微塑料对磺胺甲恶唑的吸附特性
2024-01-01闫晓娜李敏罗静罗婉均刘娟刘艳梅



摘 要:以聚氯乙烯和聚乙烯为微塑料代表,探究这两种微塑料对磺胺甲恶唑的吸附动力学及热力学特性。结果表明,聚氯乙烯对磺胺甲恶唑的吸附量较大,准二级模型更适合用于描述两种微塑料对磺胺甲恶唑的吸附动力学过程;聚氯乙烯和聚乙烯对磺胺甲恶唑的吸附热力学过程用Langmuir模型拟合较好,说明吸附过程是单层吸附;两种微塑料对磺胺甲恶唑的吸附过程是一个自发进行,吸热并且熵增的过程。
关键词:聚氯乙烯;聚乙烯;磺胺甲恶唑;吸附
中图分类号:X13 文献标志码:A 文章编号:1673-9655(2024)05-00-06
0 引言
塑料因其生产成本低、用途广泛、耐用与便于携带等优点成为日常生活中必不可少的一种材料[1, 2],每年全球产量超过3.5亿t[3, 4]。全球每天产生160万t塑料废物,相当于人均每年产生75 kg的塑料垃圾[5]。当废弃塑料进入到环境后,进一步通过物理化学作用与微生物降解等过程被分解碎裂成小碎片,其中粒径<5 mm的被称为微塑料(microplastics,MPs)[6],而这些微塑料广泛存在于河流[7, 8]、湖泊[9, 10]与海洋[11]等水环境中。微塑料难降解,可长期存在于水环境中,且微塑料比表面积大,疏水性强,是众多污染物的理想载体,包括多氯联苯[12]、多环芳烃[13]、抗生素[14, 15]和重金
属[16, 17]等,进而影响污染物在水环境中的迁移转化过程。Yu[18]等人调查发现多氯联苯在微塑料上的分布系数随疏水性的增加而增加,即疏水相互作用和表面积在微塑料与多氯联苯之间的吸附行为中起着重要作用。此外,由于生物可以摄入微塑料,受污染的微塑料可能会成为生物体污染物的携带者,并可能对它们造成健康风险[19]。
抗生素可用于改善人类和畜禽的健康状况,但仍有超过一半的抗生素因为不能被消化吸收进而通过粪便和尿液等方式排入到环境中[20]。磺胺类抗生素因其性质稳定、使用简便及价格低廉被广泛使用,磺胺甲恶唑(Sulfamethoxazole, SMX)是磺胺类抗生素的典型代表,在大部分水环境中都被检测存在,在地表水中的浓度达到了纳克至微克的水平[21, 22]。磺胺甲恶唑在相当低的浓度范围内,也可能对水生生物构成重大的生态毒理风险。这些抗生素在生物体内累积,最终通过食物链对人体及生态系统带来潜在风险和危害[23]。
近年来,微塑料和抗生素的研究越来越受到关注,微塑料载体在水生环境中运输抗生素中的作用及其综合毒性作用,使得研究微塑料与抗生素之间的吸附行为至关重要[24]。微塑料的分子结构、结晶度和极性等物理化学性质会显著影响其对抗生素的吸附能力[25]。聚氯乙烯(Polyvinyl chloride,PVC)和低密度聚乙烯(Low density polyethylene, LDPE)是日常生活中广泛使用的微塑料。PVC表面积
为0.595[26],无定形聚合物[27],属于C-C 骨架聚合物,具有耐水解和生物降解性,且在骨架的每两个碳上都存在一个氯原子,猜测这可能是其生物降解性极低的原因[28];此外,与聚丙烯(PP)、聚乙烯(PE)、聚苯乙烯(PS)相比,PVC 具有更松散的凸起表面,并包含更多的折叠结构,从而提供更多的吸附位点[29]。LDPE表面积为0.043±0.001[30],半结晶性聚合物[27],具有相对较低的结晶度[31],主要由碳氢键组成的支链结构组成,具有高度的稳定性和惰性[32]。Fries等人研究发现多环芳烃在高密度聚乙烯(HDPE)中的扩散系数远小于LDPE中的扩散系数[33]。微塑料可通过污水排放和生物摄食等不同的途径进入到水环境中,进而与水环境中的抗生素发生作用。研究发现,微塑料可与抗生素的抗性基因相互作用进一步影响水产养殖环境[34]。抗生素与微塑料相互作用的机制有四种,分别是范德华吸附、静电吸附、氢键吸附和微孔填充机制[25]。Li等[15]研究了聚乙烯、聚苯乙烯、聚丙烯、聚酰胺和聚氯乙烯五种微塑料对包括磺胺嘧啶等五种抗生素的吸附特性,结果表明,磺胺嘧啶在聚乙烯、聚苯乙烯、聚丙烯和聚氯乙烯上的吸附量较小,KF与辛醇-水分配系数呈正相关。Guo等[35]研究了磺胺二甲嘧啶在包括聚乙烯和聚氯乙烯等6种微塑料上的吸附,发现吸附可在16 h内达到平衡,吸附的主要机制是静电相互作用和范德华力。此外,特定的官能团,如酰胺基团通过质子供体与受体之间形成的氢键显著影响着微塑料对抗生素的吸附[15]。Li[36]等人研究发现微塑料会增强磺胺甲恶唑对好氧颗粒污泥的毒性作用,并富集抗生素耐药基因。因此,了解磺胺类抗生素和不同的微塑料之间可能的反应对于评估它们的环境风险是必要的。
