Cytotoxic phenazine and antiallergic phenoxazine alkaloids from an arctic Nocardiopsis dassonvillei SCSIO 502F
2023-12-29YueSongQiYangLiMengJingCongXiaoYanPangBoChenYongHongLiuLiLiaoandJunFengWang
Yue Song,Qi-Yang Li,Meng-Jing Cong,Xiao-Yan Pang,Bo Chen,Yong-Hong Liu,5,Li Liao,6* and Jun-Feng Wang,5*
Abstract Microbes well-adapted to the Arctic Ocean are promising for producing novel compounds,due to their fancy strategies for adaptation and being under-investigated.Two new phenazine alkaloids (1 and 2) and one new phenoxazine (3) were isolated from Nocardiopsis dassonvillei 502F,a strain originally isolated from Arctic deep-sea sediments.AntiSMASH analysis of the genome of Nocardiopsis dassonvillei 502F revealed the presence of 16 putative biosynthetic gene clusters (BGCs),including a phenazine BGC.Most of the isolated compounds were evaluated for their antibacterial,antiallergic,and cytotoxic activities.Among them,compounds 4 and 5 exhibited potent in vitro cytotoxic activities against osteosarcoma cell line 143B with IC50 values 0.16 and 20.0 μM,respectively.Besides,the results of antiallergic activities of compounds 6-8 exhibited inhibitory activities with IC50 values of 10.88 ± 3.05,38.88 ± 3.29,and 2.44 ± 0.17 μg/mL,respectively (IC50 91.6 μM for the positive control loratadine).
Keywords Nocardiopsis dassonvillei,Arctic Ocean,Phenazine,Phenoxazine,Cytotoxic,Antiallergic
1 Introduction
Phenazines and phenoxazines consist of a large group of nitrogen-containing heterocyclic compounds that differ in their chemical and physical properties depending on the type and location of the functional groups present [1,2].More than 100 different phenazine structural derivatives have been found in nature.Microbes inhabiting the extreme environments in the polar regions are attracting increasing attention in ecology and biotechnology.The polar regions are generally characterized by permanently low temperature,high pressure,high salinity,and extreme gradients of nutrients and light etc.To survive and propagate in such harsh environments,microbes have developed various strategies including producing novel bioactive compounds [3].Therefore,polar microorganisms are promising sources for novel natural products or chemical scaffolds with pharmaceutically relevant biological activity,considering their diversity and novelty.Previous studies have shown great potential of polar microorganisms in producing natural products with various bioactivities,including antimicrobial [4],anticancer[5],and immunosuppressive activities etc.[6] (as summarized in [7] and [8]).More recently,genome-based analyses have revealed biosynthetic potential of microbes from the polar regions,especially rare Actinobacteria groups(i.e.,non-StreptomycesActinobacteria) [9-11].Much greater biosynthetic potential has been indicated by genomic data mining than previous investigation based merely on traditional isolation.
Previously,strain 502F was isolated from Arctic deep sediments and showed antimicrobial activity [10].Primary analysis based on genome sequence indicated biosynthetic potential of various secondary metabolites.As part of our continuing efforts to explore the chemical diversity of polar microorganisms for drug discovery,strain 502F was selected for chemical investigative.Multiple-step chromatographic isolation of the crude extracts afforded two new phenazine alkaloids (1and2)and one new phenoxazine (3),as well as the previously reported compounds,N-(2-hydroxyphenyl)-2-phenazinamine (4) [4],endophenazine (5) [12],2-aminophenoxazin-3-one (6) [4],2-(N-methylamino)-3-phenoxazone(7) [4],N-acetylquestiomycin A (8) [13],2-aminobenzoic acid (9) [14],2-(acetylamino)-phenol (10) [15],3-(2-oxopropyl)-3-hydroxyind-olin-2-one (11) [16],indolyl-3-carboxylic acid (12) [17],indole-3-acetic acid (13) [18],and tryptophol (14) [19].Herein,details of the isolation,structural elucidation,and antibacterial,antiallergic,and cytotoxic activities of compounds (1-14) are described(Fig.1).
