1).Chest CT showed interstitial changes and multiple old lesions in both lungs, PH, and a small amount of pericardial effusion.The pulmonary ventilation/perfusion scan result was highly possible of PE (the area where the ventilation/blood flow perfusion mismatch decreased in the upper lobe apical segment,posterior segment, lower lobe anterior basal segment, and inner basal segment of the right lung, anterior segment of the upper lobe and the dorsal segment of the lower lobe of the left lung) (Figure 2).Repeat blood culture was negative and the repeated TTE consultations did not find valve neoplasm, so the infective endocarditis was excluded.Current diagnosis: (1) right heart failure with the with New York Heart Association (NYHA) functional class III–IV; (2) PH; (3) congenital heart defect, PDA; (4)heart valve disease, including aortic valve stenosis and insufficiency, mitral valve insufficiency, and pulmonary valve insufficiency; (5) PE; and (6) arrhythmia atrial fibrillation.The clinic symptoms of this patient were improved by symptomatic treatment, such as diuresis with potassium supplementation, cardiac strengthening, anticoagulation (low molecular weight heparin), control of ventricular rate, etc.Due to the initial diagnosis of PE, warfarin anticoagulation was intended to be given for three months, and the targeting drugs riociguat was added to reduce pulmonary artery pressure after discharge.
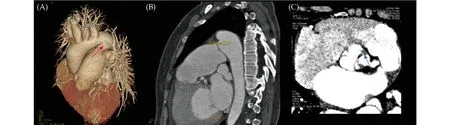
Figure 1 CTA for further diagnosis.(A): A giant PDA (red arrow) was observed from 3D volume rendering images; (B): the size of the PDA was 10.8 mm at sagittal section view; and (C): CTA revealed bicuspid aortic valves and aortic stenosis with valve neoplasm formation to be excluded (blue arrow).CTA: computed tomography angiography; PDA: patent ductus arteriosus.
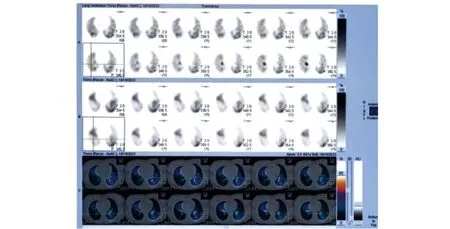
Figure 2 The ventilation/perfusion scan imagings demonstrated pulmonary embolism.
The patient was admitted after three months for further clinical management.Laboratory examination showed NT-proBNP was 3363 pg/mL.The physical examination showed that grade 2/6 systolic murmur could be heard along the 2ndto 3rdribs at the left edge of sternum.The TTE reexamination showed that PDA (1.2 cm), PH (96 mmHg), mild aortic valve stenosis and mild to moderate insufficiency, mild to moderate pulmonary valve insufficiency.The right ventricular systolic function was normal, TAPSE was 17 mm, and RV/LV was 26/40.Pulmonary ventilation/perfusion scan showed significant improvement in PE compared to before (ventilation/blood flow perfusion mismatch only decreased anterior segment of the upper lobe and the dorsal segment of the lower lobe of the left lung).Computed tomography pulmonary angiography (CTPA) indicated PDA and PH, cardiac enlargement, a small amount of pericardial effusion, and no obvious other signs of PE was found.After improving cardiac function, a right heart catheterization was performed with pulmonary artery pressure of 110/56 mmHg, descending aortic pressure of 114/70 mmHg, Qp/Qs = 1.28, and total pulmonary resistance of 13.4 woods.Pulmonary angiography showed no filling defects or stenotic lesions at the level of main branches and subsegments.Attempt occlusion using a PDA occluder for 15 min, the systolic pressure of the pulmonary artery decreased by about 20% and there was no change in aortic pressure.The patient experienced shortness of breath and chest tightness, so the intervention occlusion was terminated.In combination with the current TTE, right heart catheterization, angiocardiography and attempt occlusion results, rivaroxaban anticoagulation was used instead, and the dosage of riociguat was increased.At the same time, we decided to add macitentan to reduce PH for three months to six months until next evaluation.