以往对于微塑料与抗生素之间的相互作用主要集中在四环素类抗生素上[37-39]。微塑料与磺胺甲恶唑作为水环境中较为常见的污染物,明确两者在水环境中的环境行为,有助于更好了解微塑料与抗生素之间的相互作用。因此,该研究选择低密度聚乙烯和聚氯乙烯两种微塑料为代表,选择磺胺甲恶唑作为典型的抗生素,探究微塑料对水环境中磺胺甲恶唑的吸附行为,为实际环境中微塑料与抗生素共存的情况下二者的环境行为提供一定的理论支持。
1 材料与方法
1.1 实验仪器与材料
高效液相色谱(Agilent 1200series,USA),恒温振荡器(HZQ-X300C,上海一恒),定时恒温磁力搅拌器(90-2型,上海沪西)。
研究采用聚氯乙烯和低密度聚乙烯两种微塑料购买于东菀市华创塑化有限公司,经100~120目筛分后备用。实验所用磺胺甲恶唑购买于Sigma Aldrich,氯化钙购买于科隆,为分析纯。实验用水为Milli-Q纯水系统制备的超纯水。
1.2 实验方法
1.2.1 吸附动力学实验
以0.01 mol/LCaCl2和200 mg/L NaN3为背景溶液,使其有一定的离子浓度和减少微生物的影响,反应溶液包含10 µmol/LSMX,并且用盐酸调节pH=6,按照10 mg:5 mL的固液比投加微塑料PVC或LDPE,溶液体积为5 mL。在恒温振荡器中以200 rpm和25°C下进行处理,分别于0.5、1、1.5、2、3、4、5、12、24、36、48、60、72 h后取样后通过0.45 µm的滤膜,通过高效液相色谱测定SMX的浓度。
1.2.2 吸附等温线实验
配置5 mL溶液,使溶液包含0.01 mol/LCaCl2、200 mg/L NaN3和不同浓度的SMX,其中SMX浓度分别设置为1、3、6、10、15、20、30、40 µmol/L,
调节pH=6,分别在15、25、35°C下200 rpm的恒温振荡器中振荡24 h,0.45 µm滤膜过滤后,测定SMX的浓度。
1.3 数据分析
根据SMX的初始浓度和剩余浓度计算吸附量的公式见如下:
(1)
式中:Qt—t(h )时刻的吸附量,µg/g;C0和Ct—初始和t(h )时刻的吸附质的浓度,µg/L;m—微塑料的质量,g;V—溶液的体积,L。
采用准一级动力学和准二级动力学模型对动力学实验数据进行拟合,模型公式如下:
(2)
(3)
式中:Qe和Qt—吸附平衡时和吸附时间为t(h)时刻的吸附量,µg/g;k1和k2—准一级动力学,/h和准二级动力学的反应速率常数,g/µg·h。
采用Langmuir模型和Freundlich模型对吸附热力学过程进行拟合,模型公式如下:
(4)
(5)
式中:Ce—吸附平衡时SMX的浓度,µg/L;Qe—吸附平衡时的吸附量,µg/g;Qmax—Langmuir模型中的理论最大吸附量,µg/g;KL—Langmuir模型的吸附平衡常数,L/µg;KF和n—分别为Freundlich模型中的吸附能力(µg1-n·Ln/g)和吸附强度。
2 结果与讨论
2.1 吸附动力学
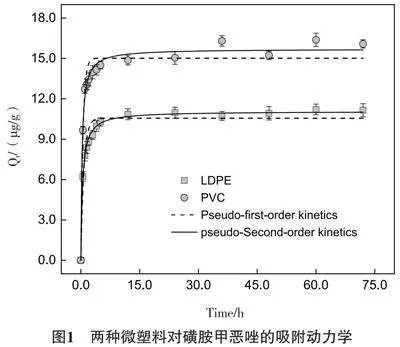
LDPE和PVC对SMX的吸附动力学模型拟合如图1所示。LDPE和PVC这两种微塑料对SMX的吸附在24 h后达到吸附平衡。两种微塑料随时间的变化趋势几乎相似,但平衡吸附能力不同,吸附容量为PVC>LDPE。吸附的过程可分为2个阶段,第一个阶段,快速吸附的过程;第二个阶段为慢速吸附的过程。在快速吸附阶段,因为微塑料表面上的吸附点位充足,SMX的浓度也比较大,能吸附更多的SMX,随着吸附时间的增加,吸附点位减少,SMX的浓度也随之减小,吸附速率逐渐变得缓慢并趋于平衡。
两种微塑料对SMX的吸附动力学拟合模型的拟合参数如表1所示。由图1和表1可得出准二级动力学模型可以较好的拟合微塑料对SMX的吸附过程,两种微塑料准二级动力学模型相关系数均大于准一级动力学模型的相关系数(LDPE:0.995>0.950;PVC:0.988>0.950);并且通过准二级动力学模型拟合两种微塑料的最大吸附量与实验得出的最大吸附量更加接近,表明两种微塑料对SMX的吸附过程以化学吸附为主。根据准二级动力学模型的理论,两种微塑料对SMX的吸附不是单一因素作用的结果,而是包括范德华力、疏水作用、静电作用和化学键等多因素共同作用的结果[35, 40, 41]。与此相同的是,Xu等[42]的研究发现准二级动力学模型对聚乙烯微塑料吸附SMX的过程拟合度更高,主要的吸附机理是范德华力。有研究证实,PE对亲水性SMX的吸附可能是因为范德华力[15, 43]。由于SMX的logKow<2.5,是一种亲水化合物[44],而LDPE[45, 46]和PVC[47]表面都是疏水性的,亲水性化合物SMX扩散到疏水性的微塑料表面较为困难,说明疏水作用不是这两种微塑料对SMX吸附的主要机理。吸附实验的pH为6,在吸附过程中SMX主要以中性分子以及阴离子形态存在,与PVC表面的负电荷相互排斥,所以静电相互作用可能是主要的机制[48]。
