Synergistic impacts of rifampicin and doxorubicin against thioacetamide-induced hepatocellular carcinoma in rats☆
2023-12-29ZhrElshhwyEntsrSdRnElSdd
Zhr R.Elshhwy ,Entsr A.Sd ,Rn R.El-Sdd
a Chemistry Department, Faculty of Science, Damietta University, Damietta, Egypt
b Gastroenterology Surgical Center, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Keywords: Hepatocellular carcinoma (HCC)Rifampicin Doxorubicin Bcl-2-associated X protein (Bax)Caspase Protein 53 (p53)Thioacetamide
ABSTRACT Background and aims: Combination therapy is a promising new strategy that has been proposed to increase the efficacy of cancer treatment.We aimed to investigate the anti-cancer activity of rifampicin monotherapy and its combination with doxorubicin against hepatocellular carcinoma (HCC).Materials and methods: The in vitro half maximal inhibitory concentration(IC50)and selectivity index(SI)of the drugs under investigation against HepG2 and human lung fibroblast (WI38) cell lines were determined.For the in vivo experiment,male Sprague-Dawley albino rats were injected with thioacetamide at 200 mg/kg twice a week for 90 days;HCC development was confirmed histopathologically.Following HCC induction,the rats were treated with intraperitoneal doxorubicin,rifampicin,or their combination for 45 or 90 days.After sacrifice,the livers were examined histopathologically.The levels of aminotransferases,albumin,bilirubin,malondialdehyde,superoxide dismutase (SOD),catalase (CAT),total antioxidant capacity (TAC),and nitric oxide were measured by spectrophotometry.Alphafetoprotein,cancer antigen 19-9,tumor necrosis factor-alpha,interleukin-6,Bcl-2-associated X protein,caspase 3,caspase 8,and p53 were estimated using ELISA.Results: In vitro,the combination of doxorubicin and rifampicin showed the highest SI of 3.43. In vivo,among the measured markers,the levels of TAC,CAT,SOD,and p53 decreased(P <0.001)and the rest of the measured marker levels increased (P <0.001) in the HCC-bearing rats;after treatment in all groups,all these changes improved toward normal in a time-dependent manner.The combination of doxorubicin and rifampicin optimized the effects of the two individual drugs and exerted the best antioxidant effects.Conclusions: In general,compared with rifampicin or doxorubicin alone,combination therapy has favorable outcomes.Based on our results,the combination of rifampicin and doxorubicin might be applicable for HCC chemotherapy.
1.Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancerrelated death worldwide.Among the types of liver cancers,HCC is the most prevalent at nearly 90%.1Among all cancers,HCC is the sixth most common,2the third most fatal,2,3and was the cause of 7.69% of global cancer-related deaths in 2020.4Hepatic cirrhosis,which is tightly connected to hepatitis B or C virus infection,is the leading risk factor of HCC,5and cirrhosis secondary to infection with both viruses poses a greater risk.6In developing countries,including Egypt,and in persons with low socioeconomic status,the HCC incidence is relatively high.7HCC is usually diagnosed at a late stage,thereby,precluding successful curative treatments,which include surgery,liver transplantation,and local ablation for the early stage and chemoembolization for the intermediate stage.In cases with late-stage HCC,systemic therapy is the only choice.8With the few availability of drugs for the treatment of HCC,efforts have been made to develop and approve numerous novel HCC drugs,such as multikinase inhibitors,immune checkpoint inhibitors,and combination therapies.9
Doxorubicin is an antibiotic and antineoplastic drug.Its mode of action depends on the intercalation of DNA and may affect gene expression and yield oxidative damage to biomolecules.On a large scale,doxorubicin had been applied systemically to treat many solid tumors,including HCC.10,11However,chemotherapy with doxorubicin for HCC has restricted efficacy and can lead to intolerable side effects,such as septicemia,neuropathy,nephrotoxicity,and cardiotoxicity.Moreover,drug resistance had been primarily responsible for the failure of chemotherapy for HCC.12-14
Rifampicin is a broad-spectrum semisynthetic antibiotic.15It is clinically used for difficult-to-treat bacterial infections,predominantly tuberculosis and leprosy,16and to prevent pruritus in chronic cholestatic liver disease.17Its antibacterial outcome depends on arresting transcription.16Moreover,it has powerful antiangiogenic effects that inhibit cancer progressionin vitro,as shown by its capability to prevent the growth of 39 varying human cancer cell lines in an experimental study.17Recently,rifampicin at a dose of 0.107 mg/kg was found to decrease the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and to improve the antioxidant defense in Ehrlich ascites carcinomain vivo.15Indeed,rifampicin is a promising anticarcinogenic agent.
Combination therapy is another new promising strategy that has been proposed to increase treatment efficacy,avoid drug resistance,and reduce treatment duration.18In fact,it has emerged as a significant advancement in cancer therapy.19However,combination therapy for HCC remains to be constrained by disease recurrence in many cases.To overcome the obstacles that face the currently used drugs for HCC,more studies on new combination therapies are required.This work was designed to study thein vitroandin vivoactivities of rifampicin and its combination with doxorubicin against HCC.
2.Materials and methods
2.1. Ethical approval
All animal experiments complied with the Animal Research:Reporting of In Vivo Experiments (ARRIVE) guidelines and were carried out following the Guide for the Care and Use of Laboratory Animals,published by the National Institutes of Health (Bethesda,Maryland,USA),and all protocols were approved by the Animal House of Biochemistry,Chemistry Department,Faculty of Science,Damietta University,Damietta,Egypt(Department Board No.203).