2 Results and discussion
Compound1was obtained as a yellow solid.The molecular formula C13H10N2O2was established upon analysis of the HRESIMS peak atm/z227.0815 [M+H]+,indicating 10 degrees of unsaturation.The UV absorptions at 233,274,371,and 433 nm implied the presence of an extended conjugated system.The1H and13C NMR spectroscopic data suggested the presence of one methoxy group,six methine,and six quaternary carbons.The1H NMR spectroscopic data of1(Table 1) revealed six aromatic protons at [δH7.67 (1H,d,J=9.4 Hz,H-3),7.93(1H,d,J=9.4 Hz,H-4),and atδH8.17 (1H,d,J=8.6 Hz,H-6),7.84 (1H,ddd,J=8.5,6.6,1.3 Hz,H-7),7.90 (1H,ddd,J=8.5,6.6,1.3 Hz,H-8),8.25 (1H,d,J=8.6 Hz,H-9)],which were assigned to be the typical AB and AA'BB' spin systems,respectively,with the aid of the COSY correlations of H-3/H-4 and H-6/H-7/H-8/H-9(Fig.2).These indicated that1had a 1,2-or 1,4-disubstituted phenazine moiety.In the HMBC spectrum(Fig.2),the key correlations of H-3 with C-1 and C-4a,and H-4 with C-2 and C-10a,confirmed that1was a 1,2-disubstituted phenazine.Combining the molecular formula and the NMR data above,the remaining one methoxy and one hydroxy were required in compound1.Dihydroxylation by the corresponding -OCH3and-OH groups in1,located at C-1 and C-2,respectively,were further supported by the HMBC correlations of H3-11 (δH4.14) with C-1 and chemical shift of C-2 (δC152.1) (Fig.2).Hence,the structure of1was identified as 1-methoxyphenazin-2-ol.
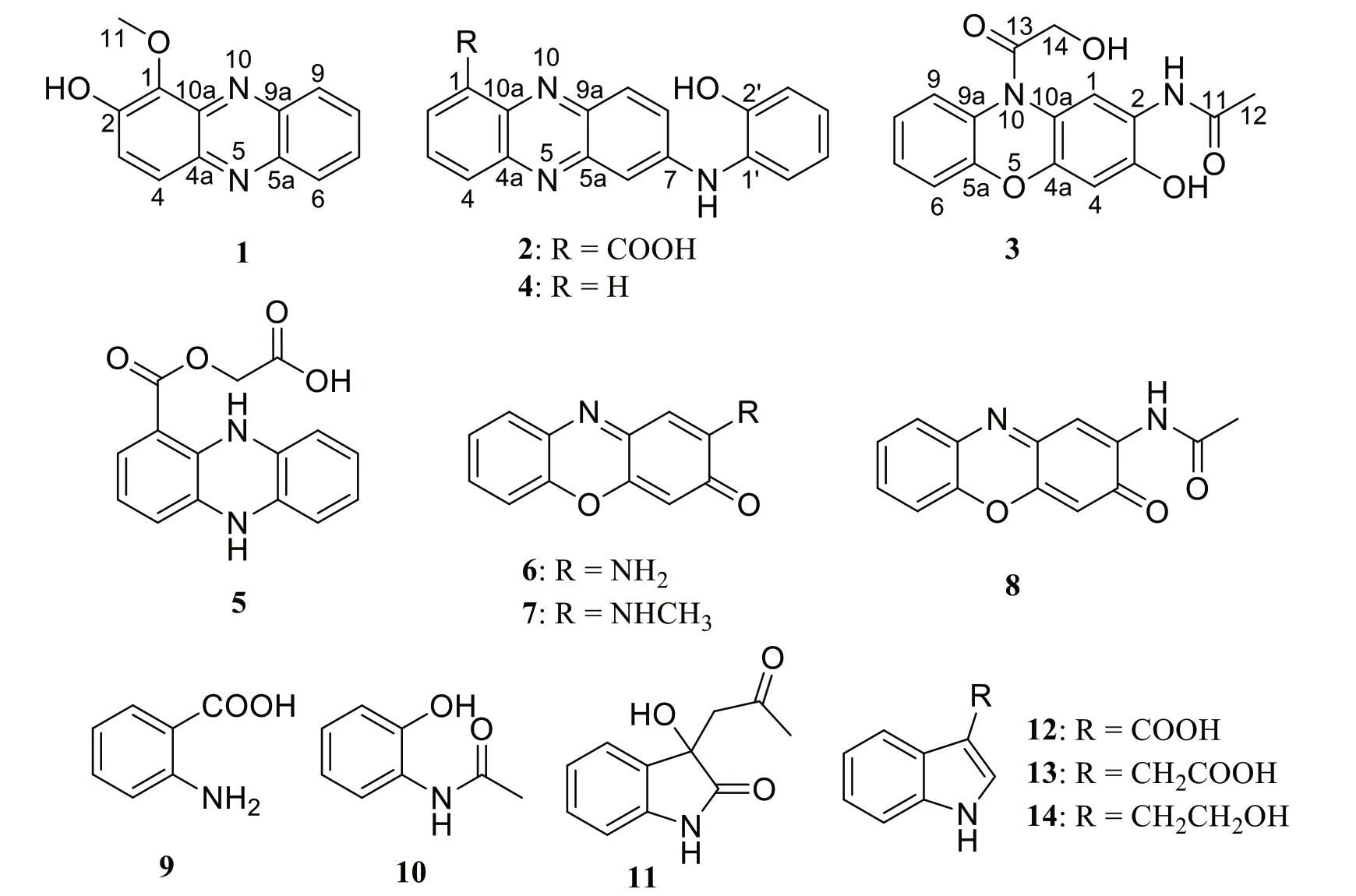
Fig. 1 Chemical structures of compounds 1-14