2.2 吸附等温线
通过Langmuir和Freundlich模型对LDPE和PVC对SMX的吸附进行拟合,如图2所示,拟合参数见表2。这两种微塑料吸附SMX的等温线变化趋势相似,不论是LDPE(图2a)或是PVC(图2b),二者在温度相对较高的情况下,反而不利于吸附SMX,但两种微塑料对SMX的吸附量随着SMX的浓度呈现出增加的趋势。相比于LDPE,PVC对SMX的吸附量更大,说明不同种类微塑料对磺胺类抗生素的吸附效果有差异。相比于LDPE,PVC是一种具有强极性的微塑料,有利于吸附SMX。另一个潜在的机制是PE被认为是橡胶塑料,而PS、PVC被认为是玻璃塑料[49]。许多研究结果表明[27, 50, 51],有机污染物对橡胶塑料上的吸附比玻璃塑料具有更高的亲和力。而本研究结果与此相反,说明抗生素的吸附程度与塑料的橡胶状态几乎没有相关性。这与Li等人的研究是一致的[15]。此外,在不同温度下,两种微塑料对SMX呈现非线性的吸附过程,说明疏水分配作用不是主要的吸附机制。
根据表2的模型拟合参数,Langmuir模型对微塑料吸附SMX的热力学曲线的拟合程度更高,表明微塑料对SMX的吸附过程是单层吸附。但是在35°C下,两种模型对微塑料吸附SMX的热力学曲线的拟合程度较低,这可能是因为微塑料自身的物理特性,两种微塑料表面的孔隙比较少,不利于后期SMX在微塑料中的内部扩散[52]。
2.3 吸附热力学
为进一步了解微塑料对SMX的吸附特性,以1/T为横坐标和lnK为纵坐标作图[53],拟合后得到热力学参数如表3所示,ΔGθlt;0,而ΔHθ和ΔSθ的值均>0,说明LDPE和PVC对磺胺甲恶唑的吸附过程是自发进行的吸热过程,而且吸附过程中的混乱度增加。同时,在两种微塑料对SMX的吸附过程中,ΔGθ的绝对值随温度的增加呈现先增加后降低的趋势,说明当温度从15°C增加至25°C,低温有利于吸附的进行,但是温度25°C增加至35°C,相对来说反应不利于进行,与吸附等温线的结果相同,在35°C时两种微塑料对SMX的吸附量最小。ΔGθ的绝对值在15°C与25°C的变化较小,表明温度对微塑料吸附SMX的影响较小。
3 结论
(1)两种微塑料对磺胺甲恶唑的吸附在24 h达到平衡,准二级动力学模型能更好地拟合动力学过程,PVC对SMX的吸附量大于LDPE的吸附量。在吸附过程中范德华力和静电作用起主要影响。
(2)PVC和LDPE对SMX的吸附热力学过程用Langmuir模型拟合较好,两种微塑料对SMX的吸附过程属于单层吸附。
(3)LDPE和PVC对磺胺甲恶唑的吸附过程是自发进行的吸热过程,而且吸附过程中的混乱度增加。
参考文献:
[1] MENG Y,KELLY F J,WRIGHT S L.Advances and challenges of microplastic pollution in freshwater ecosystems:A UK perspective [J]. Environmental Pollution,2020(256):113445.
[2] WANG C,ZHAO J,XING B.Environmental source,fate,and toxicity of microplastics [J].Journal of Hazardous Materials, 2021(407):124357.
[3] HELLWEG H W Z W A S.Deep Dive into Plastic Monomers, Additives, and Processing Aids [J].Environmental science amp; technology,2021,55(13):9339-51.
[4] HARRIS P T,WESTERVELD L,NYBERG B,et al.Exposure of coastal environments to river-sourced plastic pollution [J].Science of the Total Environment,2021(769):145222.