2.2. Cell lines and chemicals
Human lung fibroblast(WI-38)and HCC cell lines(HepG2)were purchased from the holding company for biological products and vaccines (VACSERA Co.,Cairo,Egypt).The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),Roswell Park Memorial Institute-1640 (RPMI-1640) medium,and dimethyl sulfoxide (DMSO) were obtained from Sigma,Street Louis,MO,USA.Fetal bovine serum was obtained from GIBCO,UK.
2.3. MTT assay
WI-38 and HepG2 were used to test the inhibitory effects of the drugs on cell growth using the MTT assay,as described previously by Elsayedet al.20The cells were cultured in a 96-well plate at a density of 1.0 × 104cells/well using RPMI-1640 medium with 10%fetal bovine serum,100 units/mL penicillin,and 100 μg/mL streptomycin at 37°C in a 5%CO2incubator for 48 h.Next,the cells were treated with various concentrations of the inhibitors (the drugs)and incubated again for 24 h.Twenty microlitre of MTT solution(5 mg/mL) was added and incubated for 4 h.One hundred microlitre of DMSO was added to every well,then the absorbance(A)was read at 570 nm,and the percent relative cell viability [(Atreatedsample/Auntreatedsample) × 100] was calculated.
2.4. In vivo study
2.4.1.Animals
Male Sprague-Dawley albino rats(weight:200 g and age:8-10 weeks) were received from the National Research Center of Cairo,Egypt and housed randomly at five rats per cage.Accordingly,the rats were kept warm at 23°C±2°C,with controlled humidity and a 12 h light/dark cycle.The rats were carefully observed daily;their body weights were recorded and food and water intakes were accurately measured each week to assess for any signs of toxicity or abnormality throughout the experiment.
2.4.2.Induction of HCC
The carcinogenic material thioacetamide was intraperitoneally injected into rats at 200 mg/kg twice a week.21After 45 and 90 days of administration,some liver samples were examined pathologically to follow the progression of HCC induction.The formation of HCC was ascertained in the samples that were taken from the rats administered with thioacetamide for 90 days.
2.4.3.Animal groups
A total of 60 rats were randomly divided into five sets(n=12 for each),as follows:
Normal:Healthy rats without any treatment(negative control);
HCC: Untreated HCC rats that were intraperitoneally injected with thioacetamide at 200 mg/kg twice a week for 90 days(positive control);
HCC+D: HCC rats injected with doxorubicin at a dose of 0.72 mg each,which is equivalent to a human dose of 20 mg/m2,according to Barnes and Paget,22once a week until the end of the experiment;
HCC+R: HCC rats that were intraperitoneally injected with rifampicin at a dose of 0.107 mg/kg/day until the end of the experiment;
HCC+D+R:HCC rats that were intraperitoneally injected with doxorubicin and rifampicin at the respective aforementioned doses until the end of the experiment.
On day 91 of HCC induction,all drug therapies were begun.To study the effect of drug treatment duration in each group,four rats were sacrificed on day 45 (short-term),and the remaining eight rats were sacrificed on day 90(long-term).Blood samples from the retroorbital bleeding plexus and liver samples were collected from each sacrificed rat under general anesthesia with ketamine and xylazine.The freshly excised livers were washed with saline,dried carefully,weighed,photographed,and fixed in 10% formalin until use for the histopathology study.The blood samples were left to clot and centrifuged for collection of serum samples,which were kept at -20°C until use for the biochemical assessments.
2.4.4.Histopathology
The routinely fixed liver tissues in formalin were processed,dipped in paraffin wax,cut into 5-μm-thick slices,and stained using hematoxylin and eosin(H&E).At least three different sections were examined per liver sample.
2.4.5.Biochemical assessments
Albumin,AST,and ALT levels were determined using kits (Biomerieux,Durham,USA).Total bilirubin,alpha-fetoprotein (AFP),and cancer antigen 19-9 (CA 19-9) concentrations were estimated using diagnostic kits (Roche,Mannheim,Germany).The levels of malondialdehyde (MDA),superoxide dismutase (SOD),catalase(CAT),total antioxidant capacity (TAC),and nitric oxide (NO) were determined using kits (BIODIAGNOSTIC,Giza,Egypt).Enzymelinked immunosorbent assay kits were used for immunological measurements of interleukin-6 (IL-6),Bcl-2-associated X protein(Bax),and caspase 3 (CASP3) levels (Cloud-crone Corp.,TX,USA);tumor necrosis factor alpha(TNFα) (Rat TNFα,Abbexa,Cambridge,UK);caspase 8 (CASP8) (Rat CASP8 quantitative detection,Novus Biological,USA);and p53 (Cusabio kit,Huston,TX,USA).
2.5. Statistical analysis
The data were expressed as mean ± standard error (SE) and analyzed using Statistical Package for the Social Sciences version 17(SPSS Software,SPSS Inc.,Chicago,USA).One-way analysis of variance followed by the Bonferroni multiple comparisons test was used for data with Gaussian distribution.The Kruskal-Wallis test followed by the Dunnett’s test for multiple comparisons was used for data with non-Gaussian distribution.The differences were considered significant atP<0.05.