Compound2was isolated as dark purple powder with the molecular formula C19H13N3O3as determined by HRESIMS indicating 15 degrees of unsaturation.The1H NMR spectrum showed characteristic signals for one typical 1,2,3-substituted benzene system [δH8.61 (d,J=7.6 Hz,H-2),7.96 (dd,J=8.6,7.1 Hz,H-3),and 8.27(d,J=7.6 Hz,H-4)],one ABX spin system [δH7.21 (d,J=2.4 Hz,H-6),7.86 (dd,J=9.3,2.4 Hz,H-8),and 8.11(d,J=9.3 Hz,H-9)],and one AA’BB’ spin system [δH7.00(dd,J=8.1,1.4 Hz,H-3'),7.13 (td,J=8.1,1.5 Hz,H-4'),6.95 (td,J=7.6,1.4 Hz,H-5'),and 7.43 (dd,J=7.8,1.5 Hz,H-6')],respectively.The1H-1H COSY correlation of H-2/H-3/H-4,H-8/H-9,and H-3'/H-4'/H-5'/H-6' displayed the key spin systems.Careful comparison of1H and13C NMR data with those ofN-(2-hydroxyphenyl)-2-phenazinamine (4),revealed a high degree of similarity between them,except for the presence of carboxyl group(C-11) instead of the corresponding aromatic proton(H-1),which was confirmed by the key HMBC correlations from H-2 to C-4,C-10a,and C-11 (COOH) (Fig.2).To further define the structure of2,the13C NMR spectra of2-1(carboxylic group located at C-1) and2-2(carboxylic group located at C-4) were calculated using the gauge invariant atomic orbitals (GIAO) method at the B3LYP/6-31G(d,p)/PCM (Methanol) (Additional file 1:Tables S2-S4).Subsequent analyses of the calculated13C NMR values led to a deduction that2-1adopted the suitable structure with a DP4+probability of 100%,which allowed the assignment of the carboxylic group located at C-1 in compound2.Therefore,the structure of2was identified asN-(2-hydroxyphenyl)-2-phenazinamine-1-carboxylic acid.