[5] IGALAVITHANA A D,YUAN X,ATTANAYAKE C P,et al.Sustainable management of plastic wastes in COVID-19 pandemic:The biochar solution [J].Environmental Research,2022(212):113495.
[6] LI J,LIU H,PAUL CHEN J.Microplastics in freshwater systems A review on occurrence,environmental effects,and methods for microplastics detection [J].Water Research,2018(137):362-74.
[7] YUAN W,CHRISTIE-OLEZA J A,XU E G,et al.Environmental fate of microplastics in the world’s third-largest river:Basin-wide investigation and microplastic community analysis [J].Water Research,2022(210):118002.
[8] ADOMAT Y,GRISCHEK T. Sampling and processing methods of microplastics in river sediments - A review [J]. Science of the Total Environment, 2021(758):143691.
[9] MAO R, SONG J, YAN P, et al. Horizontal and vertical distribution of microplastics in the Wuliangsuhai Lake sediment, northern China [J]. Science of the Total Environment, 2021(754) 142426.
[10] YANG S, ZHOU M, CHEN X, et al. A comparative review of microplastics in lake systems from different countries and regions [J]. Chemosphere, 2022, 286(Pt 2): 131806.
[11] YUAN Z, NAG R, CUMMINS E. Human health concerns regarding microplastics in the aquatic environment - From marine to food systems [J]. Science of the Total Environment, 2022(823): 153730.
[12] PASCALL M A, ZABIK M E, ZABIK M J, et al. Uptake of Polychlorinated Biphenyls (PCBs) from an Aqueous Medium by Polyethylene, Polyvinyl Chloride, and Polystyrene Films [J]. Journal of Agricultural and Food Chemistry, 2005, 53(1): 164-9.
[13] BAO Z Z, CHEN Z F, ZHONG Y, et al. Adsorption of phenanthrene and its monohydroxy derivatives on polyvinyl chloride microplastics in aqueous solution: Model fitting and mechanism analysis [J]. Science of the Total Environment, 2021(764): 142889.
[14] YU F, LI Y, HUANG G, et al. Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution [J]. Chemosphere, 2020(260): 127650.
[15] LI J, ZHANG K, ZHANG H. Adsorption of antibiotics on microplastics [J]. Environmental Pollution, 2018(237): 460-7.