3.Results
3.1. In vitro MTT cytotoxicity and selectivity index (SI)
As shown in Fig.1,doxorubicin monotherapy exhibited very strong cytotoxicity against HepG2 (half maximal inhibitory concentration (IC50)=8.35 ± 0.70 μg/mL) and stronger cytotoxicity against WI38(IC50=6.84± 0.50 μg/mL).Rifampicin monotherapy showed moderate cytotoxicity against HepG2(IC50=25.25 ± 1.90 μg/mL) and weak cytotoxicity against WI38(IC50=57.90 ± 3.50 μg/mL).The combination of doxorubicin and rifampicin displayed potent cytotoxicity against HepG2(IC50=15.33 ± 1.30 μg/mL) and weak cytotoxicity against WI38(IC50=52.55 ± 3.30 μg/mL).The SI was the highest with the combination of doxorubicin and rifampicin,followed by rifampicin and doxorubicin (3.43,2.29,and 0.82,respectively).
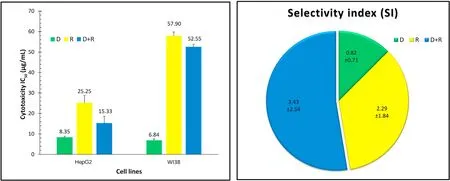
Fig.1.The IC50 and SI of the study drugs. The SI is calculated as follows: IC50 (WI38)/IC50 (HepG2).For IC50,1-10 is very strong,11-20 is strong,21-50 is moderate,51-100 is weak,and >100 μg/mL is noncytotoxic.Abbreviations:D,doxorubicin;HepG2,human hepatocellular carcinoma cell line;IC50,half maximal inhibitory concentration;R,rifampicin;SI,selectivity index;WI38,human lung fibroblast cell line.
3.2. Changes in the liver surface
On gross examination,the untreated HCC rat liver was hard and showed an atypical surface (i.e.,many small nodules and granulated appearance)and faint color,compared with the normal liver.After HCC drug treatment for 45 days,the smooth surface of the liver was restored.Among the treatment groups,the color of the liver treated with rifampicin was the closest to normal.After drug treatment for 90 days,the liver surface remained to have few abnormalities (i.e.,tiny nodules with less coarse appearance) with doxorubicin monotherapy but was normally smooth with rifampicin monotherapy or combination therapy (Fig.2).
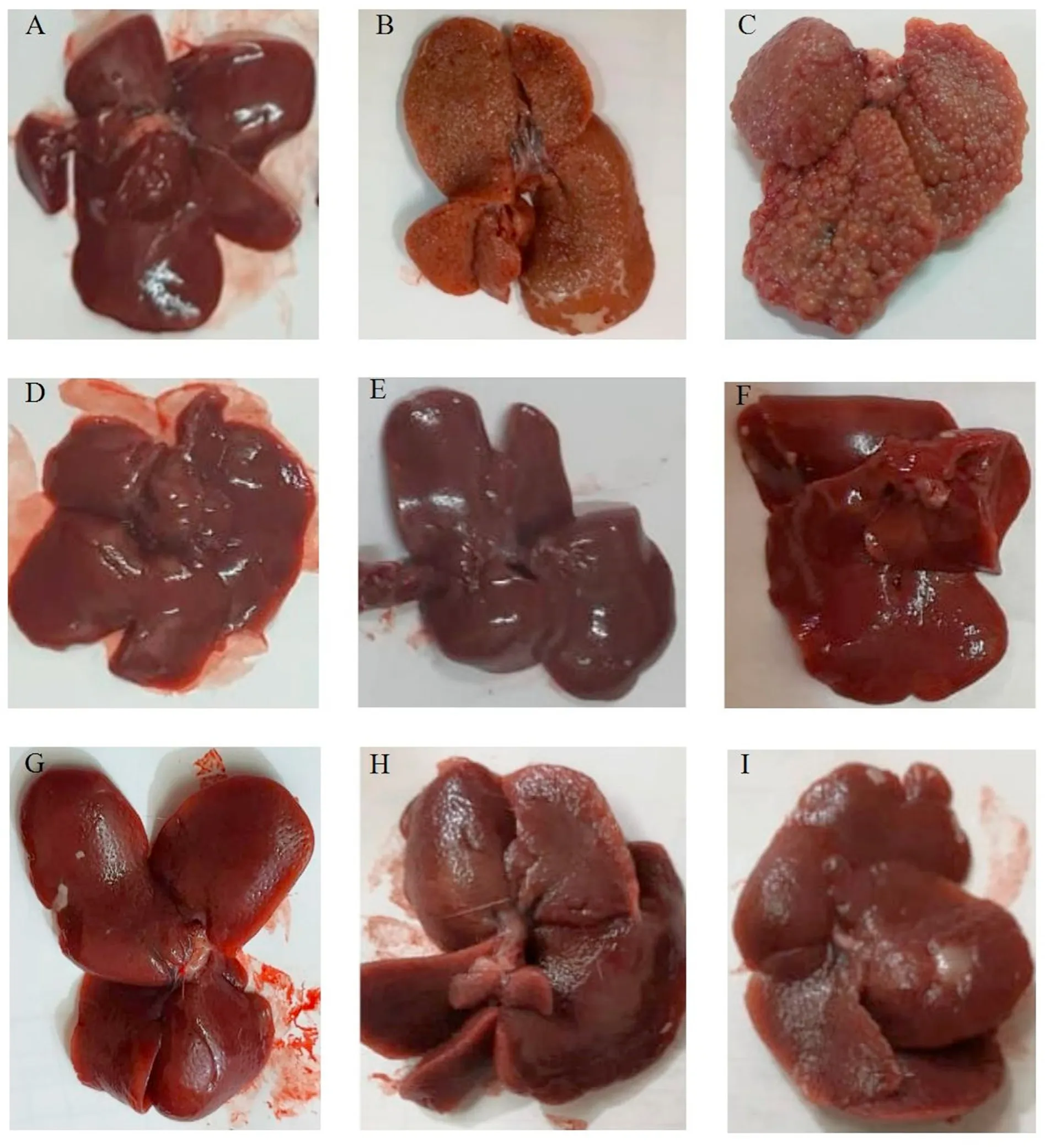
Fig.2.Representative images of the excised livers from rats before and after treatment with the different compounds.(A)represents normal liver.(B,C)represent untreated HCC liver after 45 days and 90 days,respectively.(D,G)represent HCC liver treated with doxorubicin for 45 days and 90 days,respectively.(E,H)represent HCC liver treated with rifampicin for 45 days and 90 days,respectively. (F,I) represent HCC liver treated with doxorubicin+rifampicin for 45 days and 90 days,respectively.Abbreviation: HCC,hepatocellular carcinoma.