The patient was admitted after six months since last hospitalization for condition deteriorates in recent 10 days.Chest CT shows pneumonic changes (Figure 3A &3B).The NT-proBNP was 6630 pg/mL, the hemoglobin was 88 g/L, and the procalcitonin was 0.06 ng/mL.The upper limb oxygen saturation was 90%, and the lower limb oxygen saturation was 92%.On auscultation, the breathing sounds in both lungs are thick, and wet rales can be heard on the right lung.The 3/6 grade systolic murmur can be heard in the second intercostal space on the left edge of the sternum, and there was no significant change in other cardiac murmurs.The pulmonary pressure was 86 mmHg, and the TAPSE was 19 mm, and RV/LV was 26/54 under TTE reexamination (Table 1).No signs of PE was found in CTPA reexamination (Figure 3C & 3D).Pulmonary ventilation/perfusion scan showed that PE was basically the same as last examination.Through antiinfection, anemia correction, improving cardiac function and other symptomatic treatment, the NT-proBNP decreased to 2022 pg/mL, the hemoglobin increased to 112 g/L, and the procalcitonin became normal level.
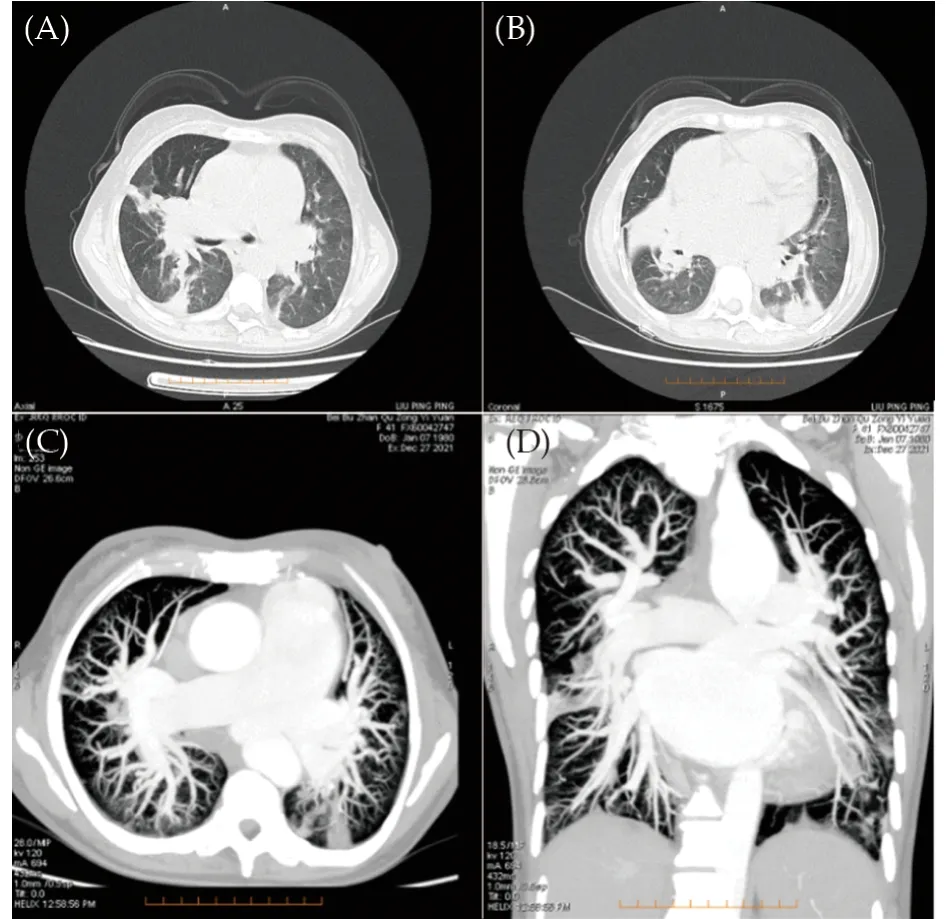
Figure 3 Chest computed tomography and CTPA results at the third hospitalization.(A & B): Pneumonic changes, multiple nodules in both lungs, mediastinal lymphadenopathy, and pulmonary hypertension were observed under chest computed tomography; and (C & D): no signs of pulmonary embolism was found under CTPA reexamination.CTPA: computed tomography pulmonary angiography.

Table 1 Main indicators changes of TTE during three hospitalizations.