[16] MAO R, LANG M, YU X, et al. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals [J]. Journal of Hazardous Materials, 2020(393): 122515.
[17] FU Q, TAN X, YE S, et al. Mechanism analysis of heavy metal lead captured by natural-aged microplastics [J]. Chemosphere, 2021(270): 128624.
[18] YU F, YANG C, ZHU Z, et al. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment [J]. Science of The Total Environment, 2019(694): 133643.
[19] GUO X, CHEN C, WANG J. Sorption of sulfamethoxazole onto six types of microplastics [J]. Chemosphere, 2019(228): 300-8.
[20] MA Y, YANG L, WU L, et al. Carbon nanotube supported sludge biochar as an efficient adsorbent for low concentrations of sulfamethoxazole removal [J]. Science of the Total Environment, 2020(718): 137299.
[21] LV Y, LI Y, LIU X, et al. The tolerance mechanism and accumulation characteristics of Phragmites australis to sulfamethoxazole and ofloxacin [J]. Chemosphere, 2020(253): 126695.
[22] DUAN W, CUI H, JIA X, et al. Occurrence and ecotoxicity of sulfonamides in the aquatic environment: A review [J]. Science of the Total Environment, 2022(820): 153178.
[23] WU Q, PAN C G, WANG Y H, et al. Antibiotics in a subtropical food web from the Beibu Gulf, South China: Occurrence, bioaccumulation and trophic transfer [J]. Science of the Total Environment, 2021(751): 141718.
[24] ZHUANG S, WANG J. Interaction between antibiotics and microplastics: Recent advances and perspective [J]. Science of The Total Environment, 2023(897): 165414.
[25] KUANG B, CHEN X, ZHAN J, et al. Interaction behaviors of sulfamethoxazole and microplastics in marine condition: Focusing on the synergistic effects of salinity and temperature [J]. Ecotoxicology and Environmental Safety, 2023(259): 115009.
[26] ABRAMOVA A V, ABRAMOV V O, FEDULOV I S, et al. Strong Antibacterial Properties of Cotton Fabrics Coated with Ceria Nanoparticles under High-Power Ultrasound [J/OL] 2021, 11(10):10.3390/nano11102704.
[27] GUO X, WANG J. The chemical behaviors of microplastics in marine environment: A review [J]. Marine Pollution Bulletin, 2019(142): 1-14.
[28] KRUEGER M C, HARMS H, SCHLOSSER D. Prospects for microbiological solutions to environmental pollution with plastics [J]. Applied Microbiology and Biotechnology, 2015, 99(21): 8857-74.
[29] GUO X, PANG J, CHEN S, et al. Sorption properties of tylosin on four different microplastics [J]. Chemosphere, 2018(209): 240-5.
[30] MA J, CHEN F, ZHU Y, et al. Joint effects of microplastics and ciprofloxacin on their toxicity and fates in wheat: A hydroponic study [J]. Chemosphere, 2022(303): 135023.
[31] KARAPANAGIOTI H K, WERNER D. Sorption of Hydrophobic Organic Compounds to Plastics in the Marine Environment: Sorption and Desorption Kinetics [M]//TAKADA H, KARAPANAGIOTI H K. Hazardous Chemicals Associated with Plastics in the Marine Environment. Cham; Springer International Publishing. 2019: 205-19.
[32] AHMED M, BASHAR I, ALAM S T, et al. An overview of Asian cement industry: Environmental impacts, research methodologies and mitigation measures [J]. Sustainable Production and Consumption, 2021(28): 1018-39.
[33] FRIES E, ZARFL C. Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE) [J]. Environmental Science and Pollution Research, 2012, 19(4): 1296-304.
[34] DONG H, CHEN Y, WANG J, et al. Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments [J]. Journal of Hazardous Materials, 2021(403): 123961.
[35] XUAN GUO Y L, JIANLONG WANG. Sorption of sulfamethazine onto different types of microplastics: A combined experimental and molecular dynamics simulation study [J]. Marine Pollution Bulletin, 2019(145): 547-54.
[36] JI J, PENG L, GAO T, et al. Microplastics enhanced the toxic effects of sulfamethoxazole on aerobic granular sludge and enriched antibiotic resistance genes [J]. Chemical Engineering Journal, 2023(464): 142783.
[37] CHEN Y, LI J, WANG F, et al. Adsorption of tetracyclines onto polyethylene microplastics: A combined study of experiment and molecular dynamics simulation [J]. Chemosphere, 2021(265): 129133.