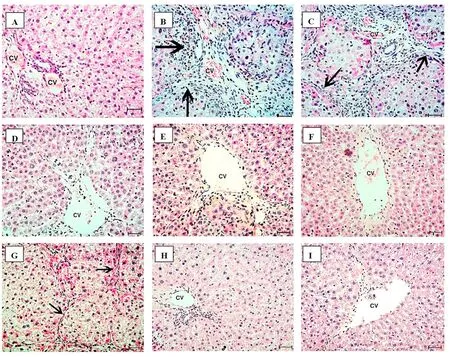
Fig.3.Histopathology of the excised livers stained with H&E.(A)The normal control liver of rats shows normal structures of the central vein,hepatocytes,and sinusoids.(B)In the HCC liver at 45 days,there are cellular infiltrates (++),masses,disruption of hepatocyte organization with pleomorphism and pseudoacini separated by fibrous trabeculae(arrows).The individual hepatocytes show nonuniform eosinophilia in the cytoplasm with high N/C ratio. (C) In the HCC liver at 90 days,there are fibrosis,masses,and more disturbed cell organization and denser trabeculae(arrow)and neovascularization.(D,G)Representative images of HCC treated with doxorubicin at 45 days(necrosis+)and 90 days(persistent signs of cancer (arrow) are predominant),respectively. (E,H) Representative images of HCC treated with rifampicin at 45 days (regenerative nodules ++and fibrosis)and 90 days(signs of carcinogenicity are less clear,with loss of fibrous trabeculations),respectively.(F,I)Representative images of HCC treated with doxorubicin+rifampicin at 45 days and 90 days,respectively;near-normal changes without obvious signs of cancer are predominant.The bars represent 50 μm.Abbreviations: (+),mild;(++),moderate;CV,central vein;HCC,hepatocellular carcinoma;N/C,nucleus/cytoplasm.
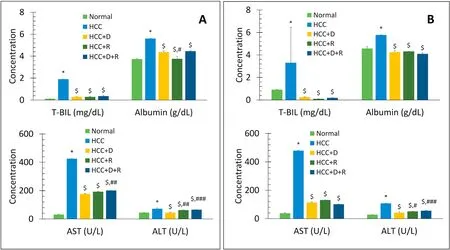
Fig.4.Comparison of mean serum levels of T-BIL(mg/dL),albumin(g/dL),AST(U/L),and ALT(U/L)between different groups at short-and long-term treatment(45 days(A)and 90 days(B)).Data are expressed as mean±SE.n=4 for 45 days,n=8 for 90 days.*P <0.001 vs.Normal group.$P <0.001 vs.HCC group.#P <0.05,##P <0.01,###P <0.001,respectively vs.HCC+D group.Abbreviations: ALT,alanine aminotransferase;AST,aspartate aminotransferase;D,doxorubicin;HCC,hepatocellular carcinoma;R,rifampicin;SE,standard error;T-BIL,total-bilirubin.
3.3. Liver histopathology
Figure 3 shows the changes in the liver tissues throughout the experiment.Compared with the healthy liver tissue sections,the liver section on day 45 after thioacetamide injection showed disruption of hepatocyte organization,pleomorphism,and formation of pseudoacini separated by fibrous trabeculae.On day 90,more disturbed organization with denser trabeculae and neovascularization was seen.Compared with the untreated HCC liver,the signs of carcinogenicity in the HCC liver tissue sections after 45 and 90 days of treatment showed some persistence in the doxorubicin group but were fewer in the rifampicin group.Conversely,the HCC liver tissues treated with the combination of rifampicin and doxorubicin had near-normal architectural appearance with no recognizable signs of cancer.