Compound3was obtained as a yellow solid and had a molecular formula of C16H14N2O5as determined by HRESIMS (m/z315.0973 [M+H]+),requiring 11 degrees of unsaturation.The1H NMR spectrum (Table 1) and COSY correlations exhibited the resonances for eight aromatic protons,of which two singlets at [δH7.88 (1H,s,H-1) and 6.70 (1H,s,H-4)],one AA’BB’ spin system at[δH7.20 (dd,J=8.1,1.3 Hz,H-6),7.26 (td,J=8.3,1.4 Hz,H-7),7.17 (td,J=7.8,1.3 Hz,H-8),and 7.58 (dd,J=7.9,1.4 Hz,H-9)] were observed.These findings in association with13C NMR data (Table 1) identified 12 aromatic resonances consistent with the molecule containing two aromatic rings.The HMBC correlations from 2-NH (δH7.91) to C-1 (δC118.8),C-2 (δC121.8),and C-3 (δC147.3)allowed it to be positioned at C-2.Further HMBC correlations from 2-NH and H3-12 (δH2.08) to C-11 (δC168.8)allowed for an acetyl moiety to be located at the nitrogen.The chemical shift of C-3 (δC147.3) indicated oxygenation at this site.Besides,the exchangeable proton 3-OH(δH10.26) in turn possessed HMBC correlations to C-2,C-3,and C-4 (δC103.4) confirmed the -OH group to be located at C-3.The oxymethylene H2-14 (δH4.28) demonstrated a HMBC correlation to C-13 (δC171.1),allowing for a hydroxyacetyl moiety to be constructed.This part has no further relevance in any 2D NMR experiments,suggesting that it is attached to the molecule by heteroatoms.Due to the presence of 2-NH and 3-OH,there was only one position for the hydroxyacetyl fragment located at 10-N,which was also consistent with the degrees of unsaturation and molecular formula.Furthermore,this deduction was supported by the ROESY correlations of H2-14 (δH4.28) with H-1 (δH7.88) and H-9(δH7.58) (Fig.2).Consequently,the structure of3was determined to beN-(3-hydroxy-10-(2-hydroxyacetyl)-10H-phenoxazin-2-yl)acetamide.
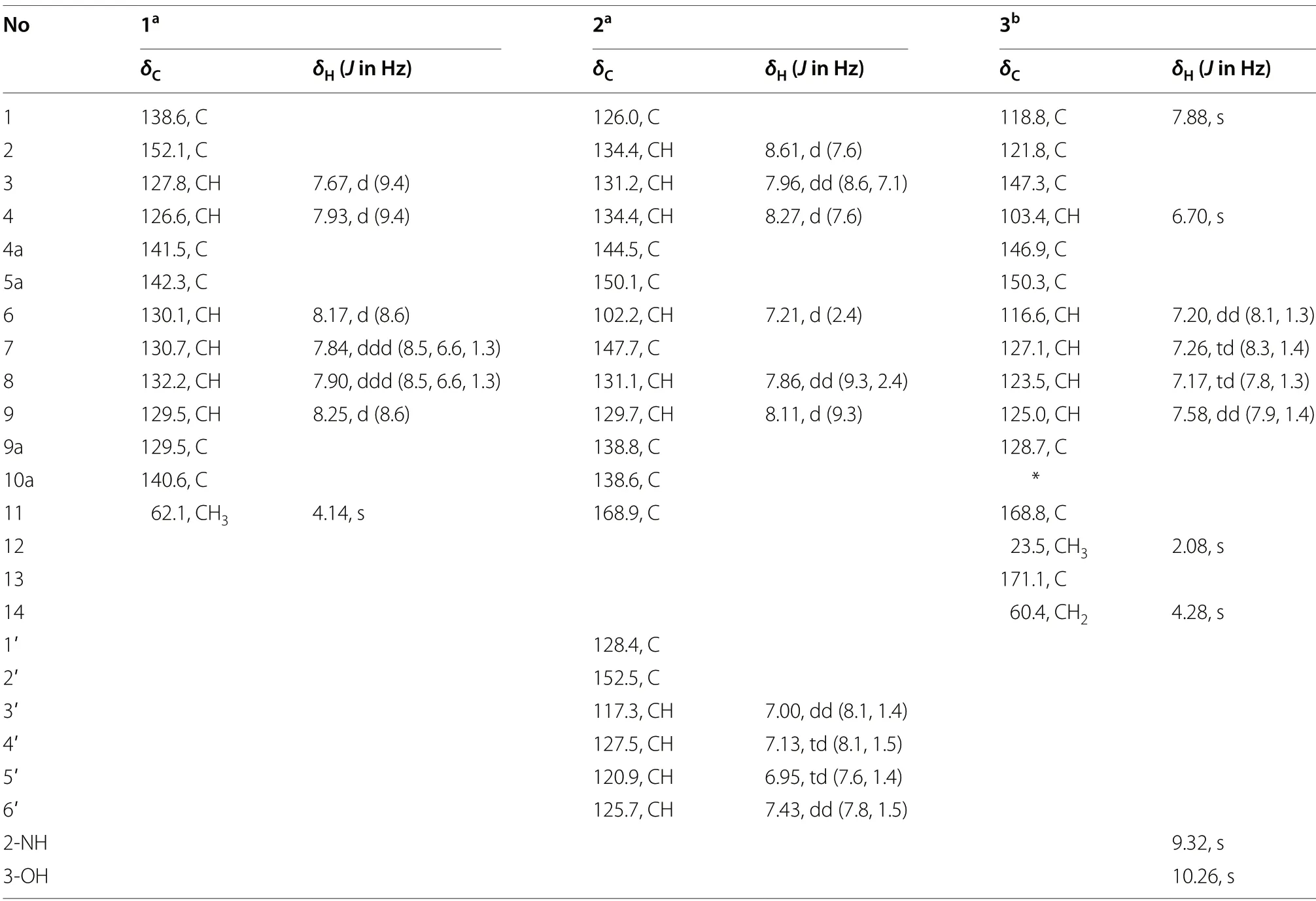
Table 1 1H and 13C NMR Data for 1-3 (700,175 MHz,TMS,δ ppm)
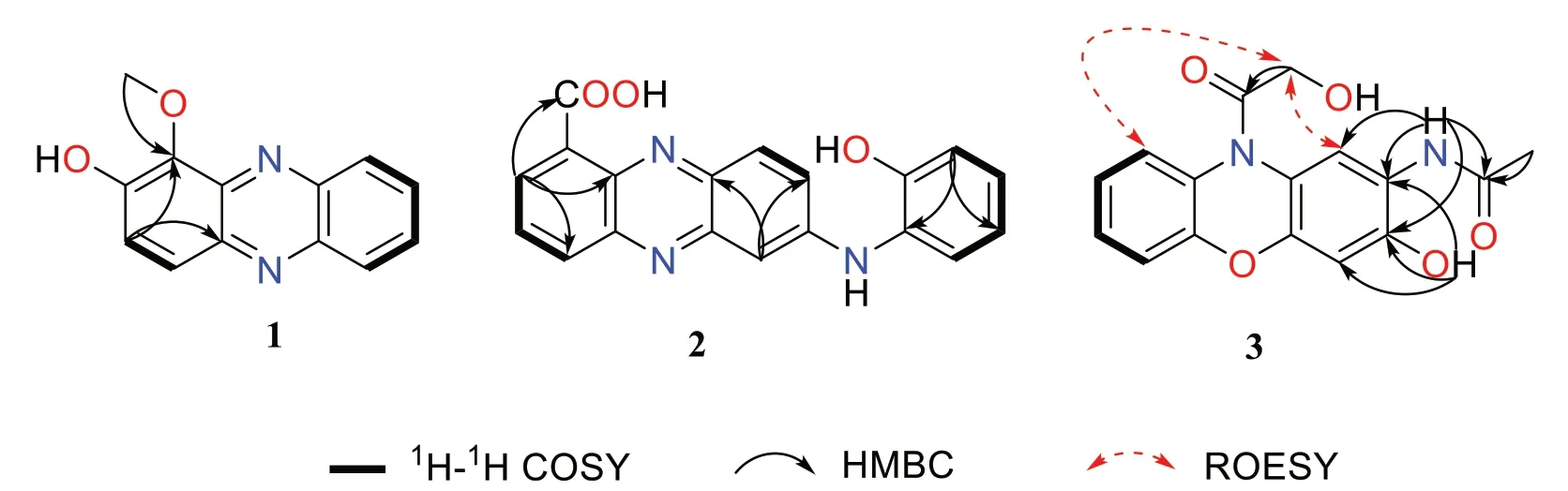
Fig.2 The key 1H-1H COSY,HMBC,and ROESY correlations of 1-3