[38] SUN M, YANG Y, HUANG M, et al. Adsorption behaviors and mechanisms of antibiotic norfloxacin on degradable and nondegradable microplastics [J]. Science of The Total Environment, 2022(807): 151042.
[39] TONG F, LIU D, ZHANG Z, et al. Heavy metal-mediated adsorption of antibiotic tetracycline and ciprofloxacin on two microplastics: Insights into the role of complexation [J]. Environmental Research, 2023(216): 114716.
[40] RAZANAJATOVO R M, DING J, ZHANG S, et al. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics [J]. Marine Pollution Bulletin, 2018(136): 516-23.
[41] GUO X, WANG J. Sorption of antibiotics onto aged microplastics in freshwater and seawater [J]. Marine Pollution Bulletin, 2019(149): 110511.
[42] XU B, LIU F, BROOKES P C, et al. The sorption kinetics and isotherms of sulfamethoxazole with polyethylene microplastics [J]. Marine pollution bulletin, 2018(131): 191-6.
[43] HUFFER T, HOFMANN T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution [J]. Environmental Pollution, 2016(214): 194-201.
[44] SEUNG-WOO NAM D-J C, SEUNG-KYU KIM, NAMGUK HER, KYUNG-DUK ZOH. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon [J]. Journal of Hazardous Materials, 2014(270): 144-52.
[45] GONG M, YANG G, ZHUANG L, et al. Microbial biofilm formation and community structure on low-density polyethylene microparticles in lake water microcosms [J]. Environmental Pollution, 2019, 252(Pt A): 94-102.
[46] DELACUVELLERIE A, CYRIAQUE V, GOBERT S, et al. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation [J]. Journal of Hazardous Materials 2019(380): 120899.
[47] XIA Y, ZHOU J J, GONG Y Y, et al. Strong influence of surfactants on virgin hydrophobic microplastics adsorbing ionic organic pollutants [J]. Environmental Pollution, 2020, 265(Pt B): 115061.
[48] BAO Z-Z, CHEN Z-F, LU S-Q, et al. Effects of hydroxyl group content on adsorption and desorption of anthracene and anthrol by polyvinyl chloride microplastics [J]. Science of the Total Environment, 2021(790): 148077.
[49] WANG F, SHIH K M, LI X Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics [J]. Chemosphere, 2015(119): 841-7.
[50] TEUTEN E L, ROWLAND S J, GALLOWAY T S, et al. Potential for Plastics to Transport Hydrophobic Contaminants [J]. Environmental Science amp; Technology, 2007, 41(22): 7759-64.
[51] ROCHMAN C M, HOH E, HENTSCHEL B T, et al. Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris [J]. Environmental Science amp; Technology, 2013, 47(3): 1646-54.
[52] 蒋晖, 刘欣, 孙姣霞, 等. 17α-乙炔基雌二醇在微塑料上的吸附和解吸行为 [J] 中国环境科学, 2021: 2258-67.
[53] ZHOU X, ZHOU X. The unit problem in the thermodynamic calculation of adsorption using the Langmuir equation [J]. Chemical Engineering Communications, 2014, 201(11): 1459-67.
Adsorption Properties of Sulfamethoxazole on Micro-plastics
YAN Xiao-na, LI Min, LUO Jing, LUO Wan-jun, LIU Juan, LIU Yan-mei
(Xihua Normal University, Nanchong Sichuan 637000,China)
Abstract: In this study, polyvinyl chloride and polyethylene were used as micro-plastics to investigate the adsorptionkinetics and thermodynamic properties of sulfamethoxazole. The results showed that the adsorption capacity of sulfamethoxazole was larger on polyvinyl chloride, and the pseudo-second-order model was more suitable for describing the adsorption kinetics of sulfamethoxazole on the two micro-plastics. The adsorption thermodynamic process of sulfamethoxazole on polyvinyl chloride and polyethylene was well fitted by the Langmuir model, indicating that the adsorption process was monolayer adsorption. The adsorption of sulfamethoxazole by the two micro-plastics was a spontaneous, endothermic and entropy-increasing process.
Key words: polyvinyl chloride; polyethylene; sulfamethoxazole; adsorption
作者简介:闫晓娜(1999- ),女,硕士,西华师范大学环境科学专业。
通信作者:刘艳梅(1993- ),女,博士,讲师,西华师范大学。