3.4. Markers of liver function, tumor activity, inflammation,apoptosis, and antioxidant activity
After 45 and 90 days of treatment with thioacetamide in the HCC model,there were significant time-dependent increases in the ALT,AST,albumin,bilirubin,AFP,CA 19-9,IL-6,and TNFα levels(P<0.001vs.normal rats).In all the treatment groups,there were significant time-dependent decreases in the levels of ALT,AST,albumin,bilirubin,IL-6,and TNFα (P<0.001vs.HCC rats),and the tumor markers AFP and CA 19-9 returned to normal levels (Figs.4 and 5).Rifampicin and not doxorubicin showed the best antiinflammatory effects (Fig.5).
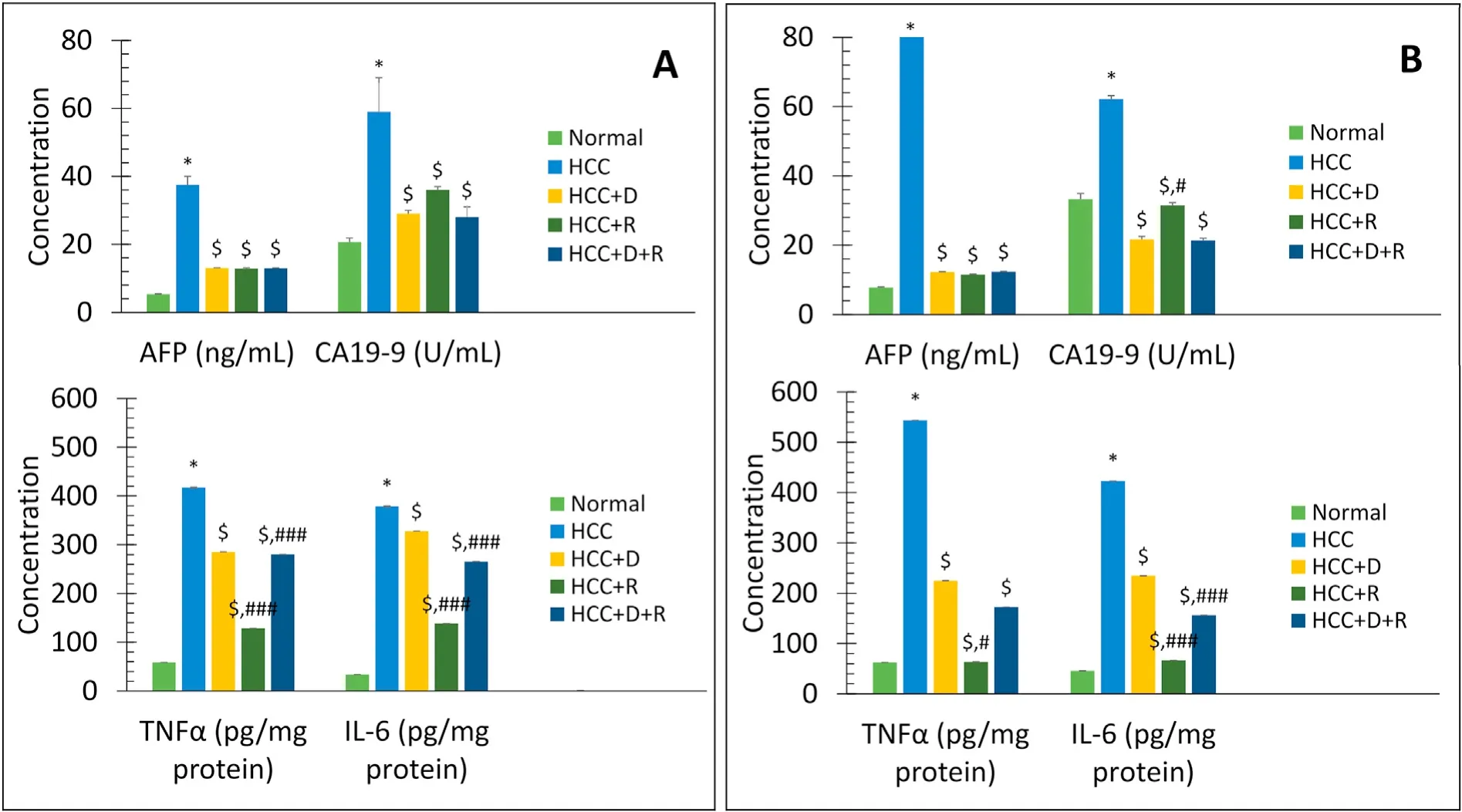
Fig.5.Comparison of mean serum levels of tumor markers AFP (ng/mL) and CA19-9 (U/mL),and inflammation markers IL-6 (pg/mg protein) and TNFα (pg/mg protein)between different groups at short-and long-term treatment(45 days(A)and 90 days(B)).Data are expressed as mean±SE.n=4 for 45 days,n=8 for 90 days.*P <0.001 vs.Normal group.$P <0.001 vs.HCC group.#P <0.05,##P <0.01,###P <0.001,respectively vs.HCC+D group.Abbreviations:AFP,alpha-fetoprotein;CA19-9,cancer antigen 19-9;D,doxorubicin;HCC,hepatocellular carcinoma;IL-6,interleukin 6;R,rifampicin;SE,standard error;TNFα,tumor necrosis factor-alpha.
For the antioxidant study,the levels of MDA and NO significantly increased in a time-dependent manner(P<0.001),but the levels of TAC,CAT,and SOD significantly decreased (P<0.001) in the HCC model,when compared with the levels in the normal rats.In all treatment groups,all these markers significantly and timedependently returned to normal.Although the antioxidant effect was better with doxorubicin than with rifampicin,it was the most potent with the combination therapy (Fig.6).