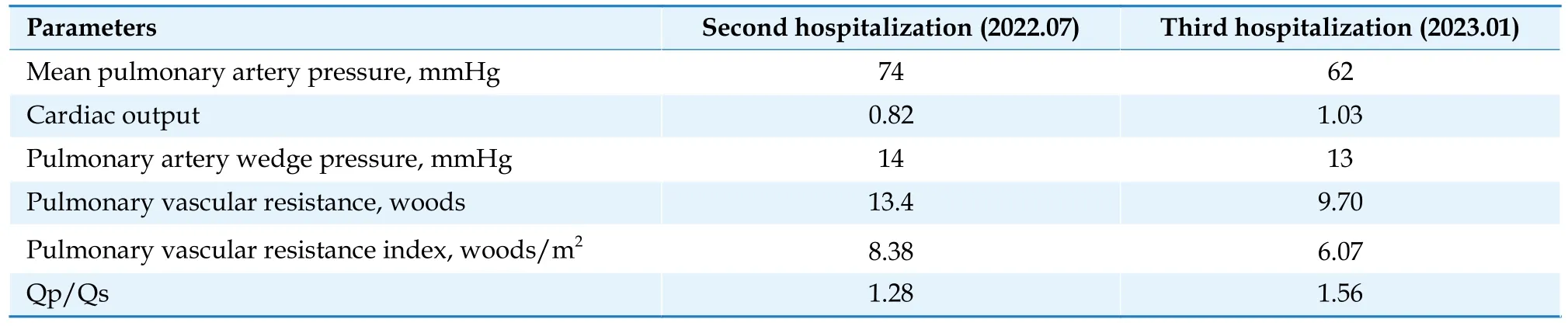
Table 2 The parameters of right heart catheterization comparison.
Right heart catheter was conducted to measure pulmonary artery pressure of 94/46 mmHg, aortic pressure of 115/70 mmHg, Qp/Qs = 1.56, and total pulmonary resistance of 9.7 woods (Table 2).The PDA was tubular type, measuring 12 mm at the aortic end, 11.0 mm at the pulmonary end, and 10.0 mm in length under angiocardiography.Using a LZPDA-16-14 occluder (Lifetech Scientific Corp., Shenzhen, China) for occlusion, the pulmonary artery pressure had decreased to 58/27 mmHg,and the pressure of ascending aorta remained unchanged, and SpO2was 93% without any discomfort after 15 min.So the transcatheter closure of PDA was successfully performed (Figure 4).TTE revealed three days after the occlusion that the systolic pulmonary artery pressure had decreased to 48 mmHg, and left and right ventricular systolic function was normal without any complication.The NT-proBNP was 807.0 pg/mL prior to discharge.Considering the possible anemia adverse reaction of macitentan, we adjusted the medication for reducing pulmonary artery pressure as follows: ambrisentan tablets 5 mg orally once a day; riociguat tablets 1.5 mg orally three times a day.Continuing anticoagulation and other symptomatic treatment.
The effect of intervention treatment was satisfactory at one, three and six-month of TTE follow-up.The patient had no complaints of discomfort.TTE showed that the position of the occluder was normal, and no residual shunt was found.There was no increase in pulmonary artery pressure with normal left and right heart function.Chest X-ray examination showed a significant reduction in cardiac boundary with the cardiothoracic ratio decreased from 0.81 to 0.68 (Figure 5), and the 6-min walk test distance was 482 m at last follow-up.The continuous follow-up observation was required.

Figure 4 Transcatheter closure of the giant PDA.(A): Right heart catheter and angiography was conducted before attempt occlusion; and (B): transcatheter closure of PDA was successfully performed using a 16/14 occluder.PDA: patent ductus arteriosus.
In the past, it was considered that the congenital heart disease with PE was rare.With the progress of disease cognition and diagnostic technology, escape diagnosis or misdiagnosis had been improved.[4]As a long history of left to right shunt in the adult giant PDA patient, the pulmonary arteriole vessels continue to spasm and become stiff, resulting in thickening of the smooth muscle intima of the pulmonary arteriole, focal necrosis, calcification of the intima, and proliferation of fibrous tissue, leading to remodeling of the vascular wall, increasing the resistance of the pulmonary artery and PH.For adult PDA patient, chronic hypoxia can also lead to the increase of red blood cells, thus making the blood volume and blood viscosity relatively increased, venous blood flow slow, and plasma fibrinogen increased.These are the main mechanisms of PE in adult patients with PDA.PH is also the most common complication of PE, which can gradually evolve into chronic pulmonary heart disease and right heart failure.[5]Due to the clear combination of PE in this case, the chronic thromboembolic PH was considered as initial application of riociguat.Because the clinical manifestations of congenital heart diseases complicated with PE were complex and diverse, it was often easy to escape diagnosis or be misdiagnosed.The accurate diagnosis of this case was the basis for subsequent successful treatment.