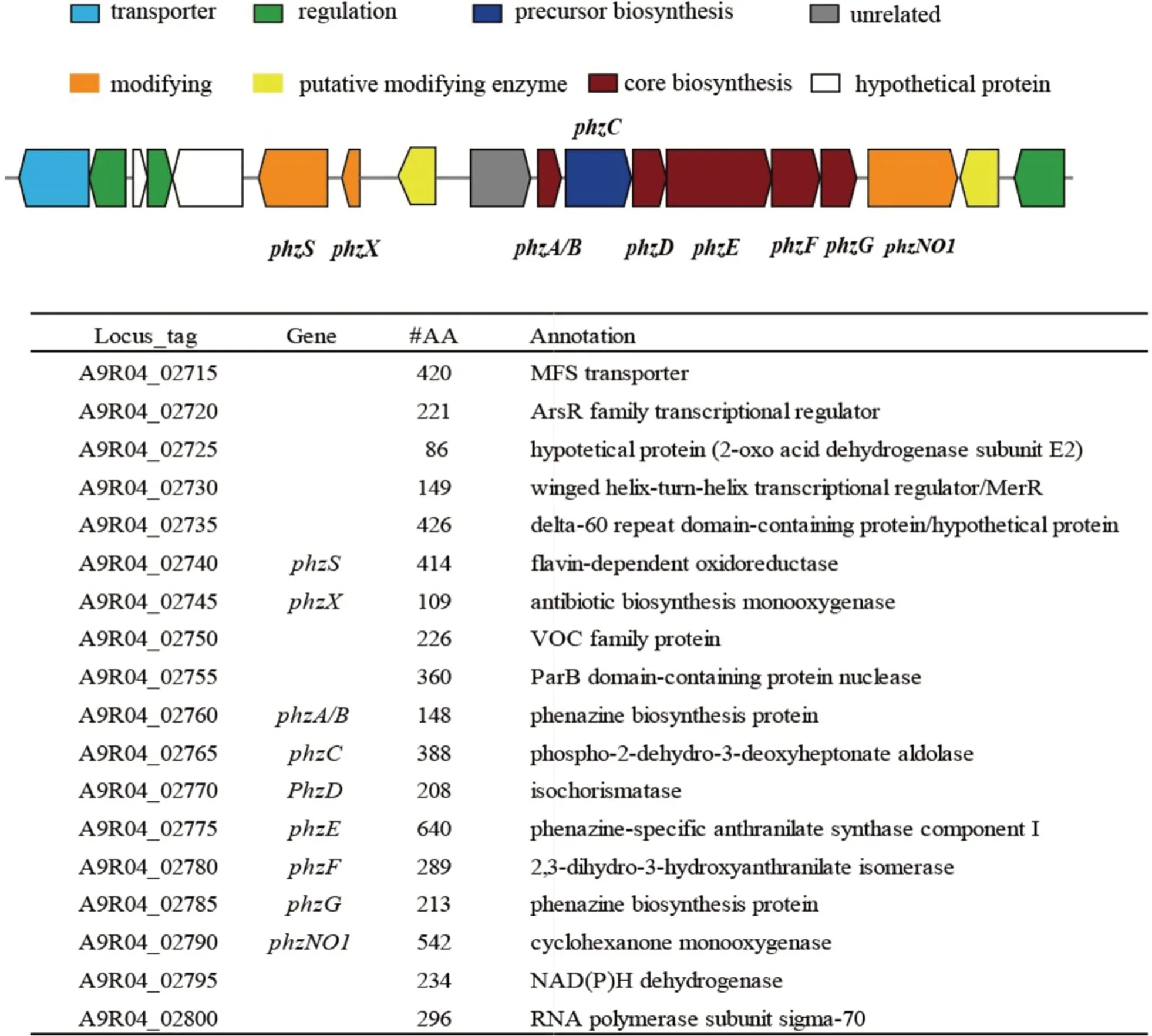
Fig. 3 Putative biosynthetic gene cluster of phenazines predicted in the genome of strain 502F
Phenazine and phenoxazine-based alkaloids are a large group of structurally unique natural products containing a tricyclic core consist of two nitrogen atoms or nitrogen and oxygen atoms,respectively.Since 1859,when Fordos reported the isolation of the blue pigment known as pyocyanin (5-N-methylphenazine-1-one),more than 100 different natural phenazines have been identified.Although phenoxazinone-type alkaloids are also widely distributed in nature,alkaloids with benzoxazine nuclei are relatively rare compared to phenazines.Furthermore,phenoxazinone-type compound3containing carbon substitution at N-10 is the third reported in nature.Naturally occurring phenazine and phenoxazine-based alkaloids have received much attention due to their interesting biological activities,including anti-bacterial,anti-cancer,anti-parasitic.