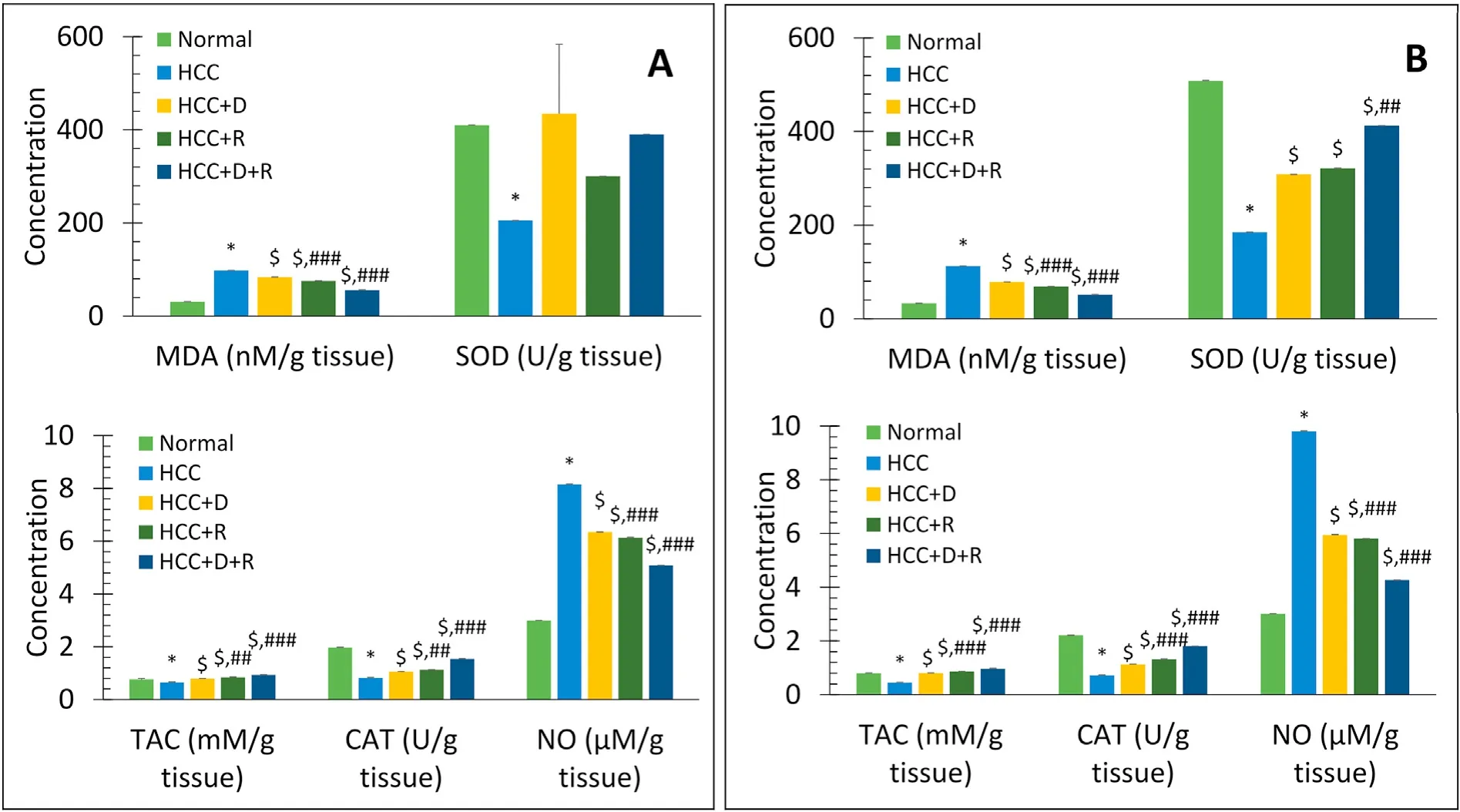
Fig.6.Comparison of mean serum levels of MDA(nM/g tissue),SOD activity(U/g tissue),TAC(mM/g tissue),CAT(U/g tissue),and NO(μM/g tissue)between different groups at short-and long-term treatment(45 days(A)and 90 days(B)).Data are expressed as mean±SE.n=4 for 45 days,n=8 for 90 days.*P <0.001 vs.Normal group.$P <0.001 vs.HCC group.##P <0.01,###P <0.001,respectively vs.HCC+D group.Abbreviations:CAT,catalase;D,doxorubicin;HCC,hepatocellular carcinoma;M,mol;MDA,malondialdehyde;NO,nitric oxide;R,rifampicin;SE,standard error;SOD,superoxide dismutase;TAC,total antioxidant capacity.
Compared with the normal rats,the HCC rats showed significant time-dependent increases in the CASP3,CASP8,and Bax levels and decrease in the p53 level(P<0.001).In all treatment groups,these changes were reversed in a time-dependent manner.Rifampicin and not doxorubicin showed the best effects (Fig.7).