Figure 5 Comparison of chest X-ray before and after transcatheter interventions.(A): Preoperative chest X-ray revealed significant enlargement of the cardiac boundary and protrusion of the pulmonary artery segment; and (B): compared to preoperative chest X-ray, a significant reduction in cardiac boundary and pulmonary artery segment at last follow-up.
After accurate diagnosis of this patient, standardized anticoagulant treatment was given for PE, and targeted drug recommended by the guidelines was applicated for PH.[6]The pulmonary ventilation/perfusion scan results showed obvious improvement when the patient was hospitalized again.No obvious signs of PE was found in CTPA and pulmonary angiography with hemodynamic indicators improved at last hospitalization.We propose a hypothesis that the patient might suffer the PE and improve situation of the condition after standardized anticoagulant treatment in time.Although there were some reports of successful surgical treatment of adult PDA complicated with chronic thromboembolic PH, most of the PDAs had adhesions, calcifications, and fragility in the surrounding tissue in elderly patients, and the risk of intraoperative and postoperative complications were extremely high when complicated with severe PH.The first trial occlusion of PDA for this case was mainly based on the patient’s significant improvement in symptoms after three months of standard anticoagulation therapy and targeted drug therapy to decrease pulmonary pressure.Through the results, we indeed realized that the decision was arbitrary and still needed to be mature considerations.Through this special case, with the progress of targeted drugs to reduce pulmonary artery pressure, some adult patients with PDA at the early stage of Eisenmenger syndrome had obtained the hope of interventional treatment through “treat and repair” therapy.Especially in the case of PE complications, the hemodynamic status of Eisenmenger syndrome may partly come from PE.According to 2022 European Society of Cardiology/European Respiratory Society guidelines for the diagnosis and treatment of PH, the risk stratification had shifted from high risk before treatment to low risk after final treatment.[7]For congenital heart disease complicated with PE, through early detection and standardized diagnosis and treatment, the connotation of “treat and repair” can be further expanded to achieve satisfactory therapeutic result.
In conclusion, the adult patients with giant PDA complicated with PE and severe PH might have opportunity of successful interventional treatment through “treat and repair” therapy.Early detection of PE, standardized anticoagulation, individualized PH targeting drug selection in different periods and other symptomatic treatments might contribute to procedural success.
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
REFERENCES
[1]Schneider DJ, Moore JW.Patent ductus arteriosus.Circulation2006; 114: 1873-1882.
[2]Baumgartner H, De Backer J, Babu-Narayan SV,et al.2020 ESC guidelines for the management of adult congenital heart disease.Eur Heart J2021; 42: 563-645.
[3]Devasia T, Kareem H, Morakhia JV,et al.Successful patent ductus arteriosus device closure in a patient with massive pulmonary embolism.J Cardiol Cases2014; 10: 62-65.
[4]Yeh TC, Liu CP, Tseng CJ,et al.Postpartum patient with congenital patent ductus arteriosus mimicking acute pulmonary embolism.BMJ Case Rep2013; 2013: bcr2012007717.
[5]Teerapuncharoen K, Bag R.Chronic thromboembolic pulmonary hypertension.Lung2022; 200: 283-299.
[6]Konstantinides SV, Meyer G, Becattini C,et al.2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS).Eur Heart J2020; 41: 543-603.
[7]Humbert M, Kovacs G, Hoeper MM,et al.2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension.Eur Respir J2023; 61: 2200879.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Discordance analysis for apolipoprotein and lipid measures for predicting myocardial infarction in statin-treated patients with coronary artery disease: a cohort study
- Efficacy and safety of sacubitril/valsartan after six months in patients with heart failure with reduced ejection fraction and asymptomatic hypotension
- Catheter ablation of atrial fibrillation via retrograde aortic approach in a patient with interrupted inferior vena cava: a case report
- Concomitant occurrences of pulmonary embolism and acute myocardial infarction in acute coronary syndrome patient undergoing percutaneous coronary intervention: a case report
- Repair of posterior ventricular septal rupture by right atrial approach
- Prescription patterns of antidiabetic and cardiovascular preventive medications in community-dwelling older adults with type 2 diabetes mellitus: a cross-sectional study