AntiSMASH analysis of the genome ofNocardiopsis dassonvillei502F revealed the presence of 16 putativebiosynthetic gene clusters (BGCs) (Additional file 1:Table S1).The BGCs contained quite diverse types,including phenazine,ectoine,PKS,NRPS,and small RPS etc.Most of the remaining BGCs shared quite low identities with known clusters,indicating their potential in producing new compounds.A putative phenazine biosynthetic gene cluster was predicted in the genome,containing the core biosynthetic genes (phzA/B,phzD,phzE,phzF,phzG and phzNO1),possible modifying genes(phzS,phzX,phzNO1,phzO and a VOC family proteinencoding gene),transporter and transcription regulation genes (Fig.3).The core biosynthetic genes were responsible for conversion of the precursor chorismate to phenazine-1-carboxylic acid (PCA) or phenazine-1,6-dicarboxylic acid (PDC).PCA or PDC are two precursors that can be modified further to create more complex phenazine derivatives,resulting in a serial of phenazines such as compounds1,2,4,and5.

Table 2 Antiallergic activities of compounds 1-8 (IC50,μM)
Most of the isolated compounds were evaluated for their antibacterial,antiallergic,and cytotoxic activities.Compounds (1-14) were evaluated in 96-well microtiter plates using a modification of the broth microdilution method.The indicator bacteria strains included three Gram-positive bacteria,methicillin-resistant Staphyloccocus aureus(MRSA),Staphyloccocus aureus(ATCC 29213),Enterococcus faecalis(ATCC 29212),respectively [20].However,no compounds showed potent antibacterial activities.Cytotoxic activities (C4-2B,MB-231,143B,MGC803,and A549 cells) were evaluated using the CCK-8 method as described previously [21].Among them,compounds4and5exhibited potent in vitro cytotoxic activities against osteosarcoma cell line 143B with IC50values 0.16 and 20.0 μM,respectively.The antiallergic activity was measured for the efficiency of the RBL-2H3 cell degranulation inhibition rate using an IgE-mediated mast cell allergic reaction [22].The results of antiallergic activity of compounds6-8showed obvious inhibition rates (86.35 ± 3.11%,38.02 ± 3.98%,and 92.84 ± 9.37% inhibition at the concentration of 20 μg/mL) with IC50values of 10.88 ± 3.05,38.88 ± 3.29,and 2.44 ± 0.17 μg/mL (Table 2),respectively,while loratadine was used as positive control with IC50value of 91.6 μM.
Supplementary Information
The online version contains supplementary material available at https:// doi.org/ 10.1007/ s13659-023-00408-w.
Additional file 1.Supplementary data.
Acknowledgements
This research was supported by the Guangdong Basic and Applied Basic Research Foundation (2021A1515011523,2021B1515120046,2022B1515120075),National Key Research and Development Program of China (2022YFC2807501),National Natural Science Foundation of China (grant no.41976224),Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City (2021JJLH0097,2021CXLH0013),Hainan Provincial Natural Science Foundation of China (823CXTD393).We thank ZH Xiao,AJ Sun,XH Zheng,X Ma,and Y Zhang in the analytical facility at SCSIO for recording spectroscopic data.
Author contributions
All authors read and approved the final manuscript.
Data availability
All relevant data are within the manuscript and its Additional files.
Declarations
Competing interests
The authors declare no conflict of interest.
Author details
1CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology/Guangdong Key Laboratory of Marine,Materia Medica/Innovation Academy of South China Sea Ecology and Environmental Engineering,South China Sea Institute of Oceanology,Chinese Academy of Sciences,Guangzhou 510301,China.2Key Laboratory for Polar Science,MNR,Polar Research Institute of China,Shanghai 200136,China.3Department of Pharmacology and Therapeutics,McGill University,Montreal H3A 0G4,Canada.4University of Chinese Academy of Sciences,19 Yuquan Road,Beijing 100049,China.5Sanya Institute of Marine Ecology and Engineering,Sanya 572000,China.6School of Oceanography,Shanghai Jiao Tong University,Shanghai 200240,China.
Received:30 July 2023
Accepted:11 October 2023
杂志排行
Natural Products and Bioprospecting的其它文章
- Quinones from Cordia species from 1972 to 2023: isolation,structural diversity and pharmacological activities
- Ginsenoside compound-K attenuates OVX-induced osteoporosis via the suppression of RANKL-induced osteoclastogenesis and oxidative stress
- The alkynyl-containing compounds from mushrooms and their biological activities
- A recent update on development,synthesis methods,properties and application of natural products derived carbon dots
- Natural product rhynchophylline prevents stress-induced hair graying by preserving melanocyte stem cells via the β2 adrenergic pathway suppression
- Kaemtakols A-D,highly oxidized pimarane diterpenoids with potent anti-inflammatory activity from Kaempferia takensis