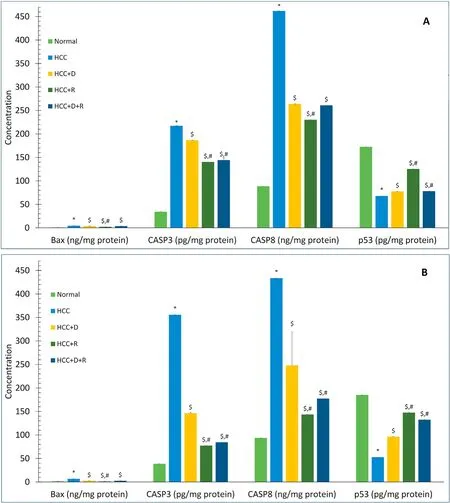
Fig.7.Comparison of mean serum levels of Bax(ng/mg protein),CASP3(pg/mg protein),CASP8(ng/mg protein),and p53(pg/mg protein)between different groups at shortand long-term treatment (45 days (A) and 90 days(B)). Data are expressed as mean ± SE. n=4 for 45 days, n=8 for 90 days.*P <0.001 vs.Normal group.$P <0.001 vs.HCC group.#P <0.001 vs.HCC+D group.Abbreviations: Bax,Bcl-2-associated X protein;D,doxorubicin;HCC,hepatocellular carcinoma;p53,protein 53;R,rifampicin;SE,standard error.
4.Discussion
In this study,although doxorubicin exhibited cytotoxic effects against cancer cells,it revealed more powerful cytotoxicity to normal cells.Compared with doxorubicin,rifampicin displayed cytotoxic activity that was lower but effective against cancer cells and much weaker against normal cells.Therefore,rifampicin seemed to be more advantageous than doxorubicin,but the combination of the drugs was preferable because of the two advantages of potent cytotoxicity against cancer cells and being safe on normal cells.Based on our results,the selectivity for cancer cells and safety for healthy cells were the best with the combination of doxorubicin and rifampicin than with either drug alone.Our findings were comparable with the study of Badmuset al.,23who reported low SI values of doxorubicin for MCF-7 (0.57),HeLa (1.31),and HT-29(0.27) cells.
In the current study,the HCC-bearing rats showed many-fold increases in the tumor markers AFP and CA 19-9,alongside severe alterations in the liver function tests.These levels were worse on day 90 than on day 45,indicating worsening of the liver status and extensive progression of HCC over time.The increase in the levels of ALT and AST is the main biochemical derangement and may be accounted for by cellular infiltration,which we found in this study.24The AST increased by more than four times than ALT;the increased mitochondrial expression and low clearance rate of AST in advanced hepatic damage may be responsible for this result.25,26The high bilirubin levels could be secondary to biliary obstruction by cancer,impaired liver function from extensive cancer cell infiltration,or shortened erythrocyte lifespan from the oxidative damage to the cell membrane and the excessive free radicals in the blood.27,28The latter was confirmed in this study by the more than three-fold increase in the serum MDA and NO levels in the HCC rats on day 90.The low levels of the antioxidant markers TAC,SOD,and CAT were the expected effects of excessive consumption to cope with the excessive production of free radicals.29If not appropriately neutralized,the free radicals can kill cells.They can prompt the peroxidation of lipids,deactivate enzymatic antioxidants,expedite DNA damage,promote carcinogenesis,and dysregulate the expression of cancerpromoting genes.20A high albumin level in HCC (>4.8 g/dL) may be attributed to increased production by cancerous hepatocytes,impaired hepatocellular osmoreceptivity leading to failure in the negative feedback,30or HCC-related impairment of renal function.Our observations were similar to the findings of other authors,5,31,32who reported hepatic functional disturbances as a result of tumor growth and advancement.Our histopathological study provided evidence to support the time-dependent progressive deterioration of the architecture of the liver.Grossly,the HCC liver appeared pale in color and grainy with small nodules on day 45 and had more faint color,coarse surface,and many small nodules on day 90.On histopathologic examination,the HCC liver section revealed disruption in the organization of cells,pleomorphism,and formation of pseudoacini separated by fibrous trabeculae on day 45 and a more severe disturbance in the organization of cells with denser trabeculae and neovascularization on day 90.
Moreover,the more than nine-fold increase in IL-6 and more than eight-fold increase in TNFα in the untreated HCC group provided strong evidence of the inflammatory response to thioacetamide.Similar to the results of Carnovaleet al.,33our results suggested that administration of thioacetamide led to loss of liver mass,which triggered an inflammatory response to remove tissue debris,followed by a regenerative response that required release of more NO.This may explain the elevated NO level seen in our HCCbearing group.However,an increase in the NO level to >100%may shift the NO effects on liver tissue from positive (i.e.,liver tissue regeneration)to negative(i.e.,destructive consequences,including apoptosis of hepatocytes).33This may have been the situation in the current study,because the NO level was elevated to >300%.Therefore,in this study,the role of NO was likely proapoptotic.The NO overproduction can result in Fas or tumor necrosis factor receptor-1 stimulation to bind FasL or TNFα,respectively,which activates CASP8.34CASP8 activates CASP3 or cuts Bid to truncated Bid,which enters the mitochondria and favors the release of cytochromec(Cytc).Together with CASP9 and other factors,Cytc forms apoptosomes and activates CASP3,thereby,initiating the apoptosis events.35In addition,the high level of reactive nitrogen species may increase the Bax levels and favor Cytc release.Furthermore,peroxynitrite may activate membrane transition pores and initiate Cytc release.33
In the current study,we expected a high rate of hepatocyte death,which was confirmed by the time-dependent overproduction of the apoptotic enzymes CASP8 and CASP3 and the proapoptotic Bax.These indicated hastened hepatocyte apoptosis and failure of liver regeneration.Moreover,the marked timedependent decrease in the p53 levels implied cancer cell survival and cancer progression.36
Younget al.37reported doxorubicin as a most widely used cytotoxic chemotherapeutic drug for first-line defense,in combination with other antitumor drugs or with surgery and radiation.It is worth mentioning that the normal rats treated with rifampicin(0.107 mg/kg) showed no liver damage (data not shown),normal liver histology(Supplemental Fig.1),and normal liver function tests and tumor marker levels (Supplemental Tables 1 and 2).In this study,the HCC rats that received the doxorubicin and rifampicin combination had normal liver surface,histological architecture,and chemistry.In all treatment groups,the surface of the treated HCC rat liver demonstrated healthy features,except for some surface abnormalities that were observed with doxorubicin monotherapy.In addition,histopathological examination indicated a limited ability of either monotherapy,especially doxorubicin,to improve the liver tissue changes but a demonstrable ability of the combination therapy to reverse the liver tissue damages to near-normal status.These suggested that the combination therapy was successful in curing HCC.Comparison of the treatment groups on the biochemical level showed that rifampicin was the most effective anti-inflammatory and antiapoptotic for normal hepatocytes,and proapoptotic for cancer cells;doxorubicin had better antioxidant effects;and the combination exhibited the best antioxidant effects.In addition,compared with short-term treatment,long-term treatment had better outcomes.In particular,our results indicated gradual time-dependent positive changes;the degree of these changes differed among the treatment groups.Restoration of liver cell function was confirmed by the decrease in the ALT,AST,albumin,and bilirubin levels;reduction of the levels of the tumor markers AFP and CA 19-9 to their baseline ranges;and normalization of the inflammatory markers IL-6 and TNFα.Improved antioxidant status was demonstrated by the increase in the TAC,CAT,and SOD levels and the decrease in the MDA and NO levels.The decrease in the CASP8,CASP3,and Bax levels was clear evidence of decreasing hepatocyte apoptosis.Moreover,the increased p53 level suggested inhibition of cancer cell survival by stimulating cancer cell apoptosis,thereby,halting cancer progression.DNA binding with rifampicin and doxorubicin is established by groove binding and intercalation,respectively,10,38-40and causes DNA damage.Once DNA is damaged,p53 is activated and a p21-dependent cell cycle arrest operates to give a chance for cell damage repair;if the repair fails because of severe DNA damage,apoptotic cell death is initiated to eliminate cancer cells.In this study,we confirmed that p53 activators are potential therapeutic drugs to remedy cancer,as previously documented by Mandinova and Lee,41and Ramoset al.42As discussed above,our results proved that the combination of doxorubicin and rifampicin was superior to doxorubicin or rifampicin alone.
5.Conclusions
In conclusion,our study provided proof that combining rifampicin and doxorubicin to treat HCC somehow led to the recruitment and targeting of all the possible pathways related to the two drugs.In vivo,rifampicin was the most effective anti-inflammatory drug,antiapoptotic for normal hepatocytes,and proapoptotic for cancer cells;doxorubicin exerted better antioxidant effects than rifampicin;and their combination optimized and exerted the best antioxidant effects.Moreover,a longer treatment duration of 90 days enhanced the chance for success.Rifampicin and doxorubicin combination therapy can be considered a promising curative strategy for HCC,but further investigation is required to validate these findings.During the course of this research,we have identified certain limitations that need to be acknowledged.These include the relatively small sample size,which restricts our ability to draw general conclusions.We were unable to investigate the potential effects of the drug treatment on vital organs other than the infected liver,such as the kidneys,heart,and so on.Furthermore,we only studied one animal model.Unfortunately,it was not possible to continue the drug treatment for more extended periods.To enhance the credibility of the research,it is necessary to replicate and validate the findings in larger and more diverse animal models.Additionally,examining the potential effects of the drug treatment on organs other than the infected liver would be beneficial for future studies.
Funding
This research did not receive any specific grant from funding agencies in the public,commercial,or not-for-profit sectors.
Authors’contributions
Zahraa R.Elshahawy,Entsar A.Saad,and Rana R.El-Sadda designed the study.Entsar A.Saad and Rana R.El-Sadda supervised the work.Zahraa R.Elshahawy and Rana R.El-Sadda drafted the manuscript.Zahraa R.Elshahawy and Entsar A.Saad collected the data and performed the statistical analyses.Entsar A.Saad critically revised the manuscript.All authors read and approved the final manuscript.
Declaration of competing interest
All authors declare that there are no competing interests.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.livres.2023.11.005.
杂志排行
Liver Research的其它文章
- Mutation of autophagy-related gene ATG7 increases the risk of severe disease in patients with non-alcoholic fatty liver disease☆
- Should they wait?Two children under 3 years old infected by HCV 1b successfully treated by ledipasvir/sofosbuvir: A report of two cases☆
- Sequential ultrasound molecular imaging for noninvasive identification and assessment of non-alcoholic steatohepatitis in mouse models☆
- Unveiling the effect of estrogen receptors in alcoholic liver disease:A novel outlook☆
- Hepatocellular carcinoma recurrence: Predictors and management☆
- Autophagy modulates physiologic and adaptive response in the liver☆
